Characterization of Glycoprotein 5-Specific Response in Pigs Vaccinated with Modified Live Porcine Reproductive and Respiratory Syndrome Virus Vaccine Derived from Two Different Lineages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Peptides, Recombinant Proteins, Cells, and Viruses
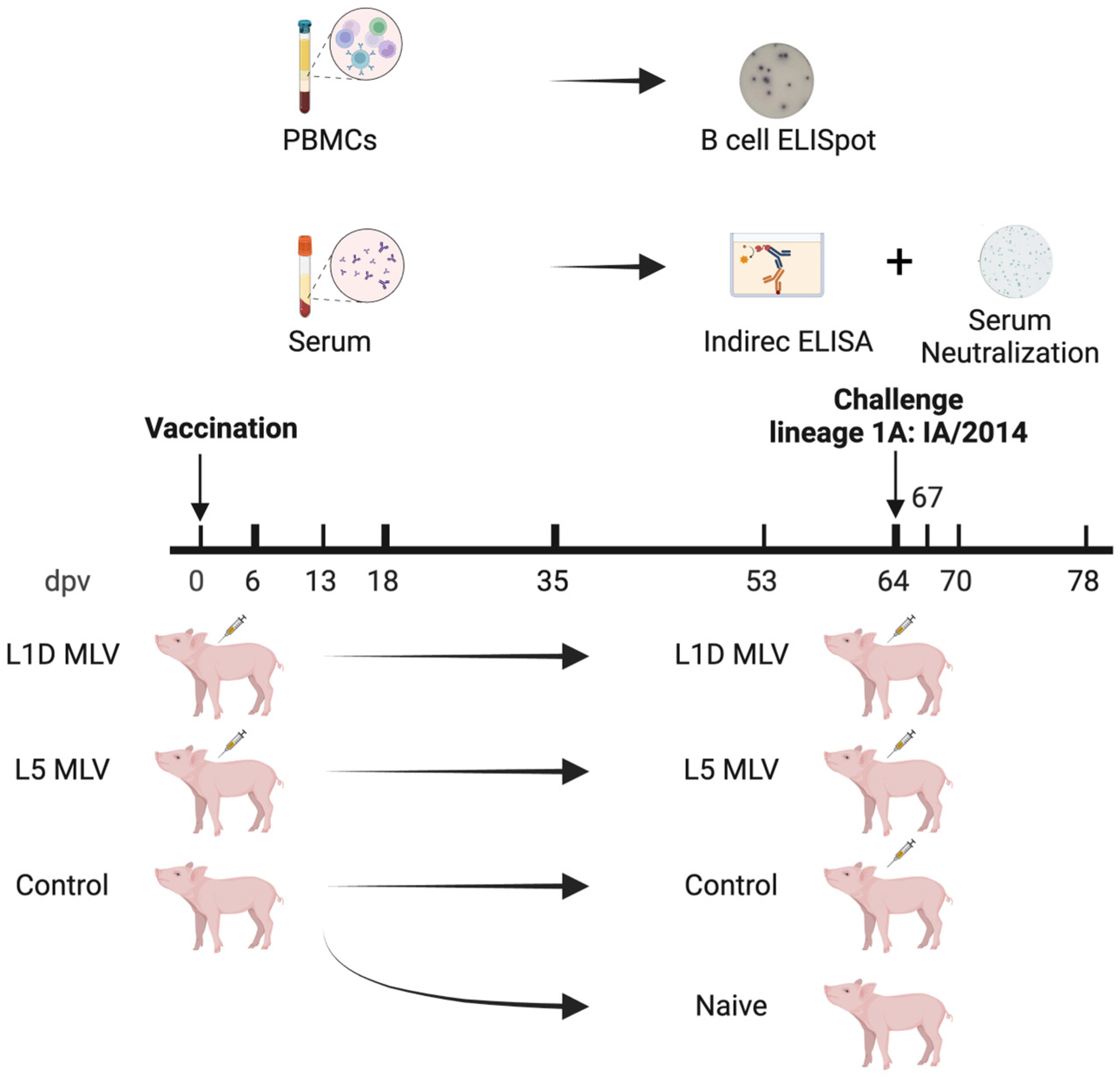
2.2. Animals
2.3. Enzyme-Linked Immunosorbent Assay (ELISA)
2.4. Microplate-Based Focus Reduction Neutralizing Assay
2.5. B-Cell Enzyme-Linked Immunosorbent Spot Assay
2.6. Statistics
3. Results
3.1. Vaccinated Groups Had Similar Antibody Responses to Different Viral Lineages
3.2. GP5-Specific Antibody Response Occurred at a Late-Stage Post-Vaccination
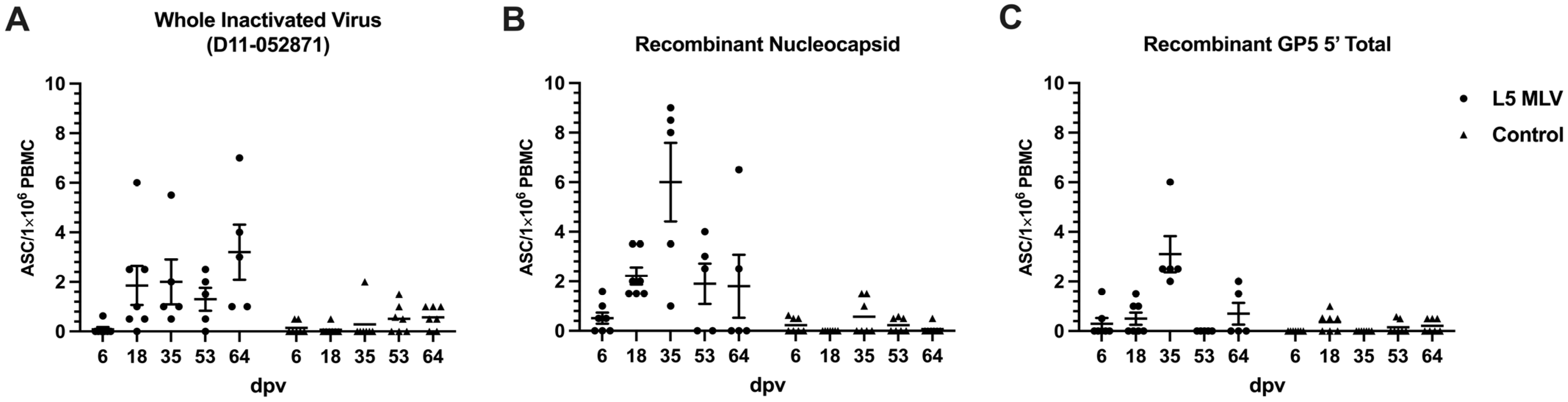
3.3. Homologous Response of GP5-Specific Antibody-Secreting Cells
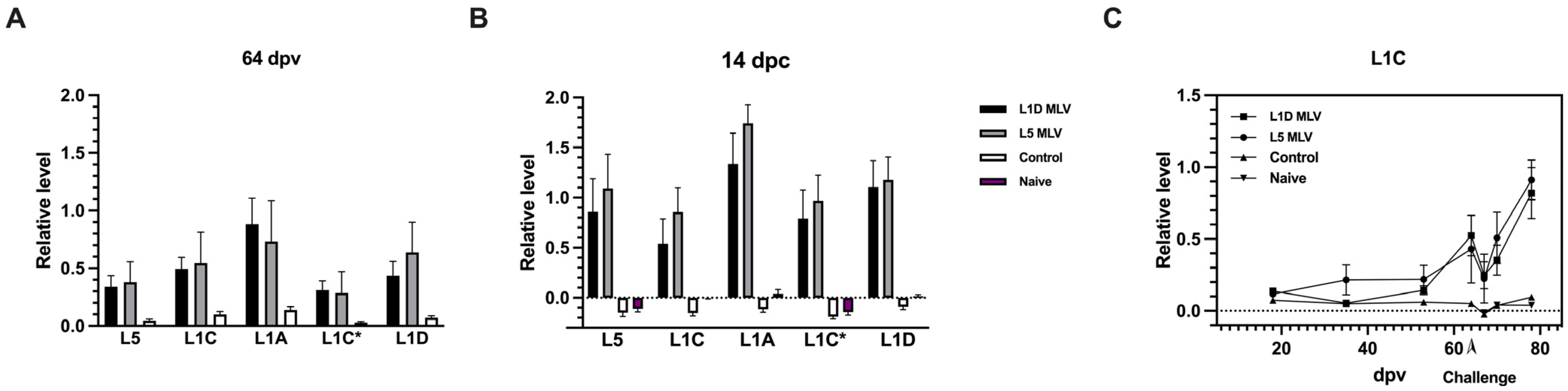
3.4. Antibody Response and Amnestic Reponse to GP5 Ectodomain Peptide (aa 32–aa 61) Representing Different Viral Lineages
3.5. Increased Serum Neutralization Activity Post-Challenge
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PRRS | Porcine reproductive and respiratory syndrome |
| PRRSV | Porcine reproductive and respiratory syndrome virus |
| MLV | Modified live virus |
| NAbs | Neutralizing antibodies |
| GP5 | Glycoprotein 5 |
| N | Nucleocapsid |
| dpv | Days post-vaccination |
| dpi | Days post-infection |
| dpc | Days post-challenge |
| aa | Amino acids |
| PBMCs | Peripheral blood mononuclear cells |
References
- Holtkamp, D.J.; Kliebenstein, J.B.; Neumann, E.J.; Zimmerman, J.J.; Rotto, H.F.; Yoder, T.K.; Wang, C.; Yeske, P.E.; Mowrer, C.L.; Haley, C.A. Assessment of the economic impact of porcine reproductive and respiratory syndrome virus on United States pork producers. J. Swine Health Prod. 2013, 21, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Osemeke, O.H.; Silva, G.D.S.E.; Corzo, C.A.; Kikuti, M.; Yue, X.; Correia Lima Linhares, D.; Holtkamp, D.J. Updating the productivity and economic costs of PRRSV in the US. In Proceedings of the 27th International Pig Veterinary Society Congress, Leipzig, Germany, 4–7 June 2024. [Google Scholar]
- Collins, J.E.; Benfield, D.A.; Christianson, W.T.; Harris, L.; Hennings, J.C.; Shaw, D.P.; Goyal, S.M.; McCullough, S.; Morrison, R.B.; Joo, H.S.; et al. Isolation of swine infertility and respiratory syndrome virus (isolate ATCC VR-2332) in North America and experimental reproduction of the disease in gnotobiotic pigs. J. Vet. Diagn. Investig. 1992, 4, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Wensvoort, G.; Terpstra, C.; Pol, J.M.; ter Laak, E.A.; Bloemraad, M.; de Kluyver, E.P.; Kragten, C.; van Buiten, L.; den Besten, A.; Wagenaar, F.; et al. Mystery swine disease in The Netherlands: The isolation of Lelystad virus. Vet. Q. 1991, 13, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Makau, D.N.; Alkhamis, M.A.; Paploski, I.A.D.; Corzo, C.A.; Lycett, S.; VanderWaal, K. Integrating animal movements with phylogeography to model the spread of PRRSV in the USA. Virus Evol. 2021, 7, veab060. [Google Scholar] [CrossRef] [PubMed]
- Paploski, I.A.D.; Pamornchainavakul, N.; Makau, D.N.; Rovira, A.; Corzo, C.A.; Schroeder, D.C.; Cheeran, M.C.; Doeschl-Wilson, A.; Kao, R.R.; Lycett, S.; et al. Phylogenetic Structure and Sequential Dominance of Sub-Lineages of PRRSV Type-2 Lineage 1 in the United States. Vaccines 2021, 9, 608. [Google Scholar] [CrossRef]
- Shi, M.; Lam, T.T.; Hon, C.C.; Murtaugh, M.P.; Davies, P.R.; Hui, R.K.; Li, J.; Wong, L.T.; Yip, C.W.; Jiang, J.W.; et al. Phylogeny-based evolutionary, demographical, and geographical dissection of North American type 2 porcine reproductive and respiratory syndrome viruses. J. Virol. 2010, 84, 8700–8711. [Google Scholar] [CrossRef]
- Rupasinghe, R.; Lee, K.; Liu, X.; Gauger, P.C.; Zhang, J.; Martinez-Lopez, B. Molecular Evolution of Porcine Reproductive and Respiratory Syndrome Virus Field Strains from Two Swine Production Systems in the Midwestern United States from 2001 to 2020. Microbiol. Spectr. 2022, 10, e0263421. [Google Scholar] [CrossRef] [PubMed]
- Chae, C. Commercial PRRS Modified-Live Virus Vaccines. Vaccines 2021, 9, 185. [Google Scholar] [CrossRef]
- Hou, F.H.; Lee, W.C.; Liao, J.W.; Chien, M.S.; Kuo, C.J.; Chung, H.P.; Chia, M.Y. Evaluation of a type 2 modified live porcine reproductive and respiratory syndrome vaccine against heterologous challenge of a lineage 3 highly virulent isolate in pigs. PeerJ 2020, 8, e8840. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Galliher-Beckley, A.; Pappan, L.; Trible, B.; Kerrigan, M.; Beck, A.; Hesse, R.; Blecha, F.; Nietfeld, J.C.; Rowland, R.R.; et al. Comparison of host immune responses to homologous and heterologous type II porcine reproductive and respiratory syndrome virus (PRRSV) challenge in vaccinated and unvaccinated pigs. BioMed Res. Int. 2014, 2014, 416727. [Google Scholar] [CrossRef]
- Madapong, A.; Temeeyasen, G.; Saeng-Chuto, K.; Tripipat, T.; Navasakuljinda, W.; Boonsoongnern, A.; Tantituvanont, A.; Nilubol, D. Humoral immune responses and viral shedding following vaccination with modified live porcine reproductive and respiratory syndrome virus vaccines. Arch. Virol. 2017, 162, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Proctor, J.; Wolf, I.; Brodsky, D.; Cortes, L.M.; Frias-De-Diego, A.; Almond, G.W.; Crisci, E.; Negrao Watanabe, T.T.; Hammer, J.M.; Kaser, T. Heterologous vaccine immunogenicity, efficacy, and immune correlates of protection of a modified-live virus porcine reproductive and respiratory syndrome virus vaccine. Front. Microbiol. 2022, 13, 977796. [Google Scholar] [CrossRef]
- Zhou, L.; Ge, X.; Yang, H. Porcine Reproductive and Respiratory Syndrome Modified Live Virus Vaccine: A “Leaky” Vaccine with Debatable Efficacy and Safety. Vaccines 2021, 9, 362. [Google Scholar] [CrossRef] [PubMed]
- Osorio, F.A.; Galeota, J.A.; Nelson, E.; Brodersen, B.; Doster, A.; Wills, R.; Zuckermann, F.; Laegreid, W.W. Passive transfer of virus-specific antibodies confers protection against reproductive failure induced by a virulent strain of porcine reproductive and respiratory syndrome virus and establishes sterilizing immunity. Virology 2002, 302, 9–20. [Google Scholar] [CrossRef]
- Luo, Q.; Zheng, Y.; Zhang, H.; Yang, Z.; Sha, H.; Kong, W.; Zhao, M.; Wang, N. Research Progress on Glycoprotein 5 of Porcine Reproductive and Respiratory Syndrome Virus. Animals 2023, 13, 813. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, M.; Galeota, J.A.; Jar, A.M.; Platt, K.B.; Osorio, F.A.; Lopez, O.J. Identification of neutralizing and nonneutralizing epitopes in the porcine reproductive and respiratory syndrome virus GP5 ectodomain. J. Virol. 2002, 76, 4241–4250. [Google Scholar] [CrossRef]
- Young, J.E.; Dvorak, C.M.T.; Graham, S.P.; Murtaugh, M.P. Isolation of Porcine Reproductive and Respiratory Syndrome Virus GP5-Specific, Neutralizing Monoclonal Antibodies From Hyperimmune Sows. Front. Immunol. 2021, 12, 638493. [Google Scholar] [CrossRef]
- Loemba, H.D.; Mounir, S.; Mardassi, H.; Archambault, D.; Dea, S. Kinetics of humoral immune response to the major structural proteins of the porcine reproductive and respiratory syndrome virus. Arch. Virol. 1996, 141, 751–761. [Google Scholar] [CrossRef]
- Lopez, O.J.; Osorio, F.A. Role of neutralizing antibodies in PRRSV protective immunity. Vet. Immunol. Immunopathol. 2004, 102, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Liu, Z.; Tong, X.; Wang, Z.; Xu, S.; Chen, Q.; Zhou, J.; Fang, L.; Wang, D.; Xiao, S. Evolutionary Dynamics of Type 2 Porcine Reproductive and Respiratory Syndrome Virus by Whole-Genome Analysis. Viruses 2021, 13, 2469. [Google Scholar] [CrossRef]
- Baker, R.B.; Yu, W.; Fuentes, M.; Johnson, C.R.; Peterson, L.; Rossow, K.; Daniels, C.S.; Daniels, A.M.; Polson, D.; Murtaugh, M.P. Prairie dog (Cynomys ludovicianus) is not a host for porcine reproductive and respiratory syndrome virus. J. Swine Health Prod. 2007, 15, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A Simple Method OF Estimating Fifty per Cent Endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Schroeder, D.C.; Odogwu, N.M.; Kevill, J.; Yang, M.; Krishna, V.D.; Kikuti, M.; Pamornchainavakul, N.; Vilalta, C.; Sanhueza, J.; Corzo, C.A.; et al. Phylogenetically Distinct Near-Complete Genome Sequences of Porcine Reproductive and Respiratory Syndrome Virus Type 2 Variants from Four Distinct Disease Outbreaks at U.S. Swine Farms over the Past 6 Years. Microbiol. Resour. Announc. 2021, 10, e0026021. [Google Scholar] [CrossRef]
- Gorcyca, D.; Schlesinger, K.; Geeding, P.; Chladek, D.; Short, J. Protection against the reproductive disease caused by porcine reproductive and respiratory syndrome (PRRS) virus by vaccinating with a modified live virus PRRS vaccine prior to breeding. In Proceedings of the 14th International Pig Veterinary Society Congress, Bologna, Italy, 7–10 July 1996; pp. 203–214. [Google Scholar]
- Renukaradhya, G.J.; Meng, X.J.; Calvert, J.G.; Roof, M.; Lager, K.M. Live porcine reproductive and respiratory syndrome virus vaccines: Current status and future direction. Vaccine 2015, 33, 4069–4080. [Google Scholar] [CrossRef]
- Cho, H.J.; Entz, S.C.; Magar, R.; Joo, H.S. Performance of ELISA antigens prepared from 8 isolates of porcine reproductive and respiratory syndrome virus with homologous and heterologous antisera. Can. J. Vet. Res. 1997, 61, 299–304. [Google Scholar] [PubMed]
- Correas, I.; Osorio, F.A.; Steffen, D.; Pattnaik, A.K.; Vu, H.L.X. Cross reactivity of immune responses to porcine reproductive and respiratory syndrome virus infection. Vaccine 2017, 35, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.I.; Lee, D.S.; Johnson, W.; Roof, M.; Cha, S.H.; Yoon, K.J. Effect of genotypic and biotypic differences among PRRS viruses on the serologic assessment of pigs for virus infection. Vet. Microbiol. 2007, 123, 1–14. [Google Scholar] [CrossRef]
- Sinkora, M.; Butler, J.E.; Lager, K.M.; Potockova, H.; Sinkorova, J. The comparative profile of lymphoid cells and the T and B cell spectratype of germ-free piglets infected with viruses SIV, PRRSV or PCV2. Vet. Res. 2014, 45, 91. [Google Scholar] [CrossRef]
- Nelson, E.A.; Christopher-Hennings, J.; Benfield, D.A. Serum Immune Responses to the Proteins of Porcine Reproductive and Respiratory Syndrome (PRRS) Virus. J. Vet. Diagn. Investig. 1994, 6, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Mulupuri, P.; Zimmerman, J.J.; Hermann, J.; Johnson, C.R.; Cano, J.P.; Yu, W.; Dee, S.A.; Murtaugh, M.P. Antigen-specific B-cell responses to porcine reproductive and respiratory syndrome virus infection. J. Virol. 2008, 82, 358–370. [Google Scholar] [CrossRef]
- Yoon, K.J.; Zimmerman, J.J.; Swenson, S.L.; McGinley, M.J.; Eernisse, K.A.; Brevik, A.; Rhinehart, L.L.; Frey, M.L.; Hill, H.T.; Platt, K.B. Characterization of the humoral immune response to porcine reproductive and respiratory syndrome (PRRS) virus infection. J. Vet. Diagn. Investig. 1995, 7, 305–312. [Google Scholar] [CrossRef]
- Biernacka, K.; Podgorska, K.; Tyszka, A.; Stadejek, T. Comparison of six commercial ELISAs for the detection of antibodies against porcine reproductive and respiratory syndrome virus (PRRSV) in field serum samples. Res. Vet. Sci. 2018, 121, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Lunney, J.K.; Fang, Y.; Ladinig, A.; Chen, N.; Li, Y.; Rowland, B.; Renukaradhya, G.J. Porcine Reproductive and Respiratory Syndrome Virus (PRRSV): Pathogenesis and Interaction with the Immune System. Annu. Rev. Anim. Biosci. 2016, 4, 129–154. [Google Scholar] [CrossRef]
- Mardassi, H.; Athanassious, R.; Mounir, S.; Dea, S. Porcine reproductive and respiratory syndrome virus: Morphological, biochemical and serological characteristics of Quebec isolates associated with acute and chronic outbreaks of porcine reproductive and respiratory syndrome. Can. J. Vet. Res. 1994, 58, 55–64. [Google Scholar] [PubMed]
- Xiao, Y.; An, T.Q.; Tian, Z.J.; Wei, T.C.; Jiang, Y.F.; Peng, J.M.; Zhou, Y.J.; Cai, X.H.; Tong, G.Z. The gene expression profile of porcine alveolar macrophages infected with a highly pathogenic porcine reproductive and respiratory syndrome virus indicates overstimulation of the innate immune system by the virus. Arch. Virol. 2015, 160, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Murtaugh, M.P. Dissociation of porcine reproductive and respiratory syndrome virus neutralization from antibodies specific to major envelope protein surface epitopes. Virology 2012, 433, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Popescu, L.N.; Trible, B.R.; Chen, N.; Rowland, R.R.R. GP5 of porcine reproductive and respiratory syndrome virus (PRRSV) as a target for homologous and broadly neutralizing antibodies. Vet. Microbiol. 2017, 209, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Kick, A.R.; Amaral, A.F.; Frias-De-Diego, A.; Cortes, L.M.; Fogle, J.E.; Crisci, E.; Almond, G.W.; Kaser, T. The Local and Systemic Humoral Immune Response Against Homologous and Heterologous Strains of the Type 2 Porcine Reproductive and Respiratory Syndrome Virus. Front. Immunol. 2021, 12, 637613. [Google Scholar] [CrossRef] [PubMed]
- Scortti, M.; Prieto, C.; Simarro, I.; Castro, J.M. Reproductive performance of gilts following vaccination and subsequent heterologous challenge with European strains of porcine reproductive and respiratory syndrome virus. Theriogenology 2006, 66, 1884–1893. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Liu, L.; Xu, B.; Hu, J.; Kuang, H.; Wang, X.; Wang, L.; Cui, X.; Sun, H.; Rong, J. Displaying epitope B and epitope 7 of porcine reproductive and respiratory syndrome virus on virus like particles of porcine circovirus type 2 provides partial protection to pigs. J. Vet. Med. Sci. 2021, 83, 1263–1272. [Google Scholar] [CrossRef]
- Kardani, K.; Bolhassani, A.; Shahbazi, S. Prime-boost vaccine strategy against viral infections: Mechanisms and benefits. Vaccine 2016, 34, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Bhagchandani, S.H.; Yang, L.; Lam, J.H.; Maiorino, L.; Ben-Akiva, E.; Rodrigues, K.A.; Romanov, A.; Suh, H.; Aung, A.; Wu, S.; et al. Two-dose priming immunization amplifies humoral immunity by synchronizing vaccine delivery with the germinal center response. Sci. Immunol. 2024, 9, eadl3755. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Culhane, M.R.; Cheeran, M.; Galina Pantoja, L.; Jansen, M.L.; Amodie, D.; Mellencamp, M.A.; Torremorell, M. Exploring heterologous prime-boost vaccination approaches to enhance influenza control in pigs. Vet. Res. 2020, 51, 89. [Google Scholar] [CrossRef] [PubMed]
- Weesendorp, E.; Stockhofe-Zurwieden, N.; Nauwynck, H.J.; Popma-De Graaf, D.J.; Rebel, J.M. Characterization of immune responses following homologous reinfection of pigs with European subtype 1 and 3 porcine reproductive and respiratory syndrome virus strains that differ in virulence. Vet. Microbiol. 2016, 182, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Rahe, M.C.; Dvorak, C.M.T.; Patterson, A.; Roof, M.; Murtaugh, M.P. The PRRSV-Specific Memory B Cell Response Is Long-Lived in Blood and Is Boosted During Live Virus Re-exposure. Front. Immunol. 2020, 11, 247. [Google Scholar] [CrossRef]
- Martinez-Lobo, F.J.; Diez-Fuertes, F.; Simarro, I.; Castro, J.M.; Prieto, C. The Ability of Porcine Reproductive and Respiratory Syndrome Virus Isolates to Induce Broadly Reactive Neutralizing Antibodies Correlates With In Vivo Protection. Front. Immunol. 2021, 12, 691145. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.; Yoo, S.J.; Sunwoo, S.Y.; Lee, D.U.; Je, S.H.; Park, J.W.; Park, C.K.; Lyoo, Y.S. Independent evolution of porcine reproductive and respiratory syndrome virus 2 with genetic heterogeneity in antigenic regions of structural proteins in Korea. Arch. Virol. 2019, 164, 213–224. [Google Scholar] [CrossRef]
- Das, P.B.; Dinh, P.X.; Ansari, I.H.; de Lima, M.; Osorio, F.A.; Pattnaik, A.K. The minor envelope glycoproteins GP2a and GP4 of porcine reproductive and respiratory syndrome virus interact with the receptor CD163. J. Virol. 2010, 84, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Moura, C.A.A.; Philips, R.; Silva, G.S.; Holtkamp, D.J.; Linhares, D.C.L. Comparison of virus detection, productivity, and economic performance between lots of growing pigs vaccinated with two doses or one dose of PRRS MLV vaccine, under field conditions. Prev. Vet. Med. 2022, 204, 105669. [Google Scholar] [CrossRef] [PubMed]


| Isolate ID | Sequence (N to C Terminus) | Lineage |
|---|---|---|
| D11-052871 | SNGSSSHLQLIYNLTLCELNGTDWLANKFD | L5 |
| 46/2020 | NNSSSSHLQLIYNLTICELNGTDWLNERFD | L1C |
| IA/2014 | NNSSSSHLQLIYNLTICELNGTDWLNKTFD | L1A |
| KP283416 | NSNSSSHLQLIYNLTICELNGTDWLSKKFD | L1C * |
| KY348849 | SSNSSSHLQLIYNLTICELNGTDWLNNEFD | L1D |
| Group/Isolate | Pig ID | 64 dpv | 14 dpc | ||
|---|---|---|---|---|---|
| IA/2014 (L1D) | D11-052871 (L5) | IA/2014 (L1D) | D11-052871 (L5) | ||
| L1D MLV | 1–5 | <4 | 4 | >128 | 32 |
| 1–7 | <4 | 64 | 64 | >128 | |
| 1–9 | <4 | 32 | >128 | >128 | |
| 1–13 | 8 | 64 | >128 | >128 | |
| 1–14 | <4 | <4 | 32 | 32 | |
| 1–15 | 32 | <4 | >128 | >128 | |
| 1–21 | 4 | 4 | 64 | >128 | |
| Neutralization (%) | 2/7 (28.6%) | 3/7 (42.9%) | 7/7 (100%) | 7/7 (100%) | |
| L5 MLV | 2–3 | <4 | 8 | >128 | >128 |
| 2–4 | <4 | 16 | 64 | >128 | |
| 2–8 | 32 | 32 | >128 | >128 | |
| 2–12 | <4 | <4 | >128 | >128 | |
| 2–20 | <4 | 4 | >128 | >128 | |
| Neutralization (%) | 1/5 (20.0%) | 3/5 (60.0%) | 5/5 (100%) | 5/5 (100%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Krishna, V.D.; Paploski, I.A.D.; VanderWaal, K.; Schroeder, D.C.; Cheeran, M.C.-J. Characterization of Glycoprotein 5-Specific Response in Pigs Vaccinated with Modified Live Porcine Reproductive and Respiratory Syndrome Virus Vaccine Derived from Two Different Lineages. Vaccines 2025, 13, 247. https://doi.org/10.3390/vaccines13030247
Huang J, Krishna VD, Paploski IAD, VanderWaal K, Schroeder DC, Cheeran MC-J. Characterization of Glycoprotein 5-Specific Response in Pigs Vaccinated with Modified Live Porcine Reproductive and Respiratory Syndrome Virus Vaccine Derived from Two Different Lineages. Vaccines. 2025; 13(3):247. https://doi.org/10.3390/vaccines13030247
Chicago/Turabian StyleHuang, Jing, Venkatramana D. Krishna, Igor A. D. Paploski, Kimberly VanderWaal, Declan C. Schroeder, and Maxim C.-J. Cheeran. 2025. "Characterization of Glycoprotein 5-Specific Response in Pigs Vaccinated with Modified Live Porcine Reproductive and Respiratory Syndrome Virus Vaccine Derived from Two Different Lineages" Vaccines 13, no. 3: 247. https://doi.org/10.3390/vaccines13030247
APA StyleHuang, J., Krishna, V. D., Paploski, I. A. D., VanderWaal, K., Schroeder, D. C., & Cheeran, M. C.-J. (2025). Characterization of Glycoprotein 5-Specific Response in Pigs Vaccinated with Modified Live Porcine Reproductive and Respiratory Syndrome Virus Vaccine Derived from Two Different Lineages. Vaccines, 13(3), 247. https://doi.org/10.3390/vaccines13030247









