SARS-CoV-2-Specific T Lymphocytes Analysis in mRNA-Vaccinated Patients with B-Cell Lymphoid Malignancies on Active Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Anti-SARS-CoV-2 Antibody Assays
2.3. Lymphocyte Profiling and SARS-CoV-2-Specific Memory CD4+ and CD8+ T Lymphocyte Responses
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Humoral Response to mRNA Vaccines
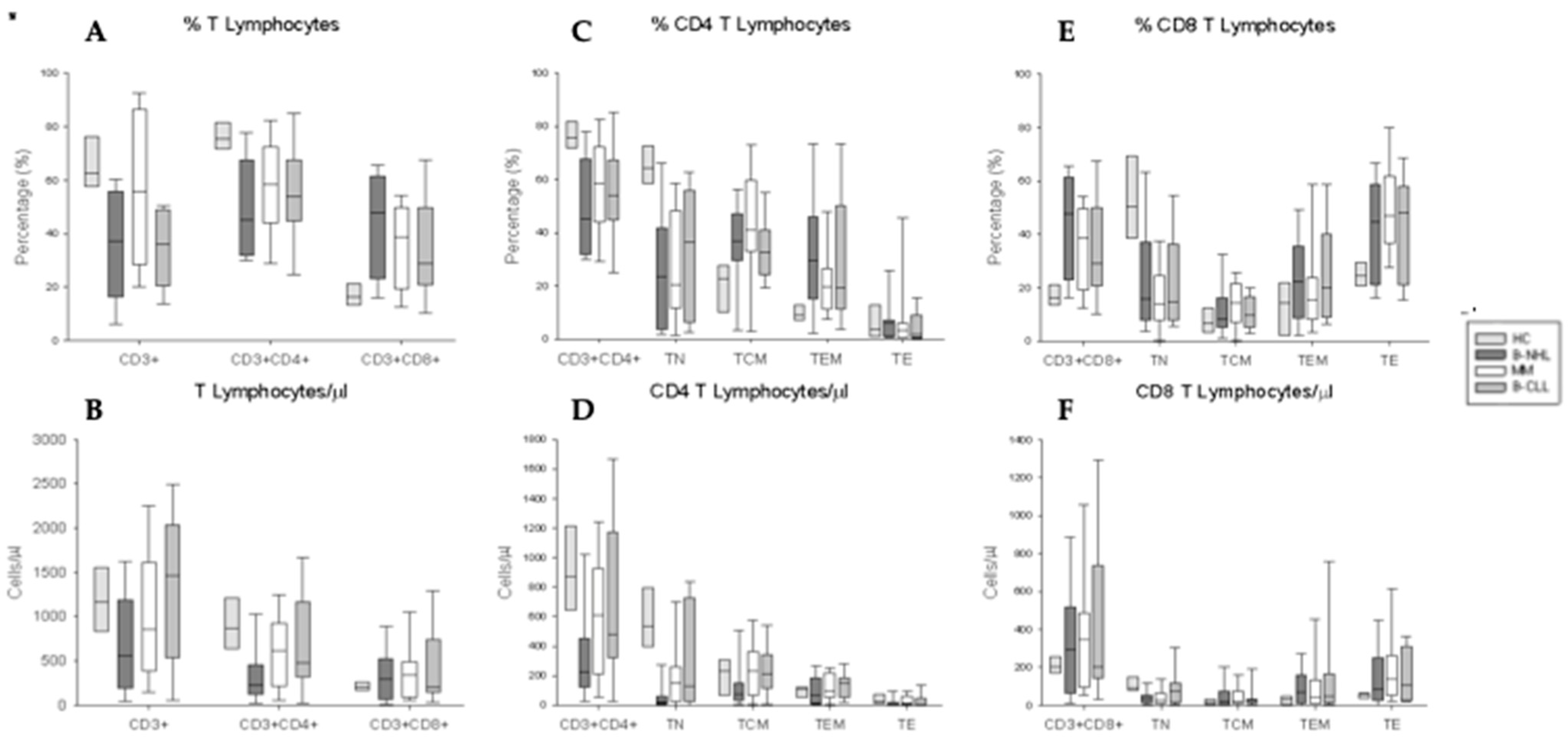
3.3. Phenotypical Characterization of CD4+ and CD8+ T Lymphocytes and Their Activation/Differentiation Stage Subpopulations
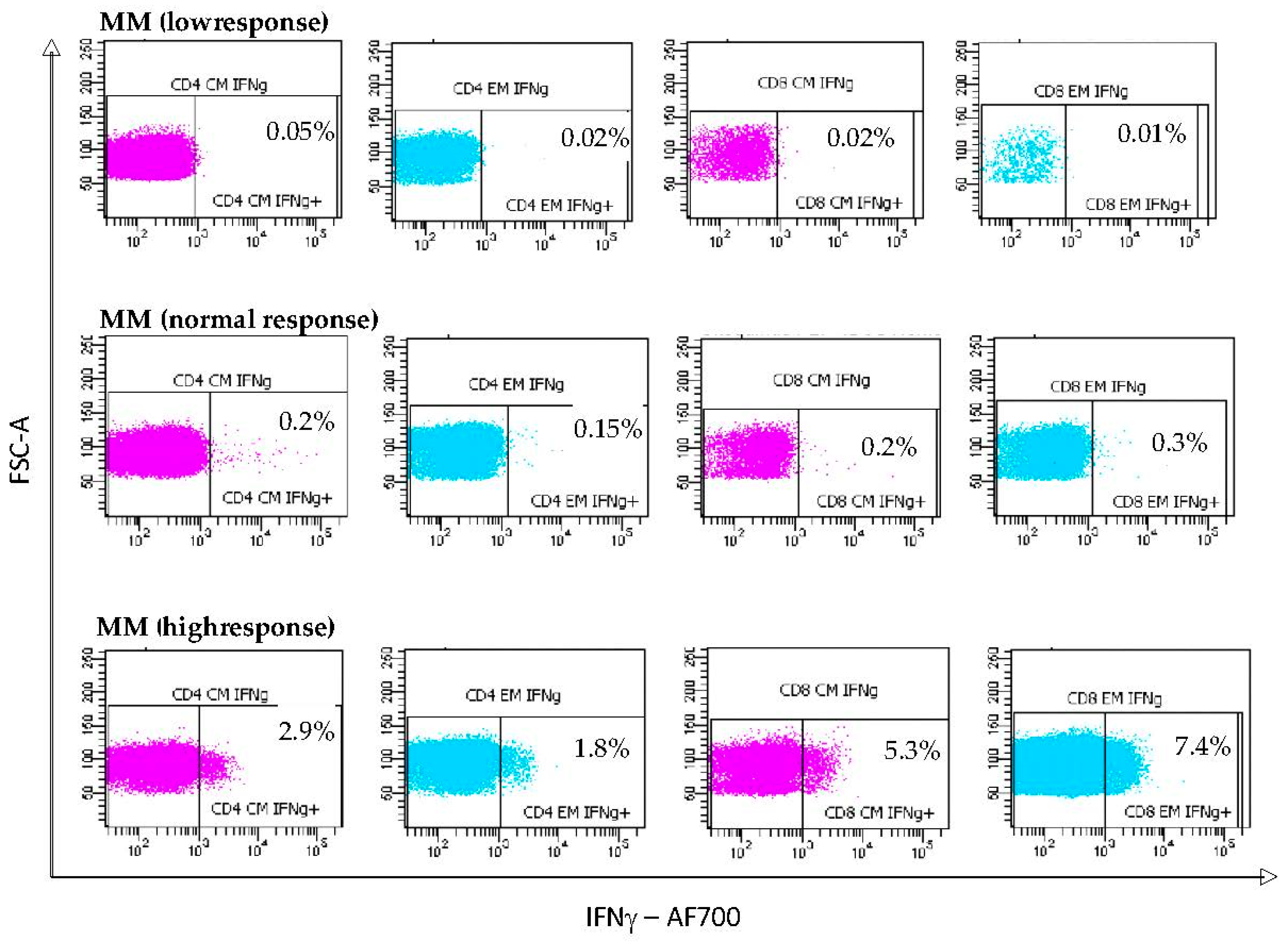
3.4. S-Specific IFNγ T Lymphocyte Responses Induced by mRNA Vaccination
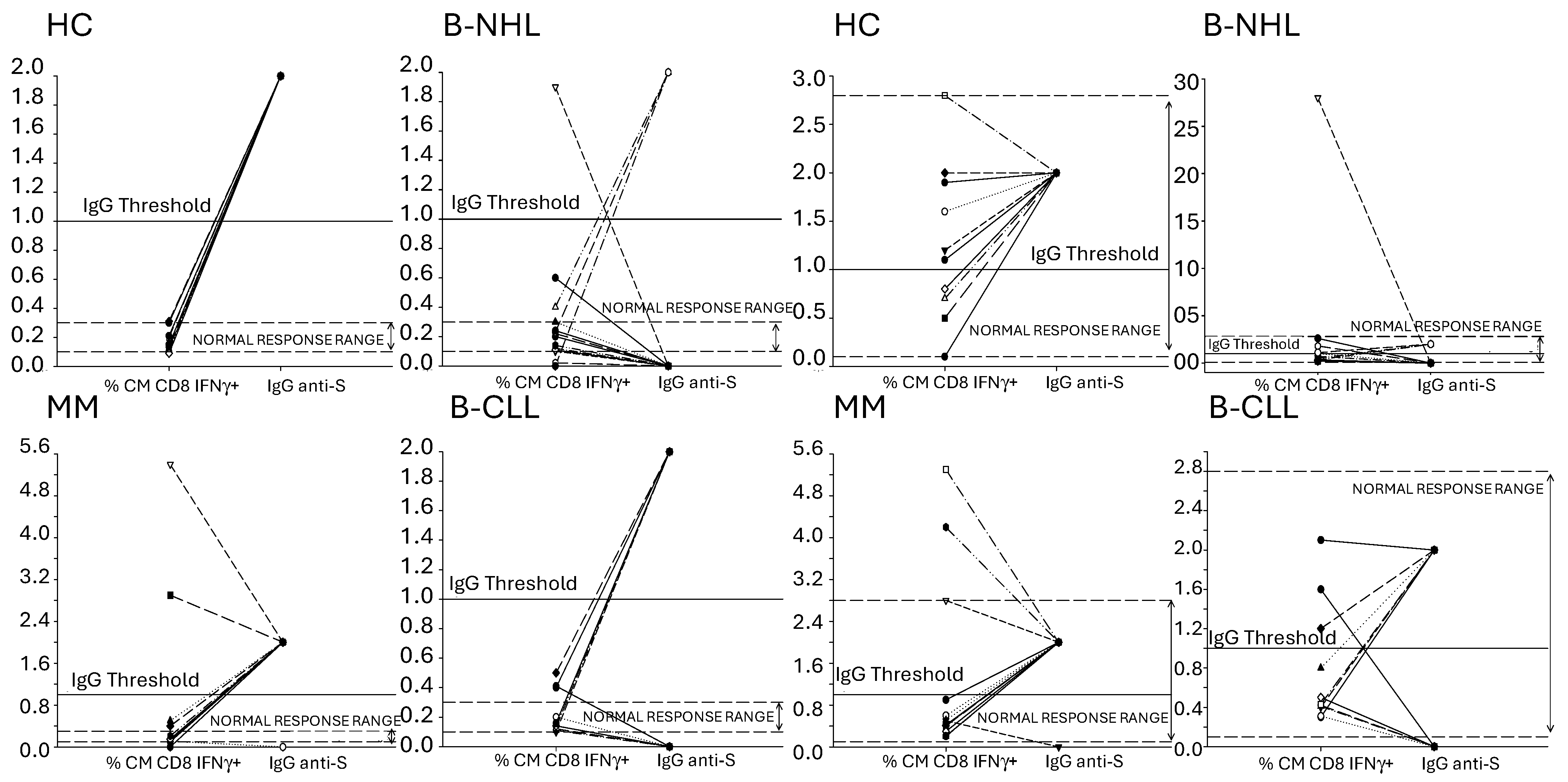
3.5. Concordance of Humoral and SARS-CoV-2-Specific T Lymphocyte Memory Response to the mRNA Vaccines
3.6. SARS-CoV-2 Breakthrough Infection in Vaccinated BCMs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chavez-MacGregor, M.; Lei, X.; Zhao, H.; Scheet, P.; Giordano, S.H. Evaluation of COVID-19 Mortality and Adverse Outcomes in US Patients With or Without Cancer. JAMA Oncol. 2022, 8, 69–78. [Google Scholar] [CrossRef]
- Mato, A.R.; Roeker, L.E.; Lamanna, N.; Allan, J.N.; Leslie, L.; Pagel, J.M.; Patel, K.; Osterborg, A.; Wojenski, D.; Kamdar, M.; et al. Outcomes of COVID-19 in patients with CLL: A multicenter international experience. Blood 2020, 136, 1134–1143. [Google Scholar] [CrossRef] [PubMed]
- Chari, A.; Samur, M.K.; Martinez-Lopez, J.; Cook, G.; Biran, N.; Yong, K.; Hungria, V.; Engelhardt, M.; Gay, F.; Feria, A.G.; et al. Clinical features associated with COVID-19 outcome in multiple myeloma: First results from the International Myeloma Society data set. Blood 2020, 136, 3033–3040. [Google Scholar] [CrossRef] [PubMed]
- Duléry, R.; Lamure, S.; Delord, M.; Di Blasi, R.; Chauchet, A.; Hueso, T.; Rossi, C.; Drenou, B.; Fischer, B.D.; Soussain, C.; et al. Prolonged in-hospital stay and higher mortality after COVID-19 among patients with non-Hodgkin lymphoma treated with B-cell depleting immunotherapy. Am. J. Hematol. 2021, 96, 934–944. [Google Scholar] [CrossRef]
- Pagano, L.; Salmanton-García, J.; Marchesi, F.; Blennow, O.; Gomes da Silva, M.; Glenthøj, A.; van Doesum, J.; Bilgin, Y.M.; López-García, A.; Itri, F.; et al. Breakthrough COVID-19 in vaccinated patients with hematologic malignancies: Results from the EPICOVIDEHA survey. Blood J. Am. Soc. Hematol. 2022, 140, 2773–2787. [Google Scholar] [CrossRef]
- Martínez-López, J.; De la Cruz, J.; Gil-Manso, R.; Alegre, A.; Ortiz, J.; Llamas, P.; Martínez, Y.; Hernández-Rivas, J.; González-Gascón, I.; Benavente, C.; et al. COVID-19 Severity and Survival over Time in Patients with Hematologic Malignancies: A Population-Based Registry Study. Cancers 2023, 15, 1497. [Google Scholar] [CrossRef]
- Hall, V.; Foulkes, S.; Insalata, F.; Kirwan, P.; Saei, A.; Atti, A.; Wellington, E.; Khawam, J.; Munro, K.; Cole, M.; et al. Protection against SARS-CoV-2 after COVID-19 Vaccination and Previous Infection. N. Engl. J. Med. 2022, 386, 1207–1220. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Beltran, W.F.; Lam, E.C.; Astudillo, M.G.; Yang, D.; Miller, T.E.; Feldman, J.; Hauser, B.M.; Caradonna, T.M.; Clayton, K.L.; Nitido, A.D.; et al. COVID-19-neutralizing antibodies predict disease severity and survival. Cell 2021, 184, 476–488.e11. [Google Scholar] [CrossRef]
- Gilbert, P.B.; Donis, R.O.; Koup, R.A.; Fong, Y.; Plotkin, S.A.; Follmann, D. A COVID-19 Milestone Attained—A Correlate of Protection for Vaccines. N. Engl. J. Med. 2022, 387, 2203–2206. [Google Scholar] [CrossRef]
- Herishanu, Y.; Avivi, I.; Aharon, A.; Shefer, G.; Levi, S.; Bronstein, Y.; Morales, M.; Ziv, T.; Arbel, Y.S.; Scarfò, L.; et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood 2021, 137, 3165–3173. [Google Scholar] [CrossRef]
- Ghione, P.; Gu, J.J.; Attwood, K.; Torka, P.; Goel, S.; Sundaram, S.; Mavis, C.; Johnson, M.; Thomas, R.; McWhite, K.M.; et al. Impaired humoral responses to COVID-19 vaccination in patients with lymphoma receiving B-cell-directed therapies. Blood 2021, 138, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Honda, A.; Kurokawa, M. COVID-19 mRNA Vaccine in Patients with Lymphoid Malignancy or Anti-CD20 Antibody Therapy: A Systematic Review and Meta-Analysis. Clin. Lymphoma Myeloma Leuk. 2022, 22, e691–e707. [Google Scholar] [CrossRef] [PubMed]
- Marasco, V.; Carniti, C.; Guidetti, A.; Farina, L.; Magni, M.; Miceli, R.; Calabretta, L.; Verderio, P.; Ljevar, S.; Serpenti, F.; et al. T-cell immune response after mRNA SARS-CoV-2 vaccines is frequently detected also in the absence of seroconversion in patients with lymphoid malignancies. Br. J. Haematol. 2022, 196, 548–558. [Google Scholar] [CrossRef]
- Perry, C.; Luttwak, E.; Balaban, R.; Shefer, G.; Morales, M.M.; Aharon, A.; Tabib, Y.; Cohen, Y.C.; Benyamini, N.; Beyar-Katz, O.; et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with B-cell non-Hodgkin lymphoma. Blood Adv. 2021, 5, 3053–3061. [Google Scholar] [CrossRef]
- Jurgens, E.M.; Ketas, T.J.; Zhao, Z.; Satlin, M.J.; Small, C.B.; Sukhu, A.; Francomano, E.; Klasse, P.J.; Garcia, A.; Nguyenduy, E.; et al. Serologic response to mRNA COVID-19 vaccination in lymphoma patients. Am. J. Hematol. 2021, 96, E410–E413. [Google Scholar] [CrossRef] [PubMed]
- Gurion, R.; Rozovski, U.; Itchaki, G.; Gafter-Gvili, A.; Leibovitch, C.; Raanani, P.; Ben-Zvi, H.; Szwarcwort, M.; Taylor-Abigadol, M.; Dann, E.J.; et al. Humoral serological response to the BNT162b2 vaccine is abrogated in lymphoma patients within the first 12 months following treatment with anti-CD2O antibodies. Haematologica 2022, 107, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Gurion, R.; Rozovski, U.; Itchaki, G.; Gafter-Gvili, A.; Leibovitch, C.; Raanani, P.; Ben-Zvi, H.; Szwarcwort, M.; Taylor-Abigadol, M.; Dann, E.J.; et al. Longitudinal assessment of IFN-I activity and immune profile in critically ill COVID-19 patients with acute respiratory distress syndrome. Crit. Care 2021, 25, 140. [Google Scholar] [CrossRef]
- Bange, E.M.; Han, N.A.; Wileyto, P.; Kim, J.Y.; Gouma, S.; Robinson, J.; Greenplate, A.R.; Hwee, M.A.; Porterfield, F.; Owoyemi, O.; et al. CD8+ T cells contribute to survival in patients with COVID-19 and hematologic cancer. Nat. Med. 2021, 27, 1280–1289. [Google Scholar] [CrossRef]
- Ayers, E.; Canderan, G.; Williams, M.E.; Keshavarz, B.; Portell, C.A.; Wilson, J.M.; Woodfolk, J.A. In-depth cellular and humoral dynamics of the response to COVID-19 vaccine booster in patients with chronic B-cell neoplasms. Blood Cancer J. 2023, 13, 114. [Google Scholar] [CrossRef]
- Atanackovic, D.; Luetkens, T.; Omili, D.; Iraguha, T.; Lutfi, F.; Hardy, N.M.; Fan, X.; Avila, S.V.; Saharia, K.K.; Husson, J.S.; et al. Vaccine-induced T-cell responses against SARS-CoV-2 and its Omicron variant in patients with B cell-depleted lymphoma after CART therapy. Blood 2022, 140, 152–156. [Google Scholar] [CrossRef]
- Pfannes, R.; Pierzchalski, A.; Maddalon, A.; Simion, A.; Zouboulis, C.C.; Behre, G.; Zenclussen, A.C.; Westphal, S.; Fest, S.; Herberth, G. Characterization of post-vaccination SARS-CoV-2 T cell subtypes in patients with different hematologic malignancies and treatments. Front. Immunol. 2023, 14, 1087996. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Muftuoglu, M.; Liang, S.; Basyal, M.; Lv, J.; Akdogan, M.E.; Chen, K.; Andreeff, M.; Flowers, C.R.; Parmar, S. In-depth analysis of SARS-CoV-2-specific T cells reveals diverse differentiation hierarchies in vaccinated individuals. JCI Insight 2022, 7, e156559. [Google Scholar] [CrossRef] [PubMed]
- Rouhani, S.J.; Yu, J.; Olson, D.; Zha, Y.; Pezeshk, A.; Cabanov, A.; Pyzer, A.R.; Trujillo, J.; A Derman, B.; O’Donnell, P.; et al. Antibody and T cell responses to COVID-19 vaccination in patients receiving anticancer therapies. J. Immunother. Cancer 2022, 10, e004766. [Google Scholar] [CrossRef] [PubMed]
- Lehmann-Horn, K.; Schleich, E.; Hertzenberg, D.; Hapfelmeier, A.; Kümpfel, T.; von Bubnoff, N.; Hohlfeld, R.; Berthele, A.; Hemmer, B.; Weber, M.S. Anti-CD20 B-cell depletion enhances monocyte reactivity in neuroimmunological disorders. J. Neuroinflamm. 2011, 8, 146. [Google Scholar] [CrossRef] [PubMed]
- Henry, J.Y.; Labarthe, M.-C.; Meyer, B.; Dasgupta, P.; Dalgleish, A.G.; Galustian, C. Enhanced cross-priming of naive CD8+ T cells by dendritic cells treated by the IMiDs® immunomodulatory compounds lenalidomide and pomalidomide. Immunology 2013, 139, 377–385. [Google Scholar] [CrossRef]
- Gonzalez-Rodriguez, A.P.; Contesti, J.; Huergo-Zapico, L.; Lopez-Soto, A.; Fernández-Guizán, A.; Acebes-Huerta, A.; Gonzalez-Huerta, A.J.; Gonzalez, E.; Fernandez-Alvarez, C.; Gonzalez, S. Prognostic significance of CD8 and CD4 T cells in chronic lymphocytic leukemia. Leuk. Lymphoma 2010, 51, 1829–1836. [Google Scholar] [CrossRef]
- Palma, M.; Gentilcore, G.; Heimersson, K.; Mozaffari, F.; Näsman-Glaser, B.; Young, E.; Rosenquist, R.; Hansson, L.; Österborg, A.; Mellstedt, H. T cells in chronic lymphocytic leukemia display dysregulated expression of immune checkpoints and activation markers. Haematologica 2017, 102, 562–572. [Google Scholar] [CrossRef]
- van Bruggen, J.A.C.; Martens, A.W.J.; Tonino, S.H.; Kater, A.P. Correction: van Bruggen et al. Overcoming the Hurdles of Autologous T-Cell-Based Therapies in B-Cell Non-Hodgkin Lymphoma. Cancers 2020, 12, 3837. Cancers 2021, 13, 4738. [Google Scholar] [CrossRef]
- Apostolidis, S.A.; Kakara, M.; Painter, M.M.; Goel, R.R.; Mathew, D.; Lenzi, K.; Rezk, A.; Patterson, K.R.; Espinoza, D.A.; Kadri, J.C.; et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021, 27, 1990–2001. [Google Scholar] [CrossRef]
- Oh, B.L.Z.; Tan, N.; de Alwis, R.; Kunasegaran, K.; Chen, Z.; Poon, M.; Chan, E.; Low, J.G.H.; Yeoh, A.E.J.; Bertoletti, A.; et al. Enhanced BNT162b2 vaccine-induced cellular immunity in anti-CD19 CAR T cell-treated patients. Blood 2022, 140, 156–160. [Google Scholar] [CrossRef]
- Puzzolo, M.C.; Del Giudice, I.; Peragine, N.; Mariglia, P.; De Propris, M.S.; Cappelli, L.V.; Trentin, L.; Reda, G.; Cuneo, A.; Molica, S.; et al. TH2/TH1 Shift Under Ibrutinib Treatment in Chronic Lymphocytic Leukemia. Front. Oncol. 2021, 11, 637186. [Google Scholar] [CrossRef] [PubMed]
- Solman, I.G.; Blum, L.K.; Burger, J.A.; Kipps, T.J.; Dean, J.P.; James, D.F.; Mongan, A. Impact of long-term ibrutinib treatment on circulating immune cells in previously untreated chronic lymphocytic leukemia. Leuk. Res. 2021, 102, 106520. [Google Scholar] [CrossRef] [PubMed]
- Renrick, A.N.; Thounaojam, M.C.; de Aquino, M.T.P.; Chaudhuri, E.; Pandhare, J.; Dash, C.; Shanker, A. Bortezomib Sustains T Cell Function by Inducing miR-155-Mediated Downregulation of SOCS1 and SHIP1. Front. Immunol. 2021, 12, 607044. [Google Scholar] [CrossRef]
- Galluzzi, L.; Buqué, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell 2015, 28, 690–714. [Google Scholar] [CrossRef] [PubMed]
- Re, D.; Seitz-Polski, B.; Brglez, V.; Carles, M.; Graça, D.; Benzaken, S.; Liguori, S.; Zahreddine, K.; Delforge, M.; Bailly-Maitre, B.; et al. Humoral and cellular responses after a third dose of SARS-CoV-2 BNT162b2 vaccine in patients with lymphoid malignancies. Nat. Commun. 2022, 13, 864. [Google Scholar] [CrossRef]
- Re, D.; Seitz-Polski, B.; Brglez, V.; Carles, M.; Graça, D.; Benzaken, S.; Liguori, S.; Zahreddine, K.; Delforge, M.; Bailly-Maitre, B.; et al. Durable spike-specific T cell responses after different COVID-19 vaccination regimens are not further enhanced by booster vaccination. Sci. Immunol. 2022, 7, eadd3899. [Google Scholar] [CrossRef]
- Zhao, Y.; Kilian, C.; Turner, J.-E.; Bosurgi, L.; Roedl, K.; Bartsch, P.; Gnirck, A.-C.; Cortesi, F.; Schultheiß, C.; Hellmig, M.; et al. Clonal expansion and activation of tissue-resident memory-like Th17 cells expressing GM-CSF in the lungs of severe COVID-19 patients. Sci. Immunol. 2021, 6, eabf6692. [Google Scholar] [CrossRef]
- Mudd, P.A.; Minervina, A.A.; Pogorelyy, M.V.; Turner, J.S.; Kim, W.; Kalaidina, E.; Petersen, J.; Schmitz, A.J.; Lei, T.; Haile, A.; et al. SARS-CoV-2 mRNA vaccination elicits a robust and persistent T follicular helper cell response in humans. Cell 2022, 185, 603–613.e15. [Google Scholar] [CrossRef]

| Variable | Entire Cohort (n = 40) | B-NHL (n = 16) | CLL (n = 11) | MM (n = 13) | Control (n = 10) |
|---|---|---|---|---|---|
| Age, yr; median (IQR) | 68 (61–72) | 69 (59.8–72) | 64 (55.5–74) | 68 (63–71) | 59 (59–59) |
| Male sex; n (%) | 27 (67.5) | 8 (50) | 8 (72.7) | 11 (84.6) | 6 (60) |
| Previous COVID-19, n (%) | 8 (20) | 4 (25) | 1 (9) | 3 (23) | 0 |
| Treatment, n (%) | NA | ||||
| Anti-CD20 +/− chemotherapy † | 15 (37.5) | 15 (93.8) | 0 | 0 | |
| BTK inhibitors | 11 (27.5) | 1 (6.3) | 10 (90.9) | 0 | |
| Anti-CD38 based therapy Φ | 7 (17.5) | 0 | 0 | 7 (53.8) | |
| IMIDs alone | 5 (12.5) | 0 | 0 | 5 (38.5) | |
| Bcl-2 inhibitors | 1 (2.5) | 0 | 1 (9) | 0 | |
| Other target therapies * | 1 (2.5) | 0 | 0 | 1 (7.7) | |
| Lines of therapy, n (%) | NA | ||||
| 1 | 24 (60) | 11 (68.8) | 7 (63.6) | 6 (46.2) | |
| 2 | 9 (22.5) | 2 (12.5) | 2 (18.2) | 5 (38.5) | |
| 3 or more | 7 (17.5) | 3 (18.8) | 2 (18.2) | 2 (15.4) | |
| Laboratory parameters | |||||
| Absolute lymphocyte count, 109/L median (IQR) | 1425 (902.5–2795) | 1150 (555–1462) | 3880 (1655–7280) | 1430 (850–1800) | 1770 (1542–2218) |
| IgG, mg/dL median (IQR) | 650 (493.25–969) | 790.5 (629–969) | 665 (568–1235) | 485 (443–614) | 1130 (1032–1172) |
| IgM, mg/dL median (IQR) | 23 (19–49.25) | 27.5 (19–56.2) | 25 (19.5–51) | 19 (19–34) | 70.5 (48.5–109) |
| IgA, mg/dL median (IQR) | 83 (46.25–173.5) | 124.5 (75.8–213) | 103 (59–194) | 41 (28–72) | 296 (203–381) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García Ramírez, P.; Callejas Charavia, M.; Oliva Martin, R.; Gómez La Hoz, A.M.; Ortega, M.Á.; García Suárez, J.; Álvarez-Mon, M.; Monserrat Sanz, J. SARS-CoV-2-Specific T Lymphocytes Analysis in mRNA-Vaccinated Patients with B-Cell Lymphoid Malignancies on Active Treatment. Vaccines 2024, 12, 961. https://doi.org/10.3390/vaccines12090961
García Ramírez P, Callejas Charavia M, Oliva Martin R, Gómez La Hoz AM, Ortega MÁ, García Suárez J, Álvarez-Mon M, Monserrat Sanz J. SARS-CoV-2-Specific T Lymphocytes Analysis in mRNA-Vaccinated Patients with B-Cell Lymphoid Malignancies on Active Treatment. Vaccines. 2024; 12(9):961. https://doi.org/10.3390/vaccines12090961
Chicago/Turabian StyleGarcía Ramírez, Patricia, Marta Callejas Charavia, Raquel Oliva Martin, Ana María Gómez La Hoz, Miguel Ángel Ortega, Julio García Suárez, Melchor Álvarez-Mon, and Jorge Monserrat Sanz. 2024. "SARS-CoV-2-Specific T Lymphocytes Analysis in mRNA-Vaccinated Patients with B-Cell Lymphoid Malignancies on Active Treatment" Vaccines 12, no. 9: 961. https://doi.org/10.3390/vaccines12090961







