Monitoring Immune Responses to Vaccination: A Focus on Single-Cell Analysis and Associated Challenges
Abstract
1. Introduction
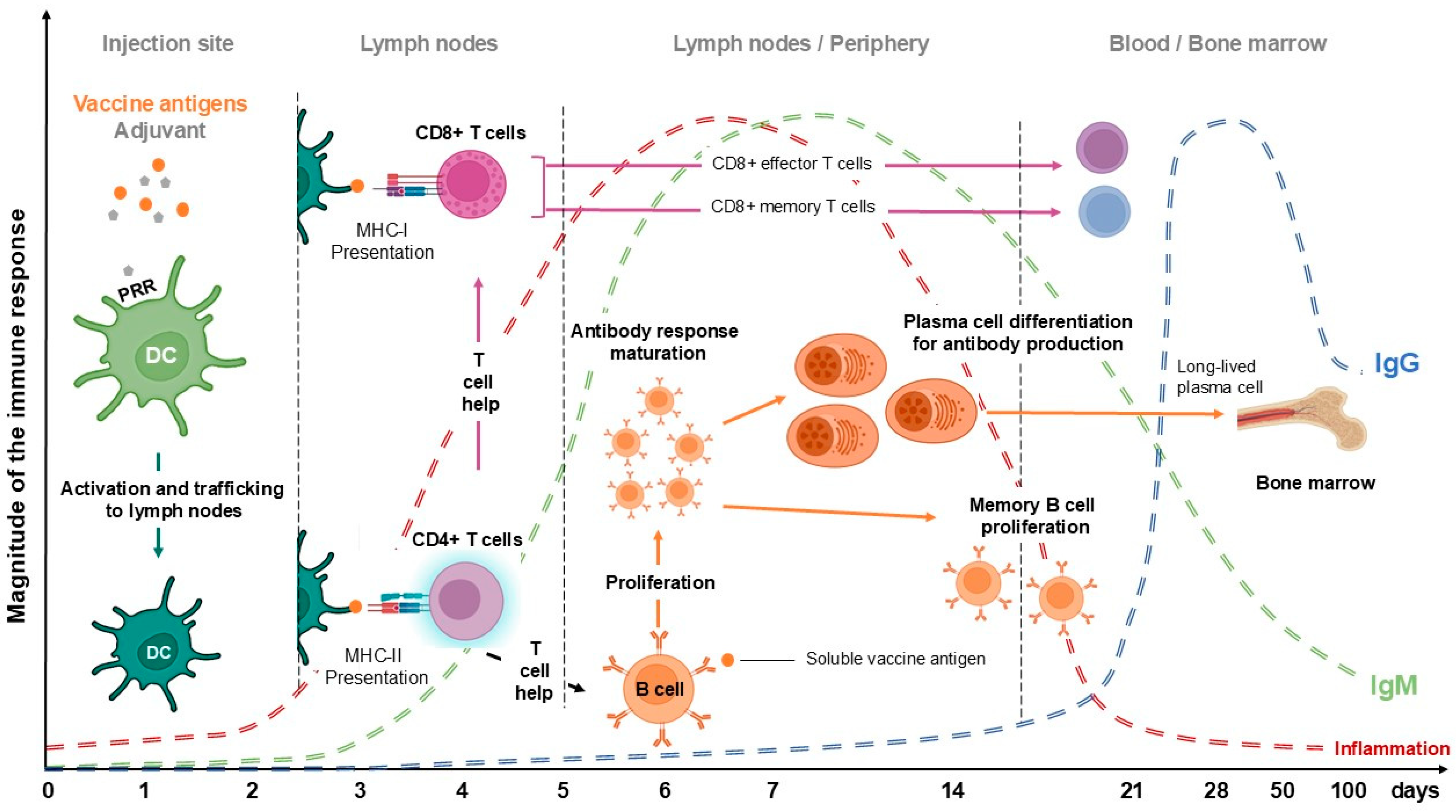
2. The Immune Response
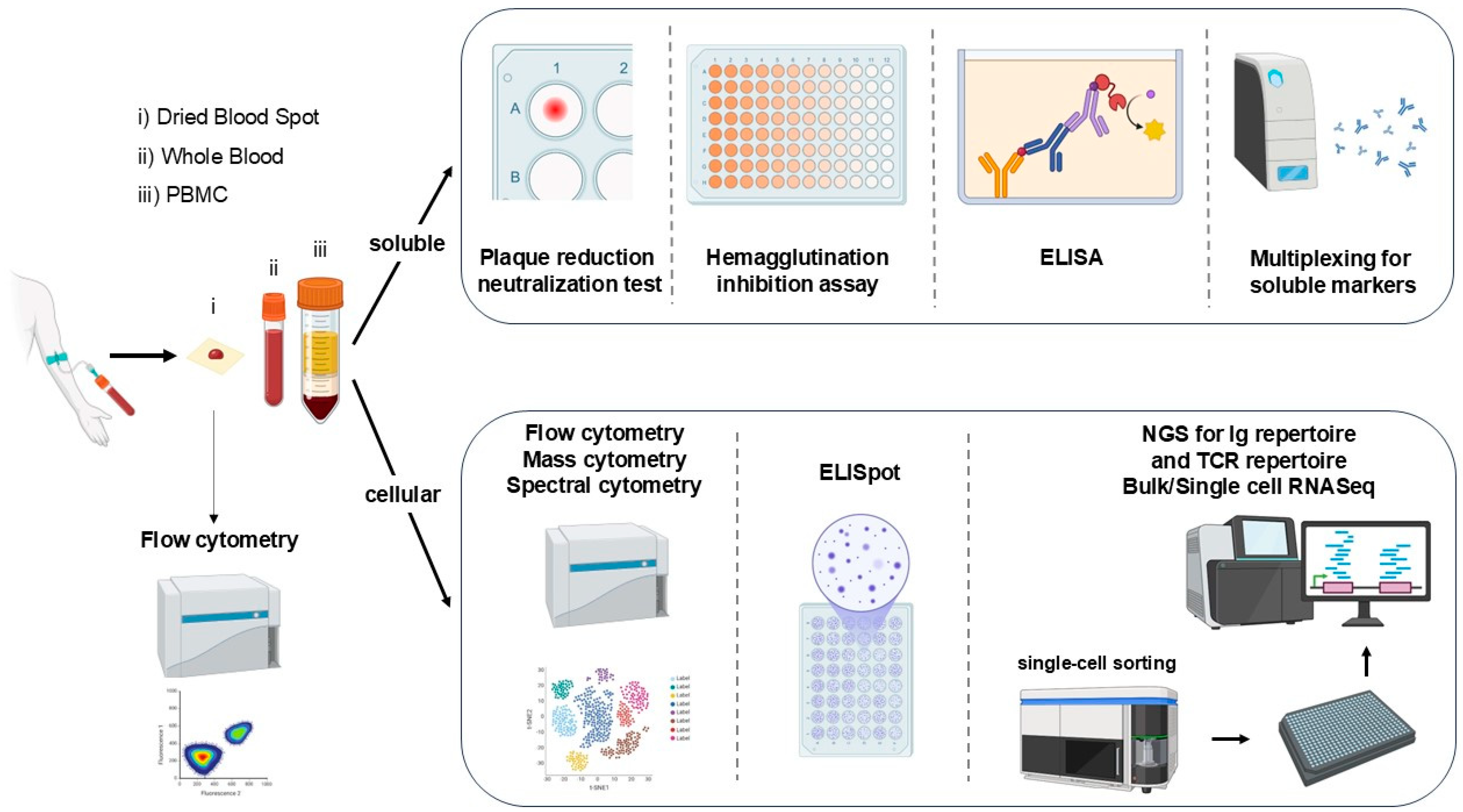
3. Assessing the Response to Vaccination
4. Other Assays Assessing the Immune Response
5. Advanced Techniques for Immune Analysis
6. Data Integration, Analysis, and Future Perspectives
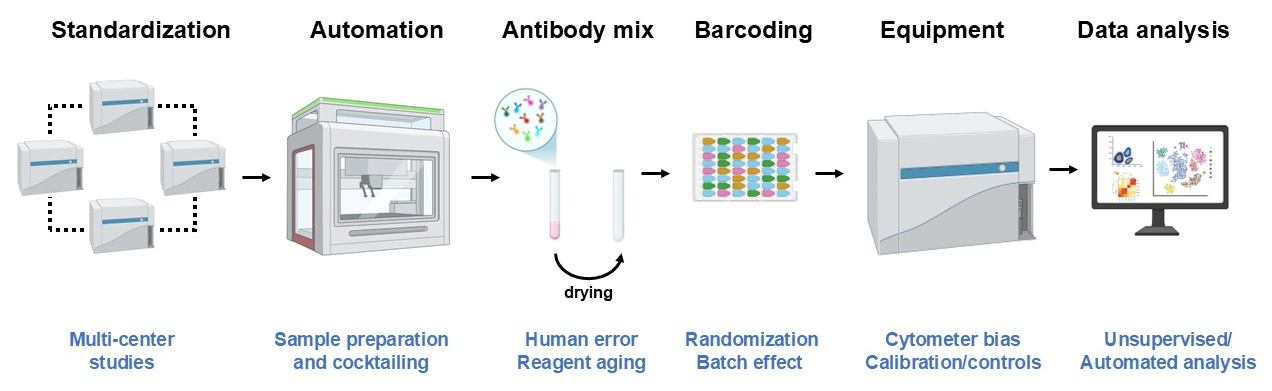
7. Generating Robust and Reproducible Data with Flow Cytometry
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dinc, G.; Ulman, Y.I. The introduction of variolation ‘A La Turca’ to the West by Lady Mary Montagu and Turkey’s contribution to this. Vaccine 2007, 25, 4261–4265. [Google Scholar] [CrossRef] [PubMed]
- Riley, A.G.; Voehler, D.; Mitrovich, R.; Carias, C.; Ollendorf, D.A.; Nelson, K.L.; Synnott, P.G.; Eiden, A.L. Documenting the Full Value of Vaccination: A Systematic Review of Value Frameworks. Value Health 2024, 27, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Zeinab, D.; Shahin, N.; Fateme, M.; Saeed, B.F. Economic evaluation of vaccination against COVID-19: A systematic review. Health Sci. Rep. 2024, 7, e1871. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Lee, H.; Wannaadisai, W.; Vietri, J.; Chaiyakunapruk, N. Systematic literature review of cost-effectiveness analyses of adult 15- and 20-valent pneumococcal vaccines. Vaccine 2025, 46, 126656. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.L. Testing vaccines: Essential steps in clinical trial design. In Vaccinology and Methods in Vaccine Research; Ashfield, R., Nnamdi Oli, A., Esimone, C., Anagu, L., Eds.; Developments in Immunology; Academic Press: Cambridge, MA, USA, 2022; pp. 295–310. [Google Scholar]
- Koff, W.C.; Burton, D.R.; Johnson, P.R.; Walker, B.D.; King, C.R.; Nabel, G.J.; Ahmed, R.; Bhan, M.K.; Plotkin, S.A. Accelerating next-generation vaccine development for global disease prevention. Science 2013, 340, 1232910. [Google Scholar] [CrossRef]
- Bok, K.; Sitar, S.; Graham, B.S.; Mascola, J.R. Accelerated COVID-19 vaccine development: Milestones, lessons, and prospects. Immunity 2021, 54, 1636–1651. [Google Scholar] [CrossRef]
- Iwasaki, A.; Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015, 16, 343–353. [Google Scholar] [CrossRef]
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef]
- Plotkin, S.A. Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 2010, 17, 1055–1065. [Google Scholar] [CrossRef]
- Ahmed, R.; Gray, D. Immunological memory and protective immunity: Understanding their relation. Science 1996, 272, 54–60. [Google Scholar] [CrossRef]
- Sallusto, F.; Lanzavecchia, A.; Araki, K.; Ahmed, R. From vaccines to memory and back. Immunity 2010, 33, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, J.; Xu, W.; Huo, X.; Wang, J.; Xu, Y.; Ding, W.; Guo, Z.; Liu, R. Advances in protein subunit vaccines against H1N1/09 influenza. Front. Immunol. 2024, 15, 1499754. [Google Scholar] [CrossRef] [PubMed]
- Ben-Akiva, E.; Chapman, A.; Mao, T.; Irvine, D.J. Linking vaccine adjuvant mechanisms of action to function. Sci. Immunol. 2025, 10, eado5937. [Google Scholar] [CrossRef]
- Vaccines and Immunizations. Available online: https://www.cdc.gov/vaccines/hcp/admin/administer-vaccines.html (accessed on 15 March 2025).
- Chen, L.; Shao, C.; Li, J.; Zhu, F. Impact of Immunosenescence on Vaccine Immune Responses and Countermeasures. Vaccines 2024, 12, 1289. [Google Scholar] [CrossRef]
- Thomas, S.J.; Yoon, I.K. A review of Dengvaxia®: Development to deployment. Hum. Vaccin Immunother. 2019, 15, 2295–2314. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B.; Ahmed, R. Immunological mechanisms of vaccination. Nat. Immunol. 2011, 12, 509–517. [Google Scholar] [CrossRef]
- Kasturi, S.P.; Skountzou, I.; Albrecht, R.A.; Koutsonanos, D.; Hua, T.; Nakaya, H.I.; Ravindran, R.; Stewart, S.; Alam, M.; Kwissa, M.; et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature 2011, 470, 543–547. [Google Scholar] [CrossRef]
- Nakayama, T. An inflammatory response is essential for the development of adaptive immunity-immunogenicity and immunotoxicity. Vaccine 2016, 34, 5815–5818. [Google Scholar] [CrossRef]
- Reed, S.G.; Orr, M.T.; Fox, C.B. Key roles of adjuvants in modern vaccines. Nat. Med. 2013, 19, 1597–1608. [Google Scholar] [CrossRef]
- Lukman, N.; Salim, G.; Kosasih, H.; Susanto, N.H.; Parwati, I.; Fitri, S.; Alisjahbana, B.; Widjaja, S.; Williams, M. Comparison of the Hemagglutination Inhibition Test and IgG ELISA in Categorizing Primary and Secondary Dengue Infections Based on the Plaque Reduction Neutralization Test. Biomed. Res. Int. 2016, 2016, 5253842. [Google Scholar] [CrossRef]
- Furman, D.; Davis, M.M. New approaches to understanding the immune response to vaccination and infection. Vaccine 2015, 33, 5271–5281. [Google Scholar] [CrossRef]
- Slota, M.; Lim, J.B.; Dang, Y.; Disis, M.L. ELISpot for measuring human immune responses to vaccines. Expert. Rev. Vaccines 2011, 10, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Moser, J.M.; Sassano, E.R.; Leistritz, D.E.L.C.; Eatrides, J.M.; Phogat, S.; Koff, W.; Drake, D.R., 3rd. Optimization of a dendritic cell-based assay for the in vitro priming of naïve human CD4+ T cells. J. Immunol. Methods 2010, 353, 8–19. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, K.M. Flow Cytometry: An Overview. Curr. Protoc. Immunol. 2018, 120, 5.1.1–5.1.11. [Google Scholar] [CrossRef]
- Tabatabaei, M.S.; Ahmed, M. Enzyme-Linked Immunosorbent Assay (ELISA). Methods Mol. Biol. 2022, 2508, 115–134. [Google Scholar] [PubMed]
- Herderschee, J.; Fenwick, C.; Pantaleo, G.; Roger, T.; Calandra, T. Emerging single-cell technologies in immunology. J. Leukoc. Biol. 2015, 98, 23–32. [Google Scholar] [CrossRef]
- Tran, T.A.T.; Grievink, H.W.; Lipinska, K.; Kluft, C.; Burggraaf, J.; Moerland, M.; Tasev, D.; Malone, K.E. Whole blood assay as a model for in vitro evaluation of inflammasome activation and subsequent caspase-mediated interleukin-1 beta release. PLoS ONE 2019, 14, e0214999. [Google Scholar] [CrossRef] [PubMed]
- Duffy, D. Milieu intérieur: Defining the boundaries of a healthy immune response for improved vaccination strategies. Hum. Vaccine Immunother. 2018, 14, 2217–2221. [Google Scholar] [CrossRef] [PubMed]
- Poland, G.A.; Ovsyannikova, I.G.; Jacobson, R.M.; Smith, D.I. Heterogeneity in vaccine immune response: The role of immunogenetics and the emerging field of vaccinomics. Clin. Pharmacol. Ther. 2007, 82, 653–664. [Google Scholar] [CrossRef]
- Maecker, H.T.; McCoy, J.P.; Nussenblatt, R. Standardizing immunophenotyping for the Human Immunology Project. Nat. Rev. Immunol. 2012, 12, 191–200. [Google Scholar] [CrossRef]
- Wang, L.; Hoffman, R.A. Standardization, Calibration, and Control in Flow Cytometry. Curr. Protoc. Cytom. 2017, 79, 1.3.1–1.3.27. [Google Scholar] [CrossRef] [PubMed]
- Ait Belkacem, I.; Mossadegh-Keller, N.; Bourgoin, P.; Arnoux, I.; Loosveld, M.; Morange, P.E.; Markarian, T.; Michelet, P.; Busnel, J.M.; Roulland, S.; et al. Cell Analysis from Dried Blood Spots: New Opportunities in Immunology, Hematology, and Infectious Diseases. Adv. Sci. 2021, 8, e2100323. [Google Scholar] [CrossRef]
- Castelletti, N.; Paunovic, I.; Rubio-Acero, R.; Beyerl, J.; Plank, M.; Reinkemeyer, C.; Kroidl, I.; Noreña, I.; Winter, S.; Olbrich, L.; et al. A Dried Blood Spot protocol for high-throughput quantitative analysis of SARS-CoV-2 RBD serology based on the Roche Elecsys system. Microbiol. Spectr. 2024, 12, e0288523. [Google Scholar] [CrossRef] [PubMed]
- Dufrusine, B.; Natale, L.; Sallese, M.; Mozzillo, E.; Di Candia, F.; Cuccurullo, I.; Iafusco, D.; Zanfardino, A.; Passariello, L.; Iannilli, A.; et al. Development and validation of a novel method for evaluation of multiple islet autoantibodies in dried blood spot using dissociation-enhanced lanthanide fluorescent immunoassays technology, specific and suitable for paediatric screening programmes. Diabetes Obes. Metab. 2025, 27, 414–418. [Google Scholar] [CrossRef]
- Reed, R.G.; Hillmann, A.R.; Nation, M.; Braksator, S.; Sigler, K. Remote dried blood spot collection for inflammatory markers in older adults is feasible, reliable, and valid. Brain Behav. Immun. 2024, 120, 545–553. [Google Scholar] [CrossRef]
- Agrawal, P.; Katragadda, S.; Hariharan, A.K.; Raghavendrachar, V.G.; Agarwal, A.; Dayalu, R.; Awasthy, D.; Sharma, S.C.; Sivasamy, Y.K.; Lakshmana, P.; et al. Validation of whole genome sequencing from dried blood spots. BMC Med. Genom. 2021, 14, 110. [Google Scholar] [CrossRef] [PubMed]
- Hubens, W.; Kluge, L.; Seitz, A.; Verjans, E.; Rink, L.; Wagner, W. Epigenetic Leukocyte Counts from Dried Blood Spots of Pediatric Patients. Clin. Chem. 2024, 70, 997–999. [Google Scholar] [CrossRef]
- Cui, H.N.; Shi, F.; Huang, G.; He, Y.; Yu, S.; Liu, L.; Li, Y.; Wen, H. Evaluation of metabolite stability in dried blood spot stored at different temperatures and times. Sci. Rep. 2024, 14, 30964. [Google Scholar] [CrossRef]
- Kumar, T.; Chauhan, R.; Kalra, B.; Patel, A.S.; Chauhan, S.; Kumar, A. Comparative Analysis of Dried Blood Spot and Serum ELISAs for AMH, FSH, and LH in Patients Seeking IVF Treatment. J. Endocr. Soc. 2024, 8 (Suppl. S1), bvae163.1579. [Google Scholar] [CrossRef]
- Blood Specimens—Shipment. Available online: https://www.cdc.gov/dpdx/diagnosticprocedures/blood/shipment.html (accessed on 16 March 2025).
- Trauet, J.; Bourgoin, P.; Schuldt, J.; Lefèvre, G.; Labalette, M.; Busnel, J.M.; Demaret, J. Studying antigen-specific T cells through a streamlined, whole blood-based extracellular approach. Cytom. A 2024, 105, 288–296. [Google Scholar] [CrossRef]
- Doss-Gollin, S.; Thomas, S.; Brook, B.; Abedi, K.; Lebas, C.; Auderset, F.; Lugo-Rodriguez, Y.; Sanchez-Schmitz, G.; Dowling, D.J.; Levy, O.; et al. Human in vitro modeling of adjuvant formulations demonstrates enhancement of immune responses to SARS-CoV-2 antigen. NPJ Vaccines 2023, 8, 163. [Google Scholar] [CrossRef]
- Morrocchi, E.; van Haren, S.; Palma, P.; Levy, O. Modeling human immune responses to vaccination in vitro. Trends Immunol. 2024, 45, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Ozulumba, T.; Montalbine, A.N.; Ortiz-Cárdenas, J.E.; Pompano, R.R. New tools for immunologists: Models of lymph node function from cells to tissues. Front. Immunol. 2023, 14, 1183286. [Google Scholar] [CrossRef]
- Hoyt, C.C. Multiplex Immunofluorescence and Multispectral Imaging: Forming the Basis of a Clinical Test Platform for Immuno-Oncology. Front. Mol. Biosci. 2021, 8, 674747. [Google Scholar] [CrossRef]
- Human Cell Atlas Project. Available online: https://www.humancellatlas.org/ (accessed on 16 March 2025).
- Liu, Y.; Yang, M.; Deng, Y.; Su, G.; Enninful, A.; Guo, C.C.; Tebaldi, T.; Zhang, D.; Kim, D.; Bai, Z.; et al. High-Spatial-Resolution Multi-Omics Sequencing via Deterministic Barcoding in Tissue. Cell 2020, 183, 1665.e18–1681.e18. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; DiStasio, M.; Su, G.; Asashima, H.; Enninful, A.; Qin, X.; Deng, Y.; Nam, J.; Gao, F.; Bordignon, P.; et al. High-plex protein and whole transcriptome co-mapping at cellular resolution with spatial CITE-seq. Nat. Biotechnol. 2023, 41, 1405–1409. [Google Scholar] [CrossRef]
- Wacleche, V.S.; Wang, R.; Rao, D.A. Identification of T Peripheral Helper (Tph) Cells. In T-Follicular Helper Cells: Methods and Protocols; Graca, L., Ed.; Methods in Molecular Biology; Humana: New York, NY, USA, 2022; Volume 2380, pp. 59–76. [Google Scholar] [CrossRef]
- Manohar, S.M.; Shah, P.; Nair, A. Flow cytometry: Principles, applications and recent advances. Bioanalysis 2021, 13, 181–198. [Google Scholar] [CrossRef] [PubMed]
- Roederer, M. Spectral compensation for flow cytometry: Visualization artifacts, limitations, and caveats. Cytometry 2001, 45, 194–205. [Google Scholar] [CrossRef]
- Nolan, J.P.; Condello, D. Spectral flow cytometry. Curr. Protoc. Cytom. 2013, 1, 1.27.1–1.27.13. [Google Scholar] [CrossRef]
- Konecny, A.J.; Mage, P.L.; Tyznik, A.J.; Prlic, M.; Mair, F. OMIP-102: 50-color phenotyping of the human immune system with in-depth assessment of T cells and dendritic cells. Cytom. A 2024, 105, 430–436. [Google Scholar] [CrossRef]
- Saeys, Y.; Van Gassen, S.; Lambrecht, B.N. Computational flow cytometry: Helping to make sense of high-dimensional immunology data. Nat. Rev. Immunol. 2016, 16, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Weiss, L.; O’Doherty, A.; Uhrig, W.; Szklanna, P.B.; Hong-Minh, M.; Wynne, K.; Blanco, A.; Zivny, J.; Lima Passos, V.; Kevane, B.; et al. Rivaroxaban, in combination with low-dose aspirin, is associated with a reduction in proinflammatory and prothrombotic circulating vesicle signatures in patients with cardiovascular disease. J. Thromb. Haemost. 2025, 23, 531–545. [Google Scholar] [CrossRef] [PubMed]
- Petukhova, G.; Korenkov, D.; Chirkova, T.; Donina, S.; Rudenko, L.; Naykhin, A. B- and T-cell memory elicited by a seasonal live attenuated reassortant influenza vaccine: Assessment of local antibody avidity and virus-specific memory T-cells using trogocytosis-based method. Influenza Other Respir. Viruses 2012, 6, 119–126. [Google Scholar] [CrossRef]
- Kannanganat, S.; Ibegbu, C.; Chennareddi, L.; Robinson, H.L.; Amara, R.R. Multiple-cytokine-producing antiviral CD4 T cells are functionally superior to single-cytokine-producing cells. J. Virol. 2007, 81, 8468–8476. [Google Scholar] [CrossRef]
- DeGottardi, Q.; Gates, T.J.; Yang, J.; James, E.A.; Malhotra, U.; Chow, I.T.; Simoni, Y.; Fehlings, M.; Newell, E.W.; DeBerg, H.A.; et al. Ontogeny of different subsets of yellow fever virus-specific circulatory CXCR5+ CD4+ T cells after yellow fever vaccination. Sci. Rep. 2020, 10, 15686. [Google Scholar] [CrossRef] [PubMed]
- Palm, A.E.; Henry, C. Remembrance of Things Past: Long-Term B Cell Memory After Infection and Vaccination. Front. Immunol. 2019, 10, 1787. [Google Scholar] [CrossRef]
- Zuo, J.; Dowell, A.C.; Pearce, H.; Verma, K.; Long, H.M.; Begum, J.; Aiano, F.; Amin-Chowdhury, Z.; Hoschler, K.; Brooks, T.; et al. Robust SARS-CoV-2-specific T cell immunity is maintained at 6 months following primary infection. Nat. Immunol. 2021, 22, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Sokal, A.; Chappert, P.; Barba-Spaeth, G.; Roeser, A.; Fourati, S.; Azzaoui, I.; Vandenberghe, A.; Fernandez, I.; Meola, A.; Bouvier-Alias, M.; et al. Maturation and persistence of the anti-SARS-CoV-2 memory B cell response. Cell 2021, 184, 1201–1213.e14. [Google Scholar] [CrossRef]
- Arbel, R.; Peretz, A.; Sergienko, R.; Friger, M.; Beckenstein, T.; Duskin-Bitan, H.; Yaron, S.; Hammerman, A.; Bilenko, N.; Netzer, D. Effectiveness of a bivalent mRNA vaccine booster dose to prevent severe COVID-19 outcomes: A retrospective cohort study. Lancet Infect. Dis. 2023, 23, 914–921. [Google Scholar] [CrossRef]
- Venet, F.; Gossez, M.; Bidar, F.; Bodinier, M.; Coudereau, R.; Lukaszewicz, A.C.; Tardiveau, C.; Brengel-Pesce, K.; Cheynet, V.; Cazalis, M.A.; et al. T cell response against SARS-CoV-2 persists after one year in patients surviving severe COVID-19. EBioMedicine 2022, 78, 103967. [Google Scholar] [CrossRef]
- Kometani, K.; Yorimitsu, T.; Jo, N.; Yamaguchi, E.; Kikuchi, O.; Fukahori, M.; Sawada, T.; Tsujimoto, Y.; Sunami, A.; Li, M.; et al. Booster COVID-19 mRNA vaccination ameliorates impaired B-cell but not T-cell responses in older adults. Front. Immunol. 2024, 15, 1455334. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Huang, H.; Yu, C.; Li, X.; Wang, Y.; Xie, L. Current status and future directions for the development of human papillomavirus vaccines. Front. Immunol. 2024, 15, 1362770. [Google Scholar] [CrossRef]
- Chua, Y.C.; Draper, S.L.; Le, S.; de Menezes, M.N.; Ganley, M.; Ge, Z.; Lee, A.; Phabmixay, T.; Hirschmann, D.; Robinson, S.A.; et al. Mechanistic insight into the induction of liver tissue-resident memory CD8+ T cells by glycolipid-peptide vaccination. Cell Rep. 2025, 44, 115295. [Google Scholar] [CrossRef] [PubMed]
- Kalimuddin, S.; Tham, C.Y.L.; Chan, Y.F.Z.; Hang, S.K.; Kunasegaran, K.; Chia, A.; Chan, C.Y.Y.; Ng, D.H.L.; Sim, J.X.Y.; Tan, H.C.; et al. Vaccine-induced T cell responses control Orthoflavivirus challenge infection without neutralizing antibodies in humans. Nat. Microbiol. 2025, 10, 374–387. [Google Scholar] [CrossRef]
- Govindan, R.; Stephenson, K.E. HIV Vaccine Development at a Crossroads: New B and T Cell Approaches. Vaccines 2024, 12, 1043. [Google Scholar] [CrossRef]
- Wang, J.; Fan, X.Y.; Hu, Z. Immune correlates of protection as a game changer in tuberculosis vaccine development. NPJ Vaccines 2024, 9, 208. [Google Scholar] [CrossRef] [PubMed]
- Mettelman, R.C.; Souquette, A.; Van de Velde, L.A.; Vegesana, K.; Allen, E.K.; Kackos, C.M.; Trifkovic, S.; DeBeauchamp, J.; Wilson, T.L.; St James, D.G.; et al. Baseline innate and T cell populations are correlates of protection against symptomatic influenza virus infection independent of serology. Nat. Immunol. 2023, 24, 1511–1526. [Google Scholar] [CrossRef] [PubMed]
- Park, L.M.; Lannigan, J.; Jaimes, M.C. OMIP-069: Forty-Color Full Spectrum Flow Cytometry Panel for Deep Immunophenotyping of Major Cell Subsets in Human Peripheral Blood. Cytom. Part A J. Int. Soc. Anal. Cytol. 2020, 97, 1044–1051. [Google Scholar] [CrossRef]
- Nolan, J.P. The evolution of spectral flow cytometry. Cytom. Part A J. Int. Soc. Anal. Cytol. 2022, 101, 812–817. [Google Scholar] [CrossRef]
- Cevirgel, A.; Vos, M.; Bijvank, E.; van Beek, J.; van der Heiden, M.; Buisman, A.M.; van Baarle, D. CD31 + naïve T cells associate with immunosenescence and responsiveness to multiple vaccines in older adults. Immun. Ageing 2025, 22, 10. [Google Scholar] [CrossRef]
- Claireaux, M.; Elias, G.; Kerster, G.; Kuijper, L.H.; Duurland, M.C.; Paul, A.G.A.; Burger, J.A.; Poniman, M.; Olijhoek, W.; de Jong, N.; et al. Deep profiling of B cells responding to various pathogens uncovers compartments in IgG memory B cell and antibody-secreting lineages. Sci. Adv. 2025, 11, eado1331. [Google Scholar] [CrossRef] [PubMed]
- Griffith, G.L.; Machmach, K.; Jian, N.; Kim, D.; Costanzo, M.C.; Creegan, M.; Swafford, I.; Kundu, G.; Yum, L.; Bolton, J.S.; et al. CD16 and CD57 expressing gamma delta T cells in acute HIV-1 infection are associated with the development of neutralization breadth. PLoS Pathog. 2025, 21, e1012916. [Google Scholar] [CrossRef] [PubMed]
- Bettini, E.; Lederer, K.; Sharpe, H.; Kaminski, M.; Jones, L.; Locci, M. A combined fine needle aspiration and spectral flow cytometry approach to assess human germinal center responses to SARS-CoV-2 vaccination. STAR Protoc. 2022, 3, 101840. [Google Scholar] [CrossRef]
- Mbow, M.; Hoving, D.; Cisse, M.; Diallo, I.; Honkpehedji, Y.J.; Huisman, W.; Pothast, C.R.; Jongsma, M.L.M.; König, M.H.; de Kroon, A.C.; et al. Immune responses to SARS-CoV-2 in sub-Saharan Africa and western Europe: A retrospective, population-based, cross-sectional study. Lancet. Microbe 2025, 6, 100942. [Google Scholar] [CrossRef]
- Japp, A.S.; Hoffmann, K.; Schlickeiser, S.; Glauben, R.; Nikolaou, C.; Maecker, H.T.; Braun, J.; Matzmohr, N.; Sawitzki, B.; Siegmund, B.; et al. Wild immunology assessed by multidimensional mass cytometry. Cytom. Part A J. Int. Soc. Anal. Cytol. 2017, 91, 85–95. [Google Scholar] [CrossRef]
- Reeves, P.M.; Sluder, A.E.; Paul, S.R.; Scholzen, A.; Kashiwagi, S.; Poznansky, M.C. Application and utility of mass cytometry in vaccine development. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2018, 32, 5–15. [Google Scholar] [CrossRef]
- Swadling, L.; Capone, S.; Antrobus, R.D.; Brown, A.; Richardson, R.; Newell, E.W.; Halliday, J.; Kelly, C.; Bowen, D.; Fergusson, J.; et al. A human vaccine strategy based on chimpanzee adenoviral and MVA vectors that primes, boosts, and sustains functional HCV-specific T cell memory. Sci. Transl. Med. 2014, 6, 261ra153. [Google Scholar] [CrossRef] [PubMed]
- Newell, E.W.; Sigal, N.; Nair, N.; Kidd, B.A.; Greenberg, H.B.; Davis, M.M. Combinatorial tetramer staining and mass cytometry analysis facilitate T-cell epitope mapping and characterization. Nat. Biotechnol. 2013, 31, 623–629. [Google Scholar] [CrossRef]
- Christophersen, A. Peptide-MHC class I and class II tetramers: From flow to mass cytometry. HLA 2020, 95, 169–178. [Google Scholar] [CrossRef]
- Fehlings, M.; Jhunjhunwala, S.; Kowanetz, M.; O’Gorman, W.E.; Hegde, P.S.; Sumatoh, H.; Lee, B.H.; Nardin, A.; Becht, E.; Flynn, S.; et al. Late-differentiated effector neoantigen-specific CD8+ T cells are enriched in peripheral blood of non-small cell lung carcinoma patients responding to atezolizumab treatment. J. Immunother. Cancer 2019, 7, 249. [Google Scholar] [CrossRef]
- Zheng, C.; Zheng, L.; Yoo, J.K.; Guo, H.; Zhang, Y.; Guo, X.; Kang, B.; Hu, R.; Huang, J.Y.; Zhang, Q.; et al. Landscape of Infiltrating T Cells in Liver Cancer Revealed by Single-Cell Sequencing. Cell 2017, 169, 1342–1356.e16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, S.; Sun, Q.; Liu, T.; Wen, Z.; Yao, M.; Zhang, S.; Duan, Q.; Zhang, X.; Pang, B.; et al. Single-cell transcriptome atlas of peripheral immune features to Omicron breakthrough infection under booster vaccination strategies. Front. Immunol. 2025, 15, 1460442. [Google Scholar] [CrossRef] [PubMed]
- Eisa, M.; Flores, N.; Khedr, O.; Gomez-Escobar, E.; Bédard, N.; Abdeltawab, N.F.; Bruneau, J.; Grakoui, A.; Shoukry, N.H. Activation-Induced Marker Assay to Identify and Isolate HCV-Specific T Cells for Single-Cell RNA-Seq Analysis. Viruses 2024, 16, 1623. [Google Scholar] [CrossRef]
- Morgan, D.M.; Zhang, Y.J.; Kim, J.H.; Murillo, M.; Singh, S.; Loschko, J.; Surendran, N.; Sekulovic, O.; Feng, E.; Shi, S.; et al. Full-length single-cell BCR sequencing paired with RNA sequencing reveals convergent responses to pneumococcal vaccination. Commun. Biol. 2024, 7, 1208. [Google Scholar] [CrossRef]
- Mzoughi, S.; Schwarz, M.; Wang, X.; Demircioglu, D.; Ulukaya, G.; Mohammed, K.; Zorgati, H.; Torre, D.; Tomalin, L.E.; Di Tullio, F.; et al. Oncofetal reprogramming drives phenotypic plasticity in WNT-dependent colorectal cancer. Nat. Genet. 2025, 57, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Scheid, J.F.; Barnes, C.O.; Eraslan, B.; Hudak, A.; Keeffe, J.R.; Cosimi, L.A.; Brown, E.M.; Muecksch, F.; Weisblum, Y.; Zhang, S.; et al. B cell genomics behind cross-neutralization of SARS-CoV-2 variants and SARS-CoV. Cell 2021, 184, 3205–3221.e24. [Google Scholar] [CrossRef]
- Kotliarov, Y.; Sparks, R.; Martins, A.J.; Mulè, M.P.; Lu, Y.; Goswami, M.; Kardava, L.; Banchereau, R.; Pascual, V.; Biancotto, A.; et al. Broad immune activation underlies shared set point signatures for vaccine responsiveness in healthy individuals and disease activity in patients with lupus. Nat. Med. 2020, 26, 618–629. [Google Scholar] [CrossRef]
- Shannon, C.P.; Blimkie, T.M.; Ben-Othman, R.; Gladish, N.; Amenyogbe, N.; Drissler, S.; Edgar, R.D.; Chan, Q.; Krajden, M.; Foster, L.J.; et al. Multi-Omic Data Integration Allows Baseline Immune Signatures to Predict Hepatitis B Vaccine Response in a Small Cohort. Front. Immunol. 2020, 11, 578801. [Google Scholar] [CrossRef]
- Marsh-Wakefield, F.M.; Mitchell, A.J.; Norton, S.E.; Ashhurst, T.M.; Leman, J.K.; Roberts, J.M.; Harte, J.E.; McGuire, H.M.; Kemp, R.A. Making the most of high-dimensional cytometry data. Immunol. Cell Biol. 2021, 99, 680–696. [Google Scholar] [CrossRef]
- Vanegas Lozano, D.M.; Devia Mejia, B.; Machuca Acevedo, C.; Jaramillo Mejia, V.; Moreno González, A.M.; Krisko, A.; Quijano Gómez, S.M.; Perdomo-Arciniegas, A.M. Immunophenotypic and Functional Interindividual Variability in Banked Cord Blood Cells: Insights for Advanced Therapies. Int. J. Mol. Sci. 2025, 26, 1208. [Google Scholar] [CrossRef]
- Nguyen, A.A.; Platt, C.D. Flow Cytometry-based Immune Phenotyping of T and B Lymphocytes in the Evaluation of Immunodeficiency and Immune Dysregulation. Clin. Lab. Med. 2024, 44, 479–493. [Google Scholar] [CrossRef]
- Tarke, A.; Coelho, C.H.; Zhang, Z.; Dan, J.M.; Yu, E.D.; Methot, N.; Bloom, N.I.; Goodwin, B.; Phillips, E.; Mallal, S.; et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell 2022, 185, 847–859.e11. [Google Scholar] [CrossRef] [PubMed]
- H-Vázquez, J.; Cal-Sabater, P.; Arribas-Rodríguez, E.; Fiz-López, A.; Perez-Segurado, C.; Martín-Muñoz, Á.; De Prado, Á.; Perez Mazzali, M.; de Castro, C.G.; Del Hierro, A.G.; et al. Unbiased immunome characterisation correlates with COVID-19 mRNA vaccine failure in immunocompromised adults. Front. Immunol. 2024, 15, 1405217. [Google Scholar] [CrossRef]
- Svensson, V.; Vento-Tormo, R.; Teichmann, S.A. Exponential scaling of single-cell RNA-seq in the past decade. Nat. Protoc. 2018, 13, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Flossdorf, M.; Rössler, J.; Buchholz, V.R.; Busch, D.H.; Höfer, T. CD8(+) T cell diversification by asymmetric cell division. Nat. Immunol. 2015, 16, 891–893. [Google Scholar] [CrossRef]
- Mou, L.; Lu, Y.; Wu, Z.; Pu, Z.; Wang, M. Integrating genomics and AI to uncover molecular targets for mRNA vaccine development in lupus nephritis. Front. Immunol. 2024, 15, 1381445. [Google Scholar] [CrossRef] [PubMed]
- Rybakowska, P.; Alarcon-Riquelme, M.E.; Maranon, C. Key steps and methods in the experimental design and data analysis of highly multi-parametric flow and mass cytometry. Comput. Struct. Biotechnol. J. 2020, 18, 874–886. [Google Scholar] [CrossRef]
- Montante, S.; Brinkman, R.R. Flow cytometry data analysis: Recent tools and algorithms. Int. J. Lab. Hematol. 2019, 41 (Suppl. S1), 56–62. [Google Scholar] [CrossRef]
- Kröger, C.; Müller, S.; Leidner, J.; Kröber, T.; Warnat-Herresthal, S.; Spintge, J.B.; Zajac, T.; Neubauer, A.; Frolov, A.; Carraro, C.; et al. Unveiling the power of high-dimensional cytometry data with cyCONDOR. Nature Commun. 2024, 15, 10702. [Google Scholar] [CrossRef]
- Melsen, J.E.; van Ostaijen-Ten Dam, M.M.; Lankester, A.C.; Schilham, M.W.; van den Akker, E.B. A Comprehensive Workflow for Applying Single-Cell Clustering and Pseudotime Analysis to Flow Cytometry Data. J. Immunol. 2020, 205, 864–871. [Google Scholar] [CrossRef]
- Pulendran, B.; SArunachalam, P.; O’Hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef] [PubMed]
- Cortese, M.; Hagan, T.; Rouphael, N.; Wu, S.Y.; Xie, X.; Kazmin, D.; Wimmers, F.; Gupta, S.; van der Most, R.; Coccia, M.; et al. System vaccinology analysis of predictors and mechanisms of antibody response durability to multiple vaccines in humans. Nat. Immunol. 2025, 26, 116–130. [Google Scholar] [CrossRef]
- Tran, T.M.; Crompton, P.D. Decoding the complexities of human malaria through systems immunology. Immunol. Rev. 2020, 293, 144–162. [Google Scholar] [CrossRef]
- Nakaya, H.I.; Pulendran, B. Systems vaccinology: Its promise and challenge for HIV vaccine development. Curr. Opin. HIV AIDS 2012, 7, 24–31. [Google Scholar] [CrossRef]
- Imani, S.; Li, X.; Chen, K.; Maghsoudloo, M.; Jabbarzadeh Kaboli, P.; Hashemi, M.; Khoushab, S.; Li, X. Computational biology and artificial intelligence in mRNA vaccine design for cancer immunotherapy. Front. Cell Infect. Microbiol. 2025, 14, 1501010. [Google Scholar] [CrossRef] [PubMed]
- Olawade, D.B.; Teke, J.; Fapohunda, O.; Weerasinghe, K.; Usman, S.O.; Ige, A.O.; Clement David-Olawade, A. Leveraging artificial intelligence in vaccine development: A narrative review. J. Microbiol. Methods 2024, 224, 106998. [Google Scholar] [CrossRef]
- Weber, J.K.; Morrone, J.A.; Kang, S.G.; Zhang, L.; Lang, L.; Chowell, D.; Krishna, C.; Huynh, T.; Parthasarathy, P.; Luan, B.; et al. Unsupervised and supervised AI on molecular dynamics simulations reveals complex characteristics of HLA-A2-peptide immunogenicity. Brief. Bioinform. 2023, 25, bbad504. [Google Scholar] [CrossRef] [PubMed]
- Gururaj, A.E.; Scheuermann, R.H.; Lin, D. AI and immunology as a new research paradigm. Nat. Immunol. 2024, 25, 1993–1996. [Google Scholar] [CrossRef]
- Kuenzi, B.M.; Park, J.; Fong, S.H.; Sanchez, K.S.; Lee, J.; Kreisberg, J.F.; Ma, J.; Ideker, T. Predicting Drug Response and Synergy Using a Deep Learning Model of Human Cancer Cells. Cancer cell 2020, 38, 672–684.e6. [Google Scholar] [CrossRef]
- Berson, E.; Chung, P.; Espinosa, C.; Montine, T.J.; Aghaeepour, N. Unlocking human immune system complexity through AI. Nat. Methods 2024, 21, 1400–1402. [Google Scholar] [CrossRef]
- Cobey, K.D.; Ebrahimzadeh, S.; Page, M.J.; Thibault, R.T.; Nguyen, P.Y.; Abu-Dalfa, F.; Moher, D. Biomedical researchers’ perspectives on the reproducibility of research. PLoS Biol. 2024, 22, e3002870. [Google Scholar] [CrossRef]
- Hunter, P. Technical bias and the reproducibility crisis: The problem of systemic errors resulting from artefacts of equipment, methods or dataset has been underappreciated. EMBO Rep. 2021, 22, e52327. [Google Scholar] [CrossRef]
- Sawitzki, B.; Harden, P.N.; Reinke, P.; Moreau, A.; Hutchinson, J.A.; Game, D.S.; Tang, Q.; Guinan, E.C.; Battaglia, M.; Burlingham, W.J.; et al. Regulatory cell therapy in kidney transplantation (The ONE Study): A harmonised design and analysis of seven non-randomised, single-arm, phase 1/2A trials. Lancet 2020, 395, 1627–1639. [Google Scholar] [CrossRef]
- Tessandier, N.; Elie, B.; Boué, V.; Selinger, C.; Rahmoun, M.; Bernat, C.; Grasset, S.; Groc, S.; Bedin, A.S.; Beneteau, T.; et al. Viral and immune dynamics of genital human papillomavirus infections in young women with high temporal resolution. PLoS Biol. 2025, 23, e3002949. [Google Scholar] [CrossRef] [PubMed]
- Cimini, E.; Tartaglia, E.; Messina, F.; Coppola, A.; Mazzotta, V.; Tempestilli, M.; Matusali, G.; Notari, S.; Mondi, A.; Prota, G.; et al. Vδ2 T-cells response in people with Mpox infection: A three-month longitudinal assessment. Emerg. Microbes Infect. 2025, 14, 2455585. [Google Scholar] [CrossRef]
- Macchia, I.; La Sorsa, V.; Ciervo, A.; Ruspantini, I.; Negri, D.; Borghi, M.; De Angelis, M.L.; Luciani, F.; Martina, A.; Taglieri, S.; et al. T Cell Peptide Prediction, Immune Response, and Host-Pathogen Relationship in Vaccinated and Recovered from Mild COVID-19 Subjects. Biomolecules 2024, 14, 1217. [Google Scholar] [CrossRef] [PubMed]
- Minutolo, A.; Petrone, V.; Fanelli, M.; Iannetta, M.; Giudice, M.; Ait Belkacem, I.; Zordan, M.; Vitale, P.; Rasi, G.; Sinibaldi-Vallebona, P.; et al. High CD169 Monocyte/Lymphocyte Ratio Reflects Immunophenotype Disruption and Oxygen Need in COVID-19 Patients. Pathogens 2021, 10, 1639. [Google Scholar] [CrossRef] [PubMed]
- Finak, G.; Langweiler, M.; Jaimes, M.; Malek, M.; Taghiyar, J.; Korin, Y.; Raddassi, K.; Devine, L.; Obermoser, G.; Pekalski, M.L.; et al. Standardizing Flow Cytometry Immunophenotyping Analysis from the Human ImmunoPhenotyping Consortium. Sci. Rep. 2016, 6, 20686. [Google Scholar] [CrossRef]
- Schulz, A.R.; Baumgart, S.; Schulze, J.; Urbicht, M.; Grützkau, A.; Mei, H.E. Stabilizing Antibody Cocktails for Mass Cytometry. Cytom. A 2019, 95, 910–916. [Google Scholar] [CrossRef]
- Lacombe, F.; Bernal, E.; Bloxham, D.; Couzens, S.; Porta, M.G.; Johansson, U.; Kern, W.; Macey, M.; Matthes, T.; Morilla, R.; et al. Harmonemia: A universal strategy for flow cytometry immunophenotyping-A European LeukemiaNet WP10 study. Leukemia 2016, 30, 1769–1772. [Google Scholar] [CrossRef]
- Le Lann, L.; Jouve, P.E.; Alarcón-Riquelme, M.; Jamin, C.; Pers, J.O.; PRECISESADS Flow Cytometry Study Group; PRECISESADS Clinical Consortium. Standardization procedure for flow cytometry data harmonization in prospective multicenter studies. Sci. Rep. 2020, 10, 11567. [Google Scholar] [CrossRef] [PubMed]
- Mair, F.; Hartmann, F.J.; Mrdjen, D.; Tosevski, V.; Krieg, C.; Becher, B. The end of gating? An introduction to automated analysis of high dimensional cytometry data. Eur. J. Immunol. 2016, 46, 34–43. [Google Scholar] [CrossRef]
- Brinkman, R.R. Improving the Rigor and Reproducibility of Flow Cytometry-Based Clinical Research and Trials Through Automated Data Analysis. Cytom. A 2020, 97, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Monaco, G.; Chen, H.; Poidinger, M.; Chen, J.; de Magalhães, J.P.; Larbi, A. flowAI: Automatic and interactive anomaly discerning tools for flow cytometry data. Bioinformatics 2016, 32, 2473–2480. [Google Scholar] [CrossRef]
- Emmaneel, A.; Quintelier, K.; Sichien, D.; Rybakowska, P.; Marañón, C.; Alarcón-Riquelme, M.E.; Van Isterdael, G.; Van Gassen, S.; Saeys, Y. PeacoQC: Peak-based selection of high-quality cytometry data. Cytom. A 2022, 101, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Ashhurst, T.M.; Marsh-Wakefield, F.; Putri, G.H.; Spiteri, A.G.; Shinko, D.; Read, M.N.; Smith, A.L.; King, N.J.C. Integration, exploration, and analysis of high-dimensional single-cell cytometry data using Spectre. Cytom. A 2022, 101, 237–253. [Google Scholar] [CrossRef]
- Bennett, H.M.; Stephenson, W.; Rose, C.M.; Darmanis, S. Single-cell proteomics enabled by next-generation sequencing or mass spectrometry. Nat. Methods 2023, 20, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ge, S.; Liao, T.; Yuan, M.; Qian, W.; Chen, Q.; Liang, W.; Cheng, X.; Zhou, Q.; Ju, Z.; et al. Integrative single-cell metabolomics and phenotypic profiling reveals metabolic heterogeneity of cellular oxidation and senescence. Nat. Commun. 2025, 16, 2740. [Google Scholar] [CrossRef]
- Zhong, C.; Zeng, H.; Deng, J.; Li, Q.; Li, Z.; Zhai, J.; Li, X.; Luan, T. High-Throughput Single-Cell Mass Spectrometry Reveals Sex-Specific Metabolic Responses to 6:2 Chlorinated Polyfluoroalkyl Ether Sulfonate in Zebrafish Liver Cells. Anal. Chem. 2025; Advance online publication. [Google Scholar] [CrossRef]
- Yan, R.; Cheng, X.; Guo, F. Protocol for scChaRM-seq: Simultaneous profiling of gene expression, DNA methylation, and chromatin accessibility in single cells. STAR Protoc. 2021, 2, 100972. [Google Scholar] [CrossRef]
- Bunne, C.; Roohani, Y.; Rosen, Y.; Gupta, A.; Zhang, X.; Roed, M.; Alexandrov, T.; AlQuraishi, M.; Brennan, P.; Burkhardt, D.B.; et al. How to build the virtual cell with artificial intelligence: Priorities and opportunities. Cell 2024, 187, 7045–7063. [Google Scholar] [CrossRef] [PubMed]

| Single-Cell Approach | Throughput | Experimental Workflow | Information Collected | Noise | Data Complexity | Cost/Expertise | |
|---|---|---|---|---|---|---|---|
| Flow cytometry | Yes | High | Well-established | Scatter and fluorescence data | Moderate (photon and electronic noise) | Low | Moderate |
| Spectral cytometry | Yes | High | Requires advanced expertise in panel design | Scatter, fluorescence, and autofluorescence data | Moderate (photon and electronic noise) | Intermediate | Moderate |
| Mass cytometry | Yes | Moderate | Requires mastering chemistry of antibody conjugation | Time-of-flight data | Low (no photon) | Intermediate | Moderate |
| RNA-seq/CITE-seq | Adaptable to single-cell | High | Requires cell sorting/enrichment and advanced data analysis expertise | Gene-expression and surface markers expression data | Moderate (ambient RNA) | High | High |
| ATAC-seq/WGSeq | Adaptable to single-cell | Moderate | Requires cell sorting/enrichment and advanced data analysis expertise | Chromatin accessibility/DNA methylation | Moderate (dropout events) | High | High |
| Proteomic | Moderately adaptable to single-cell | Moderate | Standardized process to be developed | Protein profile by mass spectrometry | Moderate (electronic, shot, and chemical noises) | High | High |
| Metabolomic | Low adaptability to single-cell | Low (imaging-based) | Standardized process to be developed | Targeted/untargeted metabolic profile by mass spectrometry | Moderate (ion suppression, ion–ion interaction, and white noise) | High | High |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montgomery, L.; Larbi, A. Monitoring Immune Responses to Vaccination: A Focus on Single-Cell Analysis and Associated Challenges. Vaccines 2025, 13, 420. https://doi.org/10.3390/vaccines13040420
Montgomery L, Larbi A. Monitoring Immune Responses to Vaccination: A Focus on Single-Cell Analysis and Associated Challenges. Vaccines. 2025; 13(4):420. https://doi.org/10.3390/vaccines13040420
Chicago/Turabian StyleMontgomery, LaToya, and Anis Larbi. 2025. "Monitoring Immune Responses to Vaccination: A Focus on Single-Cell Analysis and Associated Challenges" Vaccines 13, no. 4: 420. https://doi.org/10.3390/vaccines13040420
APA StyleMontgomery, L., & Larbi, A. (2025). Monitoring Immune Responses to Vaccination: A Focus on Single-Cell Analysis and Associated Challenges. Vaccines, 13(4), 420. https://doi.org/10.3390/vaccines13040420




