Vaccination Against RhoC in Prostate Cancer Patients Induces Potent and Long-Lasting CD4+ T Cell Responses with Cytolytic Potential in the Absence of Clinical Efficacy: A Randomized Phase II Trial
Abstract
1. Introduction
2. Materials and Methods
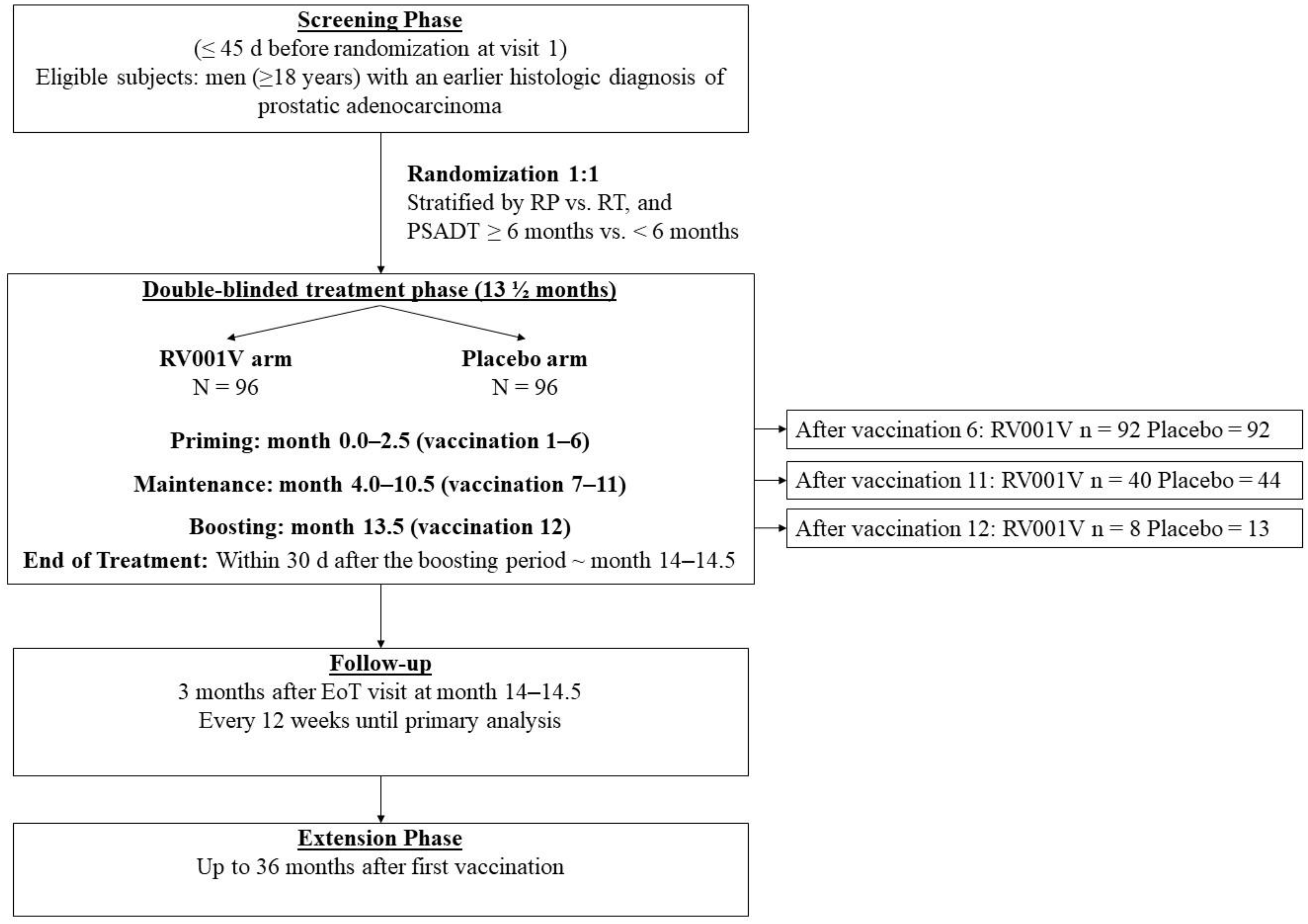
2.1. Study Design
2.2. Isolation of Peripheral Blood Mononuclear Cells from Patients
2.3. Isolation of Peripheral Blood Mononuclear Cells from Healthy Donor Blood
2.4. Culturing of the FM3 Cancer Cell Line
2.5. In Vitro Immunomonitoring for Anti-Vaccine T Cells
2.6. Establishment of RV001-Specific T Cell Cultures from a Healthy Donor
2.7. Establishment of RV001-Specific T Cell Cultures from a Patient Blood Sample
2.8. Enrichment and Sorting of RV001-Specific T Cell Cultures and T Cell Cloning
2.9. Intracellular Cytokine Staining
2.10. T-Cell Receptor Beta-Chain Variable Region Flow Cytometry Panel
2.11. T-Cell Receptor (TCR) PCR Panel
2.12. Western Blotting
2.13. xCELLigence Real-Time Cell Analysis Assay
3. Results
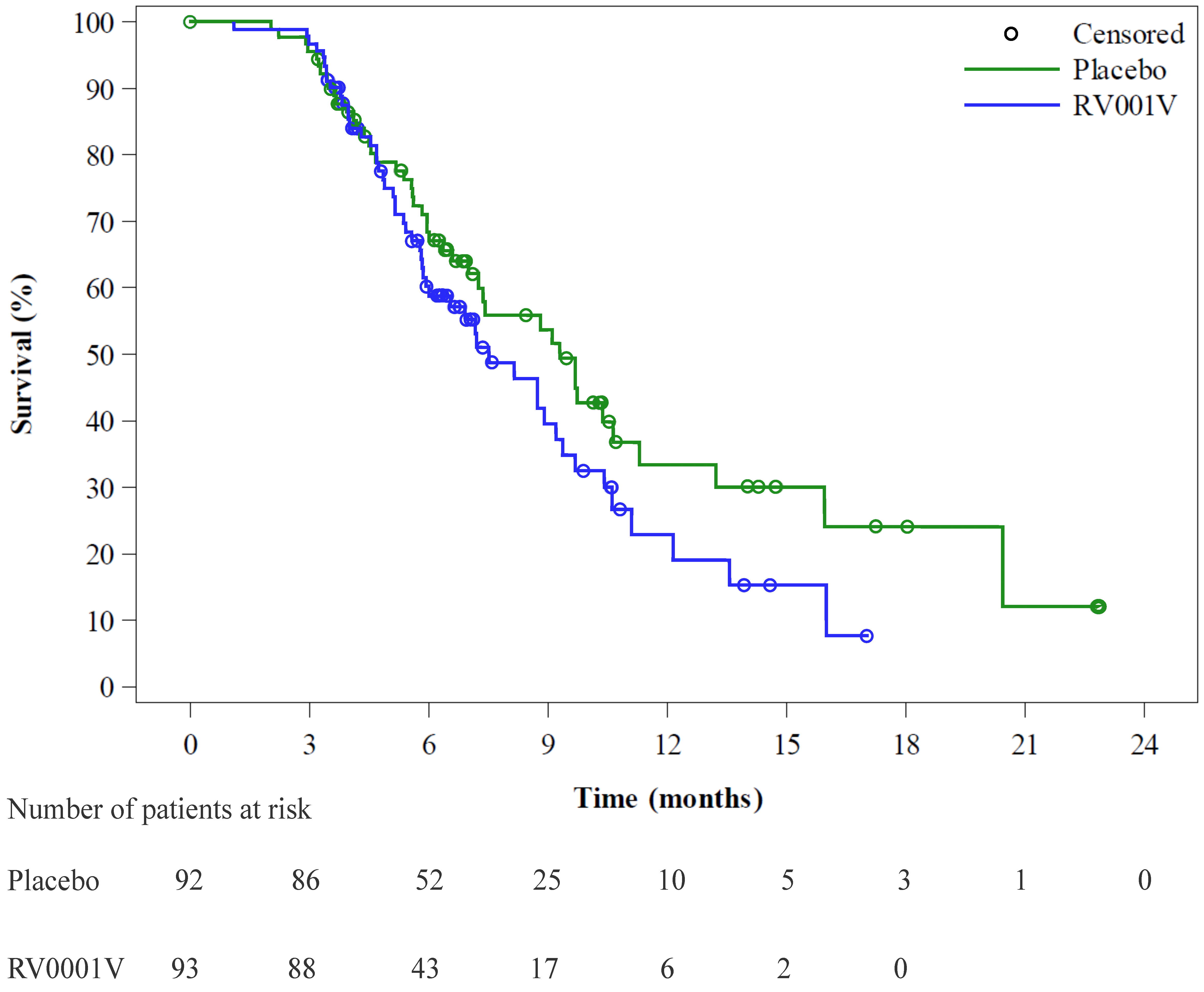
3.1. Clinical Outcome
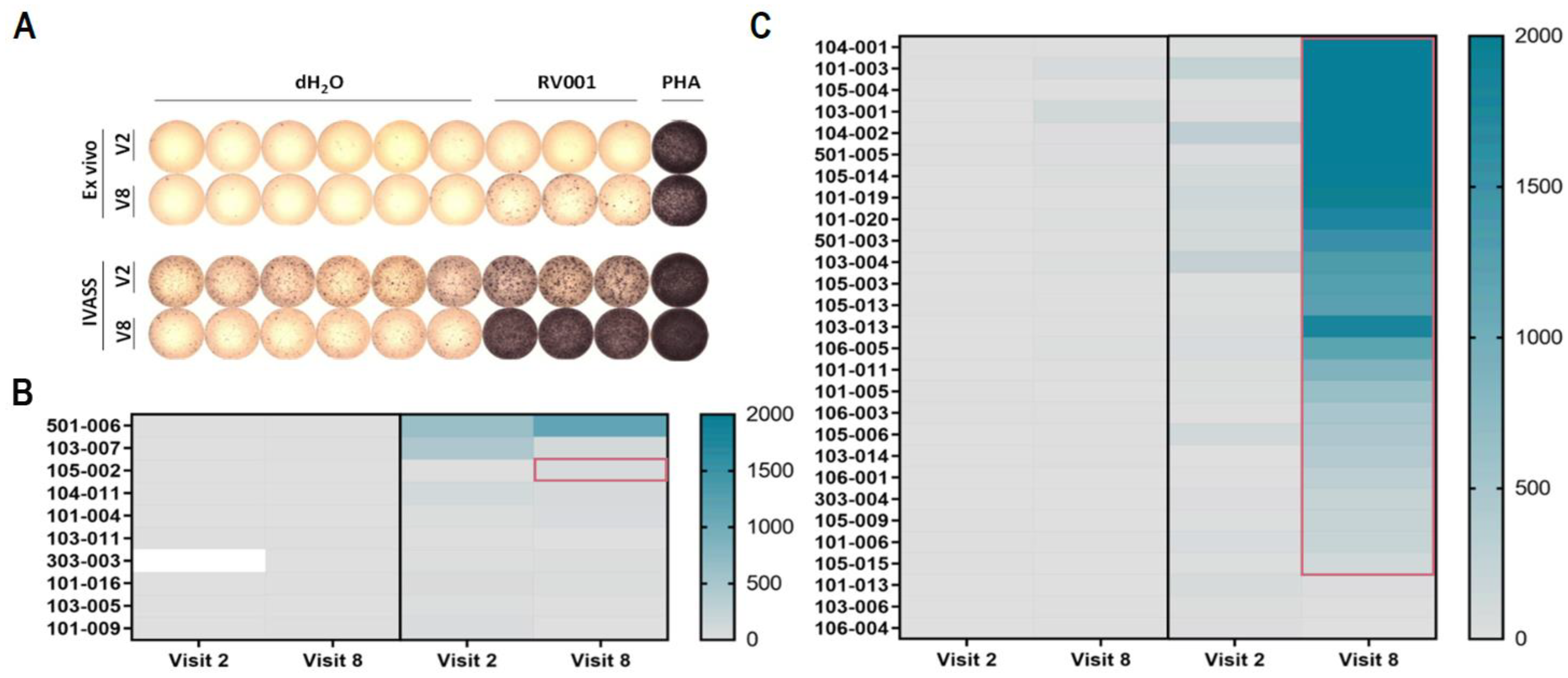
3.2. Vaccination Against RhoC Induces Potent and Lasting Vaccine-Specific T Cell Responses
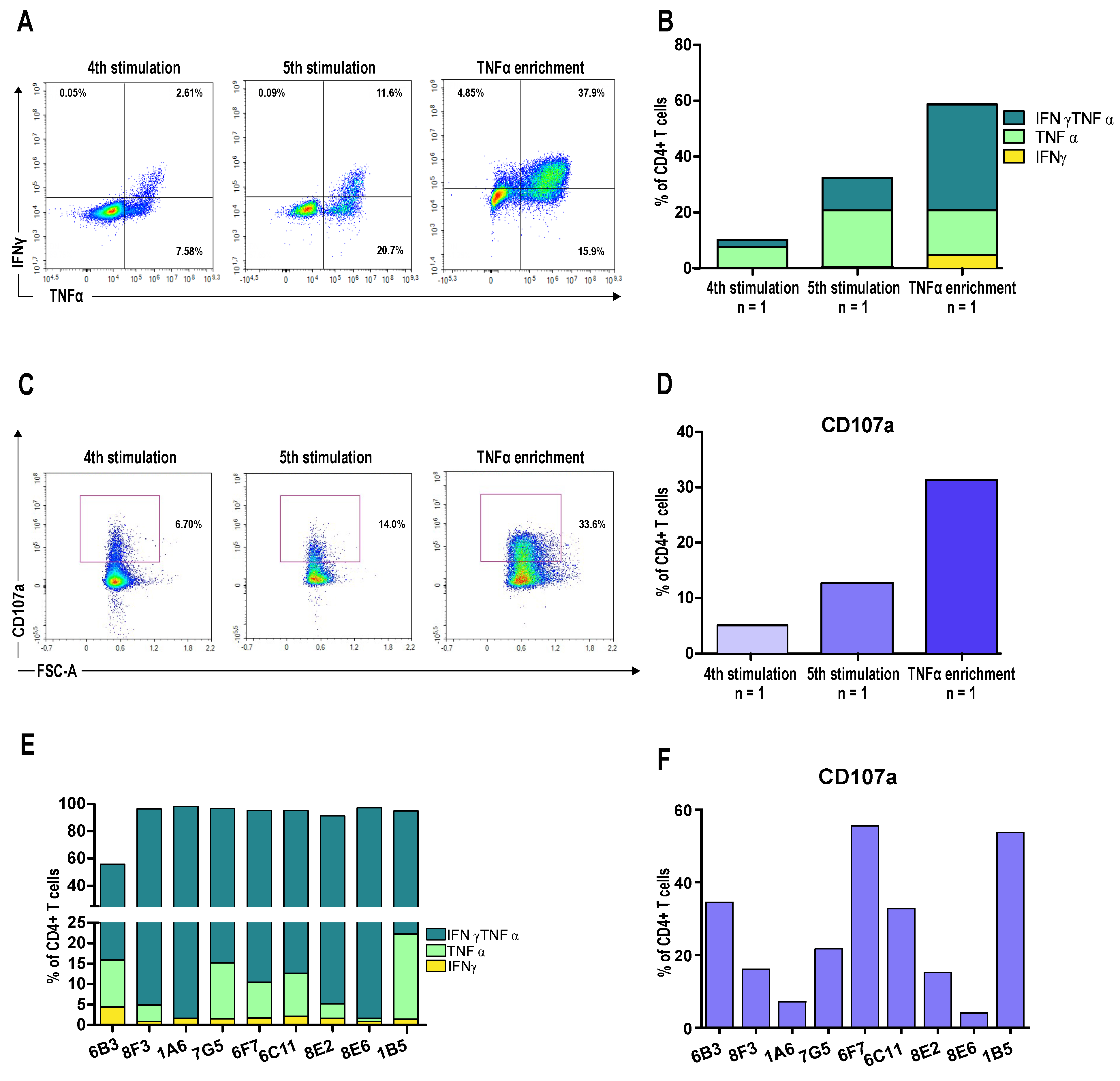
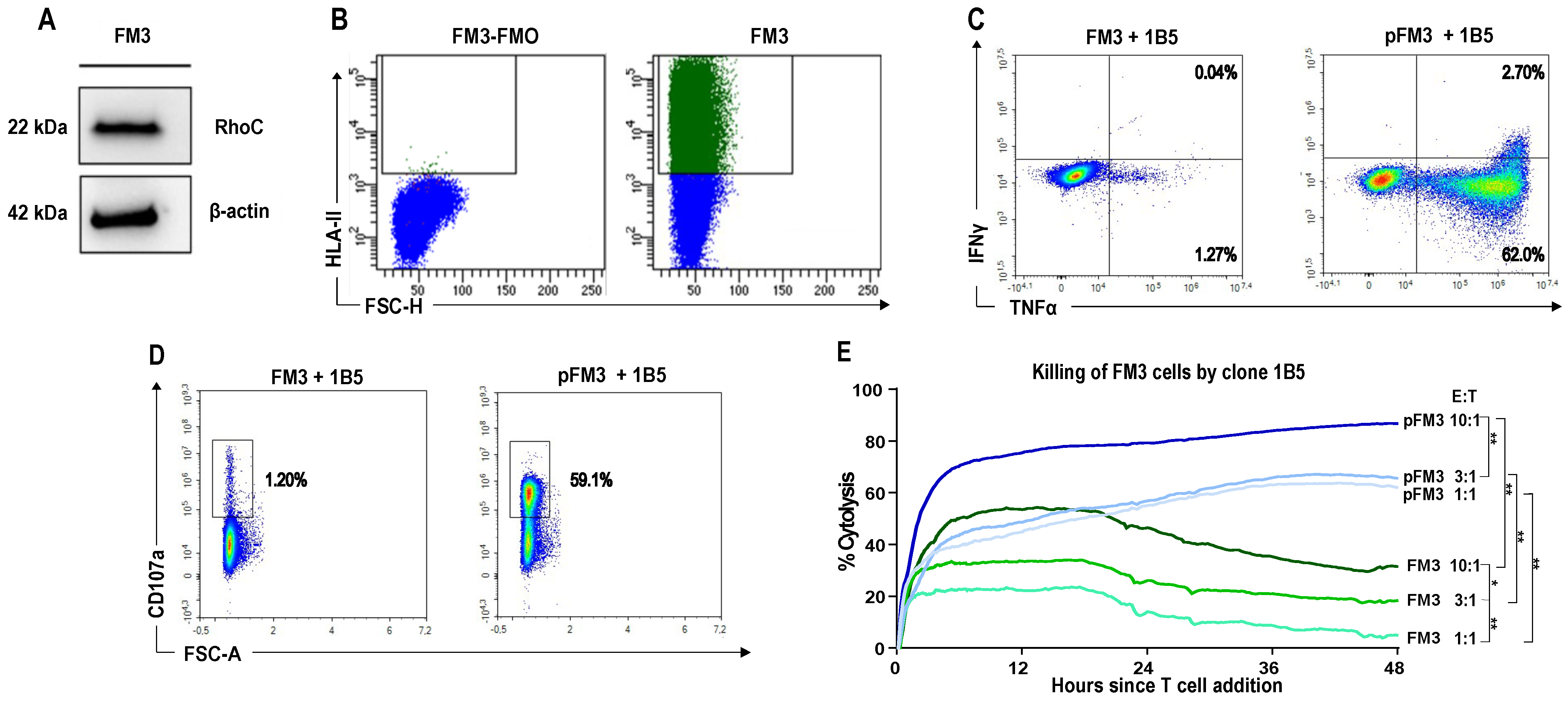
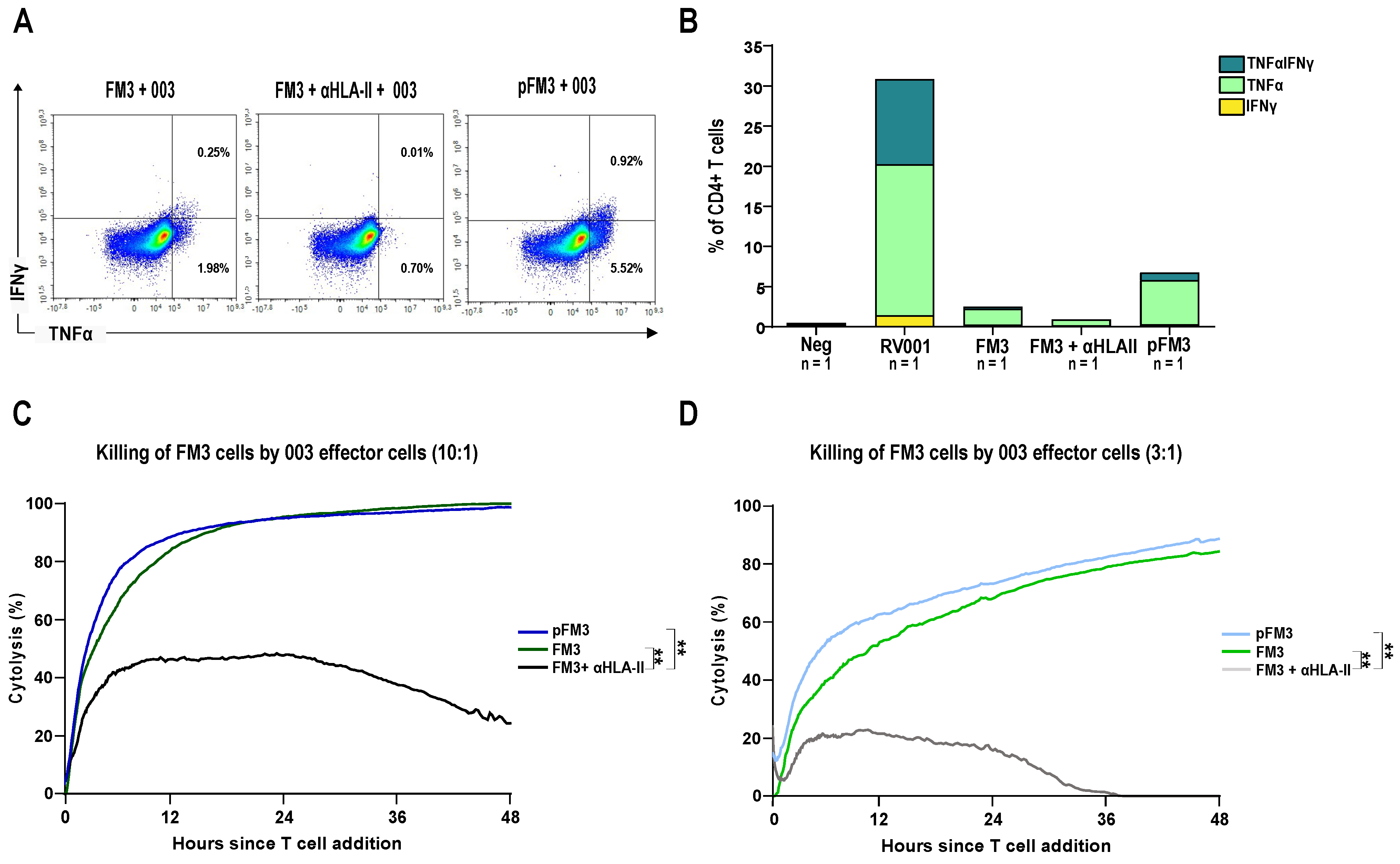
3.3. A CD4+ T Cell Clone Mediates RhoC-Specific Cancer Cell Killing
3.4. Patient-Derived CD4+ T Cells Mediate Cancer Cell Killing
3.5. Identification of TCRBV20
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
List of RhoVac II Principal Investigators
References
- Kantoff, P.; Higano, C.S. Integration of immunotherapy into the management of advanced prostate cancer. Urol. Oncol. Semin. Orig. Investig. 2012, 30, S41–S47. [Google Scholar] [CrossRef] [PubMed]
- Baxevanis, C.N.; Papamichail, M.; Perez, S.A. Immunologic biomarkers in prostate cancer: The AE37 paradigm. Hum. Vaccines Immunother. 2014, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, M.; Fujimoto, K.; Arai, G.; Hashine, K.; Matsumoto, H.; Fukasawa, S.; Kohjimoto, Y.; Nakatsu, H.; Takenaka, A.; Fujisawa, M.; et al. A randomized phase III trial of personalized peptide vaccination for castration-resistant prostate cancer progressing after docetaxel. Oncol. Rep. 2020, 45, 159–168. [Google Scholar] [CrossRef]
- Wood, L.V.; Fojo, A.; Roberson, B.D.; Hughes, M.S.B.; Dahut, W.; Gulley, J.L.; Madan, R.A.; Arlen, P.M.; Sabatino, M.; Stroncek, D.F.; et al. TARP vaccination is associated with slowing in PSA velocity and decreasing tumor growth rates in patients with Stage D0 prostate cancer. OncoImmunology 2016, 5, e1197459. [Google Scholar] [CrossRef]
- Clark, E.A.; Golub, T.R.; Lander, E.S.; Hynes, R.O. Genomic analysis of metastasis reveals an essential role for RhoC. Nature 2000, 406, 532–535. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Pranatharthi, A.; Ross, C.; Srivastava, S. RhoC: A fascinating journey from a cytoskeletal organizer to a Cancer stem cell therapeutic target. J. Exp. Clin. Cancer Res. 2019, 38, 328. [Google Scholar] [CrossRef]
- Lang, S.; Busch, H.; Boerries, M.; Brummer, T.; Timme, S.; Lassmann, S.; Aktories, K.; Schmidt, G. Specific role of RhoC in tumor invasion and metastasis. Oncotarget 2017, 8, 87364–87378. [Google Scholar] [CrossRef]
- Ridley, A. RhoA, RhoB and RhoC have different roles in cancer cell migration. J. Microsc. 2013, 251, 242–249. [Google Scholar] [CrossRef]
- Kleer, C.G.; van Golen, K.L.; Zhang, Y.; Wu, Z.-F.; Rubin, M.A.; Merajver, S.D. Characterization of RhoC expression in benign and malignant breast disease: A potential new marker for small breast carcinomas with metastatic ability. Am. J. Pathol. 2002, 160, 579–584. [Google Scholar] [CrossRef]
- Wenandy, L.; Sørensen, R.B.; Svane, I.M.; Straten, P.T.; Andersen, M.H. RhoC a new target for therapeutic vaccination against metastatic cancer. Cancer Immunol. Immunother. 2008, 57, 1871–1878. [Google Scholar] [CrossRef]
- Schuhmacher, J.; Heidu, S.; Balchen, T.; Richardson, J.R.; Schmeltz, C.; Sonne, J.; Schweiker, J.; Rammensee, H.-G.; Straten, P.T.; Røder, M.A.; et al. Vaccination against RhoC induces long-lasting immune responses in patients with prostate cancer: Results from a phase I/II clinical trial. J. Immunother. Cancer 2020, 8, e001157. [Google Scholar] [CrossRef]
- Tsuji, T.; Sabbatini, P.; Jungbluth, A.A.; Ritter, E.; Pan, L.; Ritter, G.; Ferran, L.; Spriggs, D.; Salazar, A.M.; Gnjatic, S. Effect of montanide and poly-ICLC adjuvant on human self/tumor antigen-specific CD4+ T cells in phase I overlapping long peptide vaccine trial. Cancer Immunol. Res. 2013, 1, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Moodie, Z.; Price, L.; Gouttefangeas, C.; Mander, A.; Janetzki, S.; Löwer, M.; Welters, M.J.P.; Ottensmeier, C.; van der Burg, S.H.; Britten, C.M. Response definition criteria for ELISPOT assays revisited. Cancer Immunol. Immunother. 2010, 59, 1489–1501. [Google Scholar] [CrossRef]
- Sittig, S.P.; Køllgaard, T.; Grønbæk, K.; Idorn, M.; Hennenlotter, J.; Stenzl, A.; Gouttefangeas, C.; Thor Straten, P. Clonal expansion of renal cell carcinoma-infiltrating T lymphocytes. OncoImmunology 2013, 2, e26014. [Google Scholar] [CrossRef][Green Version]
- Cheever, M.A.; Higano, C.S. PROVENGE (sipuleucel-T) in prostate cancer: The first FDA-approved therapeutic cancer vaccine. Clin. Cancer Res. 2011, 17, 3520–3526. [Google Scholar] [CrossRef] [PubMed]
- Olson, B.M.; Frye, T.P.; Johnson, L.E.; Fong, L.; Knutson, K.L.; Disis, M.L.; McNeel, D.G. HLA-A2-restricted T-cell epitopes specific for prostatic acid phosphatase. Cancer Immunol. Immunother. 2010, 59, 6. [Google Scholar] [CrossRef]
- Saxena, M.; van der Burg, S.H.; Melief, C.J.M.; Bhardwaj, N. Therapeutic cancer vaccines. Nat. Rev. Cancer 2021, 21, 360–378. [Google Scholar] [CrossRef]
- Richardson, J.R.; Schöllhorn, A.; Gouttefangeas, C.; Schuhmacher, J. CD4+ T Cells: Multitasking Cells in the Duty of Cancer Immunotherapy. Cancers 2021, 13, 596. [Google Scholar] [CrossRef] [PubMed]
- Quezada, S.A.; Simpson, T.R.; Peggs, K.S.; Merghoub, T.; Vider, J.; Fan, X.; Blasberg, R.; Yagita, H.; Muranski, P.; Antony, P.A.; et al. Tumor-reactive CD4+ T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J. Exp. Med. 2010, 207, 637–650. [Google Scholar] [CrossRef]
- Garrido, F. (Ed.) HLA Class-II Expression in Human Tumors BT—MHC Class-I Loss and Cancer Immune Escape; Springer International Publishing: Cham, Switzerland, 2019; pp. 91–95. [Google Scholar] [CrossRef]
- Halder, T.; Pawelec, G.; Kirkin, A.F.; Zeuthen, J.; Meyer, H.E.; Kun, L.; Kalbacher, H. Isolation of novel HLA-DR restricted potential tumor-associated antigens from the melanoma cell line FM3. Cancer Res. 1997, 57, 3238–3244. [Google Scholar]
- Goswami, S.; Pauken, K.E.; Wang, L.; Sharma, P. Next-generation combination approaches for immune checkpoint therapy. Nat. Immunol. 2024, 25, 2186–2199. [Google Scholar] [CrossRef]
- Venkatachalam, S.; McFarland, T.R.; Agarwal, N.; Swami, U. Immune checkpoint inhibitors in prostate cancer. Cancers 2021, 13, 2187. [Google Scholar] [CrossRef] [PubMed]
- Novysedlak, R.; Guney, M.; Al Khouri, M.; Bartolini, R.; Foley, L.K.; Benesova, I.; Ozaniak, A.; Novak, V.; Vesely, S.; Pacas, P.; et al. The immune microenvironment in prostate cancer: A comprehensive review. Oncology 2024, 1–25. [Google Scholar] [CrossRef]

| RV001V | Placebo | Overall | |
|---|---|---|---|
| (n = 96) | (n = 96) | (n = 192) | |
| Median age (range) | 70.5 (56–86) | 70 (57–86) | 70 (56–86) |
| Mean baseline PSA (ng/mL) | 1.90 (2.09) | 2.34 (2.35) | 2.12 (2.23) |
| PSADT at randomization [n (%)] | |||
| ≥6 months | 47 (49.0%) | 52 (54.2%) | 99 (51.6%) |
| <6 months | 49 (51.0%) | 44 (45.8%) | 93 (48.4%) |
| Gleason Grade group [n (%)] | |||
| Number | 95 | 96 | 191 |
| Grade 1 | 3 (3.2%) | 6 (6.3%) | 9 (4.7%) |
| Grade 2 | 14 (14.7%) | 25 (26.0%) | 39 (20.4%) |
| Grade 3 | 42 (44.2%) | 28 (29.2%) | 70 (36.6%) |
| Grade 4 | 13 (13.7%) | 21 (21.9%) | 34 (17.8%) |
| Grade 5 | 23 (24.2%) | 16 (16.7%) | 39 (20.4%) |
| Missing | 1 | 0 | 1 |
| Performance status (ECOG) [n (%)] | |||
| Number | 95 | 94 | 189 |
| 0 | 93 (97.9%) | 91 (96.8%) | 184 (97.4%) |
| 1, 2 | 2 (2.1%) | 3 (3.2%) | 5 (2.6%) |
| Missing | 1 | 2 | 3 |
| Years from diagnosis to study entry | |||
| Number | 88 | 88 | 176 |
| Mean (SD) | 5.4 (3.4) | 5.9 (3.9) | 5.7 (3.7) |
| Missing | 8 | 8 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fresnillo Saló, S.; Schuhmacher, J.; Rahbech, A.; Pedersen, S.R.; Seremet, T.; Matillas, V.A.; Schöllhorn, A.; Røder, A.; Jørgensen, S.W.; Brasso, K.; et al. Vaccination Against RhoC in Prostate Cancer Patients Induces Potent and Long-Lasting CD4+ T Cell Responses with Cytolytic Potential in the Absence of Clinical Efficacy: A Randomized Phase II Trial. Vaccines 2025, 13, 390. https://doi.org/10.3390/vaccines13040390
Fresnillo Saló S, Schuhmacher J, Rahbech A, Pedersen SR, Seremet T, Matillas VA, Schöllhorn A, Røder A, Jørgensen SW, Brasso K, et al. Vaccination Against RhoC in Prostate Cancer Patients Induces Potent and Long-Lasting CD4+ T Cell Responses with Cytolytic Potential in the Absence of Clinical Efficacy: A Randomized Phase II Trial. Vaccines. 2025; 13(4):390. https://doi.org/10.3390/vaccines13040390
Chicago/Turabian StyleFresnillo Saló, Sara, Juliane Schuhmacher, Anne Rahbech, Sara Ram Pedersen, Tina Seremet, Valero Andreu Matillas, Anna Schöllhorn, Andreas Røder, Steffen Wad Jørgensen, Klaus Brasso, and et al. 2025. "Vaccination Against RhoC in Prostate Cancer Patients Induces Potent and Long-Lasting CD4+ T Cell Responses with Cytolytic Potential in the Absence of Clinical Efficacy: A Randomized Phase II Trial" Vaccines 13, no. 4: 390. https://doi.org/10.3390/vaccines13040390
APA StyleFresnillo Saló, S., Schuhmacher, J., Rahbech, A., Pedersen, S. R., Seremet, T., Matillas, V. A., Schöllhorn, A., Røder, A., Jørgensen, S. W., Brasso, K., Gouttefangeas, C., Straten, P. t., & on behalf of the RhoVac-002 Study Group. (2025). Vaccination Against RhoC in Prostate Cancer Patients Induces Potent and Long-Lasting CD4+ T Cell Responses with Cytolytic Potential in the Absence of Clinical Efficacy: A Randomized Phase II Trial. Vaccines, 13(4), 390. https://doi.org/10.3390/vaccines13040390





