Salmonella-Based Vaccine: A Promising Strategy for Type 1 Diabetes
Abstract
:1. Introduction
2. Clinical Trials for Type 1 Diabetes Therapies: Challenges and Limitations
3. Salmonella as a Carrier for Vaccine Delivery
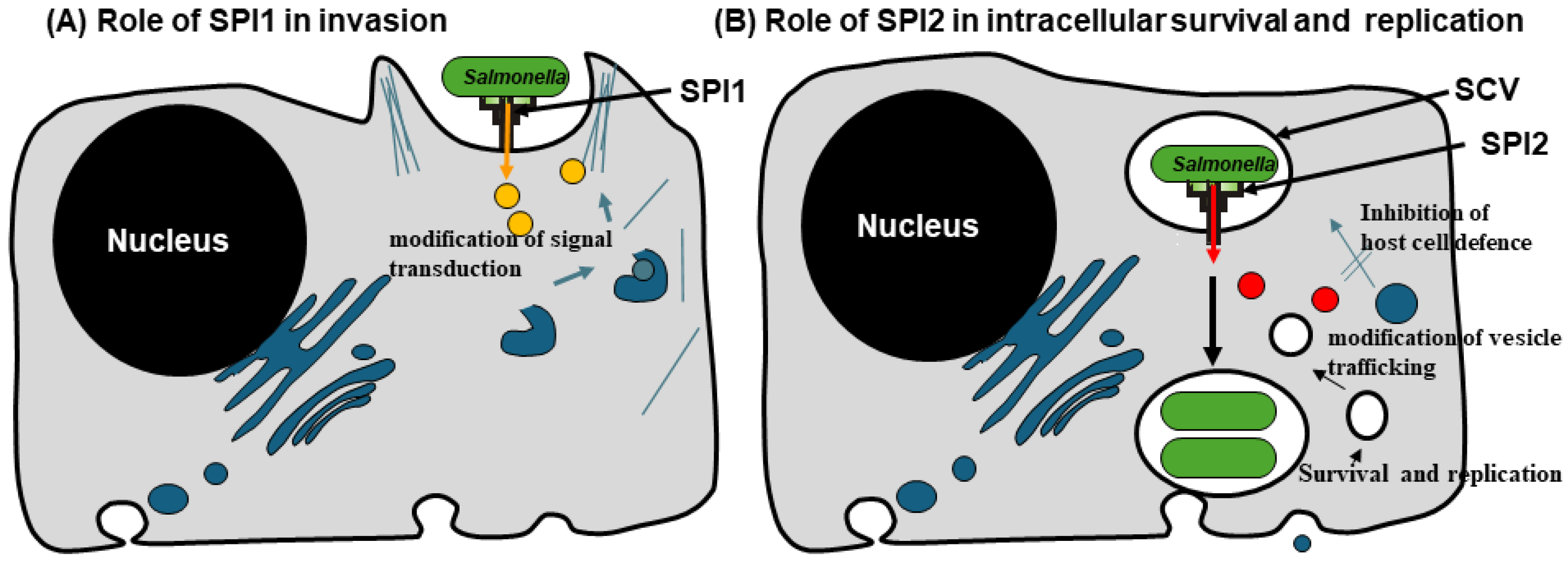
3.1. SPI-1 T3SS: An Essential System for Vaccine Delivery
3.2. SPI-2 of Salmonella: In Vivo–Inducible Promoters–A Double Edge Weapon
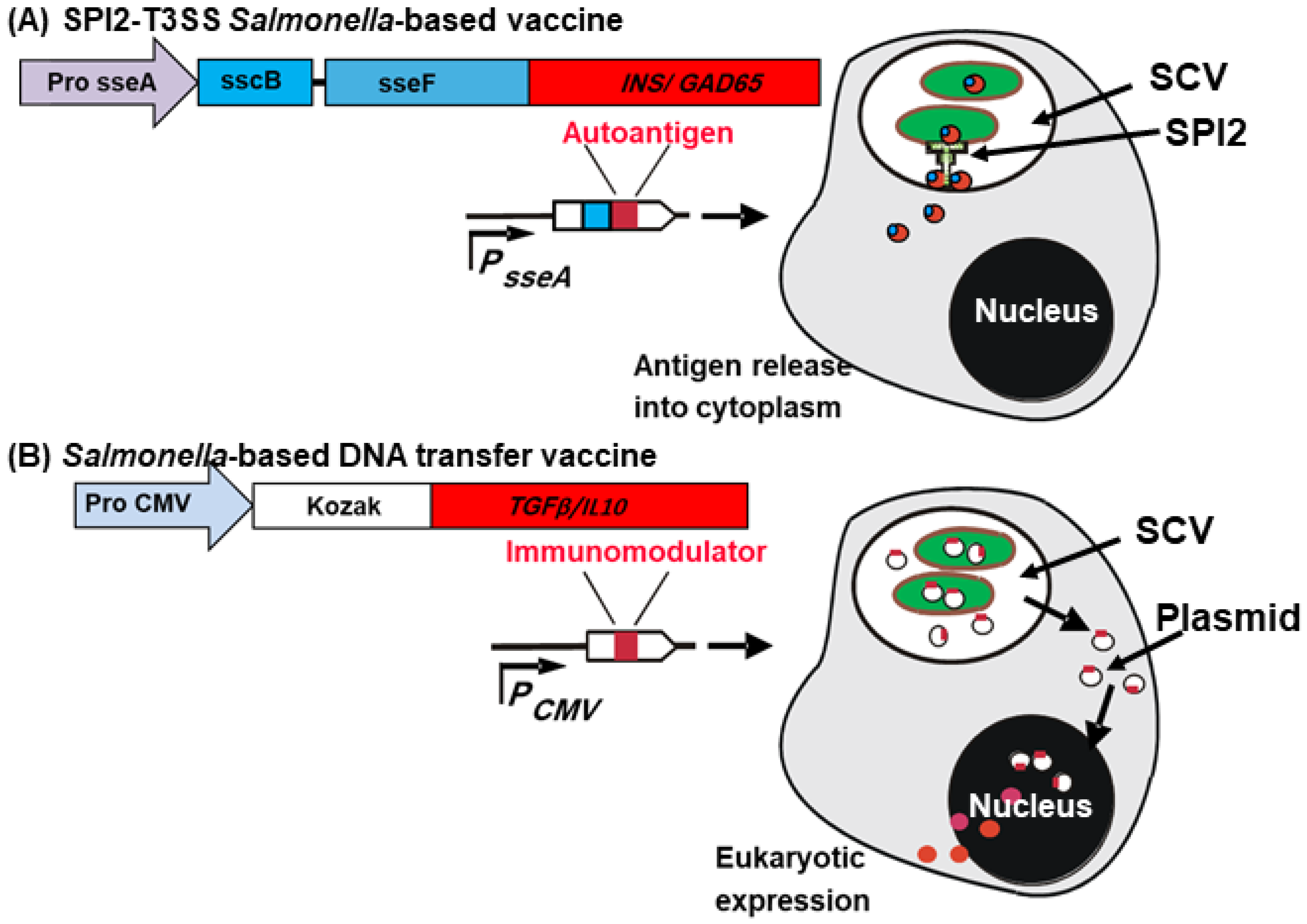
4. Innovative Use of Salmonella as a Vaccine Delivery Platform
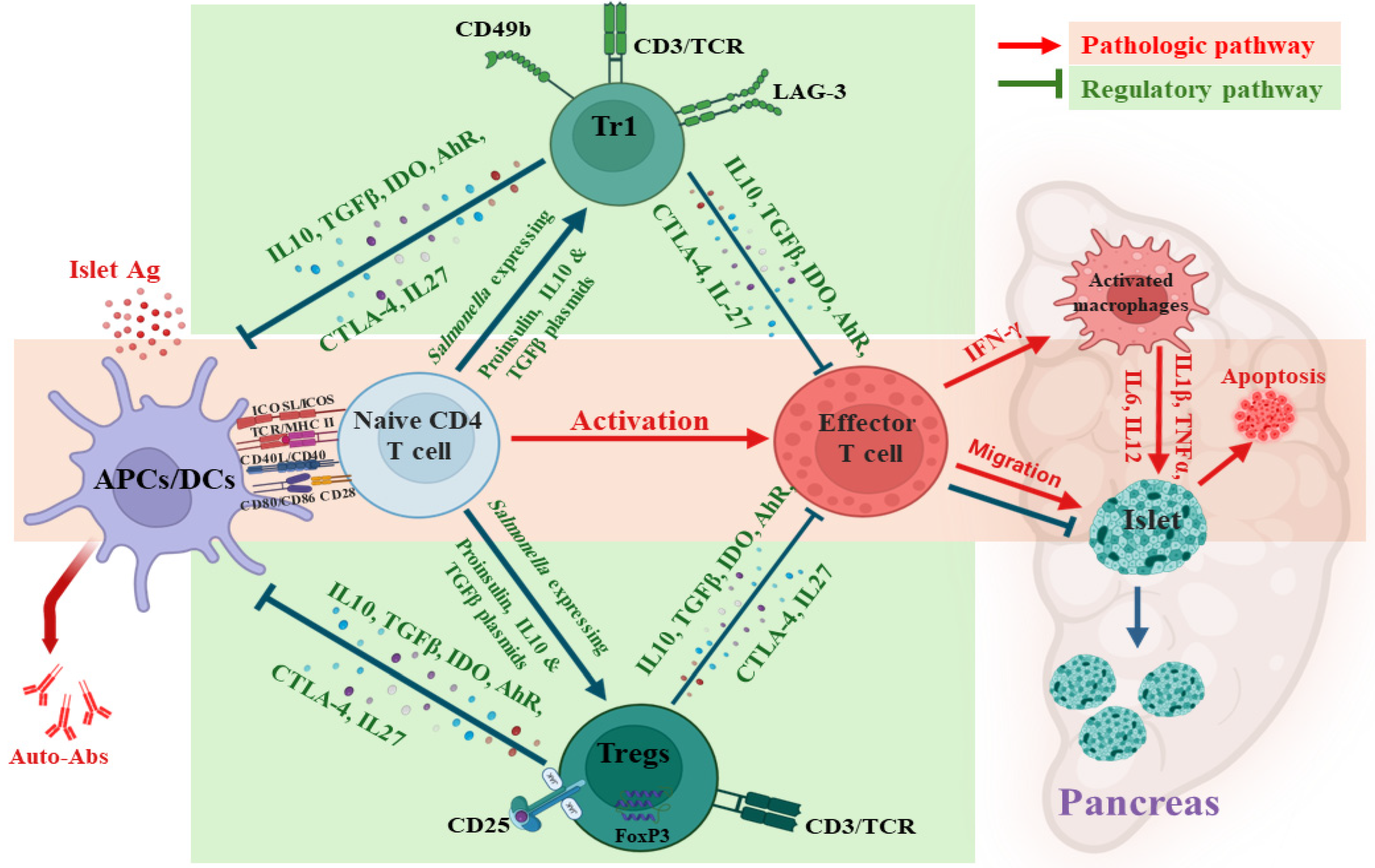
4.1. Induction of Oral Ttolerance via Salmonella-Based Vaccine
4.2. Mechanism of Oral Salmonella-Based Vaccine
5. Advantages of Salmonella-Based Vaccines
6. Limitation of Salmonella-Based Vaccine, Risks, and Mitigation Strategies
7. The Future of Salmonella-Based Vaccines: Challenges and Opportunities
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- James, E.A.; Joglekar, A.V.; Linnemann, A.K.; Russ, H.A.; Kent, S.C. The beta cell-immune cell interface in type 1 diabetes (T1D). Mol. Metab. 2023, 78, 101809. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roy, S.; Pokharel, P.; Piganelli, J.D. Decoding the immune dance: Unraveling the interplay between beta cells and type 1 diabetes. Mol. Metab. 2024, 88, 101998. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burrack, A.L.; Martinov, T.; Fife, B.T. T Cell-Mediated Beta Cell Destruction: Autoimmunity and Alloimmunity in the Context of Type 1 Diabetes. Front. Endocrinol. 2017, 8, 343. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mbongue, J.; Nicholas, D.; Firek, A.; Langridge, W. The role of dendritic cells in tissue-specific autoimmunity. J. Immunol. Res. 2014, 2014, 857143. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakayama, M.; Beilke, J.N.; Jasinski, J.M.; Kobayashi, M.; Miao, D.; Li, M.; Coulombe, M.G.; Liu, E.; Elliott, J.F.; Gill, R.G.; et al. Priming and effector dependence on insulin B:9-23 peptide in NOD islet autoimmunity. J. Clin. Investig. 2007, 117, 1835–1843. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Atkinson, M.A. The pathogenesis and natural history of type 1 diabetes. Cold Spring Harb. Perspect. Med. 2012, 2, a007641. [Google Scholar] [CrossRef] [PubMed]
- Thrower, S.L.; James, L.; Hall, W.; Green, K.M.; Arif, S.; Allen, J.S.; Van-Krinks, C.; Lozanoska-Ochser, B.; Marquesini, L.; Brown, S.; et al. Proinsulin peptide immunotherapy in type 1 diabetes: Report of a first-in-man Phase I safety study. Clin. Exp. Immunol. 2009, 155, 156–165. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rodriguez-Calvo, T.; Zapardiel-Gonzalo, J.; Amirian, N.; Castillo, E.; Lajevardi, Y.; Krogvold, L.; Dahl-Jorgensen, K.; von Herrath, M.G. Increase in Pancreatic Proinsulin and Preservation of beta-Cell Mass in Autoantibody-Positive Donors Prior to Type 1 Diabetes Onset. Diabetes 2017, 66, 1334–1345. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kronenberg, D.; Knight, R.R.; Estorninho, M.; Ellis, R.J.; Kester, M.G.; de Ru, A.; Eichmann, M.; Huang, G.C.; Powrie, J.; Dayan, C.M.; et al. Circulating Preproinsulin Signal Peptide-Specific CD8 T Cells Restricted by the Susceptibility Molecule HLA-A24 Are Expanded at Onset of Type 1 Diabetes and Kill beta-Cells. Diabetes 2012, 61, 1752–1759. [Google Scholar] [CrossRef]
- Skowera, A.; Ellis, R.J.; Varela-Calvino, R.; Arif, S.; Huang, G.C.; Van-Krinks, C.; Zaremba, A.; Rackham, C.; Allen, J.S.; Tree, T.I.M.; et al. CTLs are targeted to kill beta cells in patients with type 1 diabetes through recognition of a glucose-regulated preproinsulin epitope. J. Clin. Investig. 2008, 118, 3390–3402, Erratum in J. Clin. Investig. 2009, 119, 2843. [Google Scholar] [CrossRef]
- Ludvigsson, J. Therapy with GAD in diabetes. Diabetes Metab. Res. Rev. 2009, 25, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Danke, N.A.; Berger, D.; Reichstetter, S.; Reijonen, H.; Greenbaum, C.; Pihoker, C.; James, E.A.; Kwok, W.W. Islet-specific glucose-6-phosphatase catalytic subunit-related protein-reactive CD4+ T cells in human subjects. J. Immunol. 2006, 176, 2781–2789. [Google Scholar] [CrossRef] [PubMed]
- Dang, M.; Rockell, J.; Wagner, R.; Wenzlau, J.M.; Yu, L.P.; Hutton, J.C.; Gottlieb, P.A.; Davidson, H.W. Human Type 1 Diabetes Is Associated with T Cell Autoimmunity to Zinc Transporter 8. J. Immunol. 2011, 186, 6056–6063. [Google Scholar] [CrossRef] [PubMed]
- Badami, E.; Sorini, C.; Coccia, M.; Usuelli, V.; Molteni, L.; Bolla, A.M.; Scavini, M.; Mariani, A.; King, C.; Bosi, E.; et al. Defective differentiation of regulatory FoxP3+ T cells by small-intestinal dendritic cells in patients with type 1 diabetes. Diabetes 2011, 60, 2120–2124. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singer, M.; Elsayed, A.M.; Husseiny, M.I. Regulatory T-cells: The Face-off of the Immune Balance. Front. Biosci. Landmark Ed. 2024, 29, 377. [Google Scholar] [CrossRef] [PubMed]
- Kuhtreiber, W.M.; Faustman, D.L. BCG Therapy for Type 1 Diabetes: Restoration of Balanced Immunity and Metabolism. Trends Endocrinol. Metab. 2019, 30, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Kuhtreiber, W.M.; Takahashi, H.; Keefe, R.C.; Song, Y.; Tran, L.; Luck, T.G.; Shpilsky, G.; Moore, L.; Sinton, S.M.; Graham, J.C.; et al. BCG Vaccinations Upregulate Myc, a Central Switch for Improved Glucose Metabolism in Diabetes. iScience 2020, 23, 101085. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Doupis, J.; Kolokathis, K.; Markopoulou, E.; Efthymiou, V.; Festas, G.; Papandreopoulou, V.; Kallinikou, C.; Antikidou, D.; Gemistou, G.; Angelopoulos, T. The Role of Pediatric BCG Vaccine in Type 1 Diabetes Onset. Diabetes Ther. 2021, 12, 2971–2976. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aniagyei, W.; Mohayideen, S.; Sarfo-Kantanka, O.; Bittner, S.; Vivekanandan, M.M.; Arthur, J.F.; Boateng, A.O.; Yeboah, A.; Ahor, H.S.; Asibey, S.O.; et al. BCG Vaccination-Associated Lower HbA1c and Increased CD25 Expression on CD8+ T Cells in Patients with Type 1 Diabetes in Ghana. Vaccines 2024, 12, 452. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nikolic, T.; Zwaginga, J.J.; Uitbeijerse, B.S.; Woittiez, N.J.; de Koning, E.J.; Aanstoot, H.J.; Roep, B.O. Safety and feasibility of intradermal injection with tolerogenic dendritic cells pulsed with proinsulin peptide-for type 1 diabetes. Lancet Diabetes Endocrinol. 2020, 8, 470–472. [Google Scholar] [CrossRef] [PubMed]
- Mansilla, M.J.; Hilkens, C.M.U.; Martinez-Caceres, E.M. Challenges in tolerogenic dendritic cell therapy for autoimmune diseases: The route of administration. Immunother. Adv. 2023, 3, ltad012. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rios-Rios, W.J.; Sosa-Luis, S.A.; Torres-Aguilar, H. Current advances in using tolerogenic dendritic cells as a therapeutic alternative in the treatment of type 1 diabetes. World J. Diabetes 2021, 12, 603–615. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Passeri, L.; Marta, F.; Bassi, V.; Gregori, S. Tolerogenic Dendritic Cell-Based Approaches in Autoimmunity. Int. J. Mol. Sci. 2021, 22, 8415. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giannoukakis, N. Tolerogenic dendritic cells in type 1 diabetes: No longer a concept. Front. Immunol. 2023, 14, 1212641. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pedersen, I.B.; Kjolby, M.; Hjelholt, A.J.; Madsen, M.; Christensen, A.R.; Adolfsen, D.; Hjelle, J.S.; Kremke, B.; Stovring, H.; Jessen, N.; et al. INfluenza VaccInation to mitigate typE 1 Diabetes (INVITED): A study protocol for a randomised, double-blind, placebo-controlled clinical trial in children and adolescents with recent-onset type 1 diabetes. BMJ Open 2024, 14, e084808. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Van Rampelbergh, J.; Achenbach, P.; Leslie, R.D.; Kindermans, M.; Parmentier, F.; Carlier, V.; Bovy, N.; Vanderelst, L.; Van Mechelen, M.; Vandepapeliere, P.; et al. First-in-human, double-blind, randomized phase 1b study of peptide immunotherapy IMCY-0098 in new-onset type 1 diabetes: An exploratory analysis of immune biomarkers. BMC Med. 2024, 22, 259. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matthews, J.B.; Staeva, T.P.; Bernstein, P.L.; Peakman, M.; von Herrath, M.; Combination, I.-J.T.D. Developing combination immunotherapies for type 1 diabetes: Recommendations from the ITN-JDRF Type 1 Diabetes Combination Therapy Assessment Group. Clin. Exp. Immunol. 2010, 160, 176–184. [Google Scholar] [CrossRef]
- Kokori, E.; Olatunji, G.; Ogieuhi, I.J.; Aboje, J.E.; Olatunji, D.; Aremu, S.A.; Igwe, S.C.; Moradeyo, A.; Ajayi, Y.I.; Aderinto, N. Teplizumab’s immunomodulatory effects on pancreatic beta-cell function in type 1 diabetes mellitus. Clin. Diabetes Endocrinol. 2024, 10, 23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gaglia, J.; Kissler, S. Anti-CD3 Antibody for the Prevention of Type 1 Diabetes: A Story of Perseverance. Biochemistry 2019, 58, 4107–4111. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mullard, A. FDA approves anti-CD3 antibody to delay type 1 diabetes onset. Nat. Rev. Drug Discov. 2023, 22, 6–7. [Google Scholar] [CrossRef] [PubMed]
- Evans-Molina, C.; Oram, R.A. Teplizumab approval for type 1 diabetes in the USA. Lancet Diabetes Endocrinol. 2023, 11, 76–77. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, S.; Chopra, A.; Nagendra, L.; Kalra, S.; Bhattacharya, S. Teplizumab in Type 1 Diabetes Mellitus: An Updated Review. touchREV Endocrinol. 2023, 19, 22–30. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Herold, K.C.; Bundy, B.N.; Long, S.A.; Bluestone, J.A.; DiMeglio, L.A.; Dufort, M.J.; Gitelman, S.E.; Gottlieb, P.A.; Krischer, J.P.; Linsley, P.S.; et al. An Anti-CD3 Antibody, Teplizumab, in Relatives at Risk for Type 1 Diabetes. N. Engl. J. Med. 2019, 381, 603–613. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Daifotis, A.G.; Koenig, S.; Chatenoud, L.; Herold, K.C. Anti-CD3 clinical trials in type 1 diabetes mellitus. Clin. Immunol. 2013, 149, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, A.J.; Shaheen, Z.R.; Fife, B.T. Antigen-specific T cell responses in autoimmune diabetes. Front. Immunol. 2024, 15, 1440045. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zala, A.; Thomas, R. Antigen-specific immunotherapy to restore antigen-specific tolerance in Type 1 diabetes and Graves’ disease. Clin. Exp. Immunol. 2023, 211, 164–175. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Harrison, L.C.; Wentworth, J.M.; Zhang, Y.; Bandala-Sanchez, E.; Bohmer, R.M.; Neale, A.M.; Stone, N.L.; Naselli, G.; Bosco, J.J.; Auyeung, P.; et al. Antigen-based vaccination and prevention of type 1 diabetes. Curr. Diab Rep. 2013, 13, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, P.; Utz, P.J.; Robinson, W.; Steinman, L. Clinical optimization of antigen specific modulation of type 1 diabetes with the plasmid DNA platform. Clin. Immunol. 2013, 149, 297–306. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Postigo-Fernandez, J.; Firdessa-Fite, R.; Creusot, R.J. Preclinical evaluation of a precision medicine approach to DNA vaccination in type 1 diabetes. Proc. Natl. Acad. Sci. USA 2022, 119, e2110987119. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weiner, H.L.; da Cunha, A.P.; Quintana, F.; Wu, H. Oral tolerance. Immunol. Rev. 2011, 241, 241–259. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Holmgren, J.; Czerkinsky, C. Mucosal immunity and vaccines. Nat. Med. 2005, 11, S45–S53. [Google Scholar] [CrossRef] [PubMed]
- Estevan, M.; Irache, J.M.; Grillo, M.J.; Blasco, J.M.; Gamazo, C. Encapsulation of antigenic extracts of Salmonella enterica serovar. Abortusovis into polymeric systems and efficacy as vaccines in mice. Vet. Microbiol. 2006, 118, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Tuli, A.; Sharma, M. How to do business with lysosomes: Salmonella leads the way. Curr. Opin. Microbiol. 2019, 47, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jennings, E.; Thurston, T.L.M.; Holden, D.W. Salmonella SPI-2 Type III Secretion System Effectors: Molecular Mechanisms and Physiological Consequences. Cell Host Microbe 2017, 22, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Lisowski, C.; Dias, J.; Costa, S.; Silva, R.J.; Mano, M.; Eulalio, A. Dysregulated endolysosomal trafficking in cells arrested in the G(1) phase of the host cell cycle impairs Salmonella vacuolar replication. Autophagy 2022, 18, 1785–1800. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lilic, M.; Galkin, V.E.; Orlova, A.; VanLoock, M.S.; Egelman, E.H.; Stebbins, C.E. Salmonella SipA polymerizes actin by stapling filaments with nonglobular protein arms. Science 2003, 301, 1918–1921. [Google Scholar] [CrossRef]
- Schlumberger, M.C.; Hardt, W.D. Triggered phagocytosis by Salmonella: Bacterial molecular mimicry of RhoGTPase activation/deactivation. Curr. Top. Microbiol. 2005, 291, 29–42. [Google Scholar]
- Haneda, T.; Ishii, Y.; Shimizu, H.; Ohshima, K.; Iida, N.; Danbara, H.; Okada, N. Salmonella type III effector SpvC, a phosphothreonine lyase, contributes to reduction in inflammatory response during intestinal phase of infection. Cell Microbiol. 2012, 14, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Lostroh, C.P.; Lee, C.A. The Salmonella pathogenicity island-1 type III secretion system. Microbes Infect. 2001, 3, 1281–1291. [Google Scholar] [CrossRef] [PubMed]
- Boonyom, R.; Karavolos, M.H.; Bulmer, D.M.; Khan, C.M.A. Salmonella pathogenicity island 1 (SPI-1) type III secretion of SopD involves N- and C-terminal signals and direct binding to the InvC ATPase. Microbiology 2010, 156, 1805–1814. [Google Scholar] [CrossRef]
- Hegazy, W.A.H.; Hensel, M. Salmonella enterica as a vaccine carrier. Future Microbiol. 2012, 7, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Husseiny, M.I.; Hensel, M. Evaluation of an intracellular-activated promoter for the generation of live Salmonella recombinant vaccines. Vaccine 2005, 23, 2580–2590. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Schifferli, D.M. Construction, characterization, and immunogenicity of an attenuated Salmonella enterica serovar typhimurium pgtE vaccine expressing fimbriae with integrated viral epitopes from the spiC promoter. Infect. Immun. 2003, 71, 4664–4673. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, X.; Husseiny, M.I.; Goldwich, A.; Hensel, M. Efficacy of Intracellular Activated Promoters for Generation of Salmonella-Based Vaccines. Infect. Immun. 2010, 78, 4828–4838. [Google Scholar] [CrossRef]
- Cheminay, C.; Hensel, M. Rational design of Salmonella recombinant vaccines. Int. J. Med. Microbiol. 2008, 298, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Husseiny, M.I.; Rawson, J.; Kaye, A.; Nair, I.; Todorov, I.; Hensel, M.; Kandeel, F.; Ferreri, K. An oral vaccine for type 1 diabetes based on live attenuated Salmonella. Vaccine 2014, 32, 2300–2307. [Google Scholar] [CrossRef] [PubMed]
- Husseiny, M.I.; Du, W.; Mbongue, J.; Lenz, A.; Rawson, J.; Kandeel, F.; Ferreri, K. Factors affecting Salmonella-based combination immunotherapy for prevention of type 1 diabetes in non-obese diabetic mice. Vaccine 2018, 36, 8008–8018. [Google Scholar] [CrossRef] [PubMed]
- Galan, J.E.; Lara-Tejero, M.; Marlovits, T.C.; Wagner, S. Bacterial type III secretion systems: Specialized nanomachines for protein delivery into target cells. Annu. Rev. Microbiol. 2014, 68, 415–438. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cornelis, G.R. The type III secretion injectisome, a complex nanomachine for intracellular ‘toxin’ delivery. Biol. Chem. 2010, 391, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Marlovits, T.C.; Kubori, T.; Lara-Tejero, M.; Thomas, D.; Unger, V.M.; Galan, J.E. Assembly of the inner rod determines needle length in the type III secretion injectisome. Nature 2006, 441, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Galan, J.E.; Wolf-Watz, H. Protein delivery into eukaryotic cells by type III secretion machines. Nature 2006, 444, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.; Yoo, Y.; Chae, Y.S.; Kee, S.H.; Kim, B.M. Therapeutic advantage of genetically engineered Salmonella typhimurium carrying short hairpin RNA against inhibin alpha subunit in cancer treatment. Ann. Oncol. 2018, 29, 2010–2017. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Liu, Q.; Li, P.; Luo, H.; Wang, H.; Kong, Q. Genetically engineered Salmonella Typhimurium: Recent advances in cancer therapy. Cancer Lett. 2019, 448, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Mbongue, J.C.; Rawson, J.; Garcia, P.A.; Gonzalez, N.; Cobb, J.; Kandeel, F.; Ferreri, K.; Husseiny, M.I. Reversal of New Onset Type 1 Diabetes by Oral Salmonella-Based Combination Therapy and Mediated by Regulatory T-Cells in NOD Mice. Front. Immunol. 2019, 10, 320. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mbongue, J.C.; Alhoshani, A.; Rawson, J.; Garcia, P.A.; Gonzalez, N.; Ferreri, K.; Kandeel, F.; Husseiny, M.I. Tracking of an Oral Salmonella-Based Vaccine for Type 1 Diabetes in Non-obese Diabetic Mice. Front. Immunol. 2020, 11, 712. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cobb, J.; Rawson, J.; Gonzalez, N.; Hensel, M.; Kandeel, F.; Husseiny, M.I. Oral Salmonella msbB Mutant as a Carrier for a Salmonella-Based Vaccine for Prevention and Reversal of Type 1 Diabetes. Front. Immunol. 2021, 12, 667897. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cobb, J.; Soliman, S.S.M.; Retuerto, M.; Quijano, J.C.; Orr, C.; Ghannoum, M.; Kandeel, F.; Husseiny, M.I. Changes in the gut microbiota of NOD mice in response to an oral Salmonella-based vaccine against type 1 diabetes. PLoS ONE 2023, 18, e0285905. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Commins, S.P. Mechanisms of Oral Tolerance. Pediatr. Clin. N. Am. 2015, 62, 1523–1529. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ngan, J.; Kind, L.S. Suppressor T cells for IgE and IgG in Peyer’s patches of mice made tolerant by the oral administration of ovalbumin. J. Immunol. 1978, 120, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, E.C. Generation of improved mucosal vaccines by induction of innate immunity. Cell. Mol. Life Sci. 2005, 62, 2750–2770. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cobb, J.; Rawson, J.; Gonzalez, N.; Orr, C.; Kandeel, F.; Husseiny, M.I. Reversal of diabetes by an oral Salmonella-based vaccine in acute and progressive diabetes in NOD mice. PLoS ONE 2024, 19, e0303863. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cobb, J.; Rawson, J.; Gonzalez, N.; Singer, M.; Kandeel, F.; Husseiny, M.I. Mechanism of Action of Oral Salmonella-Based Vaccine to Prevent and Reverse Type 1 Diabetes in NOD Mice. Vaccines 2024, 12, 276. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Frorup, C.; Gerwig, R.; Svane, C.A.S.; Mendes Lopes de Melo, J.; Henriksen, K.; Floyel, T.; Pociot, F.; Kaur, S.; Storling, J. Characterization of the functional and transcriptomic effects of pro-inflammatory cytokines on human EndoC-betaH5 beta cells. Front. Endocrinol. 2023, 14, 1128523. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Preisser, T.M.; da Cunha, V.P.; Santana, M.P.; Pereira, V.B.; Cara, D.C.; Souza, B.M.; Miyoshi, A. Recombinant Lactococcus lactis Carrying IL-4 and IL-10 Coding Vectors Protects against Type 1 Diabetes in NOD Mice and Attenuates Insulitis in the STZ-Induced Model. J. Diabetes Res. 2021, 2021, 6697319. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bender, C.; Rajendran, S.; von Herrath, M.G. New Insights into the Role of Autoreactive CD8 T Cells and Cytokines in Human Type 1 Diabetes. Front. Endocrinol. 2020, 11, 606434. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xiong, G.; Husseiny, M.I.; Song, L.; Erdreich-Epstein, A.; Shackleford, G.M.; Seeger, R.C.; Jackel, D.; Hensel, M.; Metelitsa, L.S. Novel cancer vaccine based on genes of Salmonella pathogenicity island 2. Int. J. Cancer 2010, 126, 2622–2634. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Husseiny, M.I.; Wartha, F.; Hensel, M. Recombinant vaccines based on translocated effector proteins of Salmonella Pathogenicity Island 2. Vaccine 2007, 25, 185–193. [Google Scholar] [CrossRef]
- Takiishi, T.; Korf, H.; Van Belle, T.L.; Robert, S.; Grieco, F.A.; Caluwaerts, S.; Galleri, L.; Spagnuolo, I.; Steidler, L.; Van Huynegem, K.; et al. Reversal of autoimmune diabetes by restoration of antigen-specific tolerance using genetically modified Lactococcus lactis in mice. J. Clin. Investig. 2012, 122, 1717–1725. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Daniel, C.; Weigmann, B.; Bronson, R.; von Boehmer, H. Prevention of type 1 diabetes in mice by tolerogenic vaccination with a strong agonist insulin mimetope. J. Exp. Med. 2011, 208, 1501–1510. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Greenbaum, C.J.; Beam, C.A.; Boulware, D.; Gitelman, S.E.; Gottlieb, P.A.; Herold, K.C.; Lachin, J.M.; McGee, P.; Palmer, J.P.; Pescovitz, M.D.; et al. Fall in C-peptide during first 2 years from diagnosis: Evidence of at least two distinct phases from composite Type 1 Diabetes TrialNet data. Diabetes 2012, 61, 2066–2073. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Husseiny, M.I.; Kaye, A.; Zebadua, E.; Kandeel, F.; Ferreri, K. Tissue-specific methylation of human insulin gene and PCR assay for monitoring beta cell death. PLoS ONE 2014, 9, e94591. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Husseiny, M.I.; Kuroda, A.; Kaye, A.N.; Nair, I.; Kandeel, F.; Ferreri, K. Development of a quantitative methylation-specific polymerase chain reaction method for monitoring beta cell death in type 1 diabetes. PLoS ONE 2012, 7, e47942. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yue, T.; Sun, F.; Yang, C.; Wang, F.; Luo, J.; Yang, P.; Xiong, F.; Zhang, S.; Yu, Q.; Wang, C.Y. The AHR Signaling Attenuates Autoimmune Responses During the Development of Type 1 Diabetes. Front. Immunol. 2020, 11, 1510. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anquetil, F.; Mondanelli, G.; Gonzalez, N.; Rodriguez Calvo, T.; Zapardiel Gonzalo, J.; Krogvold, L.; Dahl-Jorgensen, K.; Van den Eynde, B.; Orabona, C.; Grohmann, U.; et al. Loss of IDO1 Expression from Human Pancreatic beta-Cells Precedes Their Destruction During the Development of Type 1 Diabetes. Diabetes 2018, 67, 1858–1866. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Orabona, C.; Mondanelli, G.; Pallotta, M.T.; Carvalho, A.; Albini, E.; Fallarino, F.; Vacca, C.; Volpi, C.; Belladonna, M.L.; Berioli, M.G.; et al. Deficiency of immunoregulatory indoleamine 2,3-dioxygenase 1in juvenile diabetes. JCI Insight 2018, 3, e96244. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abram, D.M.; Fernandes, L.G.R.; Ramos Filho, A.C.S.; Simioni, P.U. The modulation of enzyme indoleamine 2,3-dioxygenase from dendritic cells for the treatment of type 1 diabetes mellitus. Drug Des. Dev. Ther. 2017, 11, 2171–2178. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raker, V.K.; Domogalla, M.P.; Steinbrink, K. Tolerogenic Dendritic Cells for Regulatory T Cell Induction in Man. Front. Immunol. 2015, 6, 569. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Castenmiller, C.; Keumatio-Doungtsop, B.C.; van Ree, R.; de Jong, E.C.; van Kooyk, Y. Tolerogenic Immunotherapy: Targeting DC Surface Receptors to Induce Antigen-Specific Tolerance. Front. Immunol. 2021, 12, 643240. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Manicassamy, S.; Pulendran, B. Dendritic cell control of tolerogenic responses. Immunol. Rev. 2011, 241, 206–227. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morita, Y.; Masters, E.A.; Schwarz, E.M.; Muthukrishnan, G. Interleukin-27 and Its Diverse Effects on Bacterial Infections. Front. Immunol. 2021, 12, 678515. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meka, R.R.; Venkatesha, S.H.; Dudics, S.; Acharya, B.; Moudgil, K.D. IL-27-induced modulation of autoimmunity and its therapeutic potential. Autoimmun. Rev. 2015, 14, 1131–1141. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jantsch, J.; Chikkaballi, D.; Hensel, M. Cellular aspects of immunity to intracellular Salmonella enterica. Immunol. Rev. 2011, 240, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Husseiny, M.I. Immunological Considerations for the Development of an Effective Herpes Vaccine. Microorganisms 2024, 12, 1846. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Husseiny, M.I.; Hensel, M. Evaluation of Salmonella live vaccines with chromosomal expression cassettes for translocated fusion proteins. Vaccine 2009, 27, 3780–3787. [Google Scholar] [CrossRef] [PubMed]
- Husseiny, M.I.; Hensel, M. Rapid method for the construction of Salmonella enterica Serovar Typhimurium vaccine carrier strains. Infect. Immun. 2005, 73, 1598–1605. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kong, W.; Brovold, M.; Koeneman, B.A.; Clark-Curtiss, J.; Curtiss, R. Turning self-destructing Salmonella into a universal DNA vaccine delivery platform. Proc. Natl. Acad. Sci. USA 2012, 109, 19414–19419. [Google Scholar] [CrossRef]
- Yu, X.; Jia, R.; Huang, J.; Shu, B.; Zhu, D.; Liu, Q.; Gao, X.; Lin, M.; Yin, Z.; Wang, M.; et al. Attenuated Salmonella typhimurium delivering DNA vaccine encoding duck enteritis virus UL24 induced systemic and mucosal immune responses and conferred good protection against challenge. Vet. Res. 2012, 43, 56. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Robert, S.; Gysemans, C.; Takiishi, T.; Korf, H.; Spagnuolo, I.; Sebastiani, G.; Van Huynegem, K.; Steidler, L.; Caluwaerts, S.; Demetter, P.; et al. Oral delivery of glutamic acid decarboxylase (GAD)-65 and IL10 by Lactococcus lactis reverses diabetes in recent-onset NOD mice. Diabetes 2014, 63, 2876–2887. [Google Scholar] [CrossRef] [PubMed]
- Poirier, T.P.; Kehoe, M.A.; Beachey, E.H. Protective immunity evoked by oral administration of attenuated aroA Salmonella typhimurium expressing cloned streptococcal M protein. J. Exp. Med. 1988, 168, 25–32. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Simanjuntak, C.H.; Paleologo, F.P.; Punjabi, N.H.; Darmowigoto, R.; Totosudirjo, S.H.; Haryanto, P.; Suprijanto, E.; Witham, N.D.; Hoffman, S.L. Oral Immunization against Typhoid-Fever in Indonesia with Ty21a Vaccine. Lancet 1991, 338, 1055–1059. [Google Scholar] [CrossRef]
- Tennant, S.M.; Levine, M.M. Live attenuated vaccines for invasive Salmonella infections. Vaccine 2015, 33 (Suppl. S3), C36–C41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, B.; Yang, M.; Wong, H.Y.; Watt, R.M.; Song, E.; Zheng, B.J.; Yuen, K.Y.; Huang, J.D. A method to generate recombinant Salmonella typhi Ty21a strains expressing multiple heterologous genes using an improved recombineering strategy. Appl. Microbiol. Biotechnol. 2011, 91, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Frahm, M.; Felgner, S.; Kocijancic, D.; Rohde, M.; Hensel, M.; Curtiss, R., 3rd; Erhardt, M.; Weiss, S. Efficiency of conditionally attenuated Salmonella enterica serovar Typhimurium in bacterium-mediated tumor therapy. MBio 2015, 6, 10-1128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Claes, A.K.; Steck, N.; Schultz, D.; Zahringer, U.; Lipinski, S.; Rosenstiel, P.; Geddes, K.; Philpott, D.J.; Heine, H.; Grassl, G.A. Salmonella enterica serovar Typhimurium DeltamsbB triggers exacerbated inflammation in Nod2 deficient mice. PLoS ONE 2014, 9, e113645. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Di Lorenzo, T.P.; Peakman, M.; Roep, B.O. Translational mini-review series on type 1 diabetes: Systematic analysis of T cell epitopes in autoimmune diabetes. Clin. Exp. Immunol. 2007, 148, 1–16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barker, J.M.; Barriga, K.J.; Yu, L.; Miao, D.; Erlich, H.A.; Norris, J.M.; Eisenbarth, G.S.; Rewers, M. Prediction of autoantibody positivity and progression to type 1 diabetes: Diabetes autoimmunity study in the young (DAISY). J. Clin. Endocrinol. Metab. 2004, 89, 3896–3902. [Google Scholar] [CrossRef] [PubMed]
- Verge, C.F.; Stenger, D.; Bonifacio, E.; Colman, P.G.; Pilcher, C.; Bingley, P.J.; Eisenbarth, G.S. Combined use of autoantibodies (IA-2 autoantibody, GAD autoantibody, insulin autoantibody, cytoplasmic islet cell antibodies) in type 1 diabetes: Combinatorial Islet Autoantibody Workshop. Diabetes 1998, 47, 1857–1866. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Eisenbarth, G.S. Prediction and prevention of type 1 diabetes mellitus. J. Diabetes 2011, 3, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Stadinski, B.D.; Zhang, L.; Crawford, F.; Marrack, P.; Eisenbarth, G.S.; Kappler, J.W. Diabetogenic T cells recognize insulin bound to IAg7 in an unexpected, weakly binding register. Proc. Natl. Acad. Sci. USA 2010, 107, 10978–10983. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moser, A.; Hsu, H.T.; van Endert, P. Beta cell antigens in type 1 diabetes: Triggers in pathogenesis and therapeutic targets. F1000 Biol. Rep. 2010, 2, 75. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Daniel, C.; von Boehmer, H. Extrathymic generation of regulatory T cells-chances and challenges for prevention of autoimmune disease. Adv. Immunol. 2011, 112, 177–213. [Google Scholar] [CrossRef] [PubMed]
- Serr, I.; Furst, R.W.; Achenbach, P.; Scherm, M.G.; Gokmen, F.; Haupt, F.; Sedlmeier, E.M.; Knopff, A.; Shultz, L.; Willis, R.A.; et al. Type 1 diabetes vaccine candidates promote human Foxp3+Treg induction in humanized mice. Nat. Commun. 2016, 7, 10991. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Donath, M.Y.; Hess, C.; Palmer, E. What is the role of autoimmunity in type 1 diabetes? A clinical perspective. Diabetologia 2014, 57, 653–655. [Google Scholar] [CrossRef] [PubMed]
- Luce, S.; Guinoiseau, S.; Gadault, A.; Letourneur, F.; Blondeau, B.; Nitschke, P.; Pasmant, E.; Vidaud, M.; Lemonnier, F.; Boitard, C. Humanized mouse model to study type 1 diabetes. Diabetes 2018, 67, 1816–1829. [Google Scholar] [CrossRef] [PubMed]
- Danner, R.; Chaudhari, S.N.; Rosenberger, J.; Surls, J.; Richie, T.L.; Brumeanu, T.D.; Casares, S. Expression of HLA Class II molecules in humanized NOD.Rag1KO.IL2RgcKO mice is critical for development and function of human T and B cells. PLoS ONE 2011, 6, e19826. [Google Scholar] [CrossRef]
- Gu, A.; Torres-Coronado, M.; Tran, C.A.; Vu, H.; Epps, E.W.; Chung, J.; Gonzalez, N.; Blanchard, S.; DiGiusto, D.L. Engraftment and Lineage Potential of Adult Hematopoietic Stem and Progenitor Cells Is Compromised Following Short-Term Culture in the Presence of an Aryl Hydrocarbon Receptor Antagonist. Hum. Gene Ther. Method. 2014, 25, 221–231. [Google Scholar] [CrossRef]
- Tran, C.A.; Torres-Coronado, M.; Gardner, A.; Gu, A.; Vu, H.; Rao, A.; Cao, L.F.; Ahmed, A.; Digiusto, D. Optimized processing of growth factor mobilized peripheral blood CD34+ products by counterflow centrifugal elutriation. Stem Cells Transl. Med. 2012, 1, 422–429. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bansal, G.; Ghanem, M.; Sears, K.T.; Galen, J.E.; Tennant, S.M. Genetic engineering of Salmonella spp. for novel vaccine strategies and therapeutics. EcoSal Plus 2024, 12, eesp00042023. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kong, Q.; Yang, J.; Liu, Q.; Alamuri, P.; Roland, K.L.; Curtiss, R., 3rd. Effect of deletion of genes involved in lipopolysaccharide core and O-antigen synthesis on virulence and immunogenicity of Salmonella enterica serovar typhimurium. Infect. Immun. 2011, 79, 4227–4239. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Covarrubias, C.E.; Rivera, T.A.; Soto, C.A.; Deeks, T.; Kalergis, A.M. Current GMP standards for the production of vaccines and antibodies: An overview. Front. Public Health 2022, 10, 1021905. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baral, K.C.; Bajracharya, R.; Lee, S.H.; Han, H.K. Advancements in the Pharmaceutical Applications of Probiotics: Dosage Forms and Formulation Technology. Int. J. Nanomed. 2021, 16, 7535–7556. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, H.; Fan, Y.; Zhong, J.; Malkoch, M.; Cai, Z.; Wang, Z. Advance in oral delivery of living material. Biomed. Technol. 2023, 3, 26–39. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singer, M.; Kandeel, F.; Husseiny, M.I. Salmonella-Based Vaccine: A Promising Strategy for Type 1 Diabetes. Vaccines 2025, 13, 405. https://doi.org/10.3390/vaccines13040405
Singer M, Kandeel F, Husseiny MI. Salmonella-Based Vaccine: A Promising Strategy for Type 1 Diabetes. Vaccines. 2025; 13(4):405. https://doi.org/10.3390/vaccines13040405
Chicago/Turabian StyleSinger, Mahmoud, Fouad Kandeel, and Mohamed I. Husseiny. 2025. "Salmonella-Based Vaccine: A Promising Strategy for Type 1 Diabetes" Vaccines 13, no. 4: 405. https://doi.org/10.3390/vaccines13040405
APA StyleSinger, M., Kandeel, F., & Husseiny, M. I. (2025). Salmonella-Based Vaccine: A Promising Strategy for Type 1 Diabetes. Vaccines, 13(4), 405. https://doi.org/10.3390/vaccines13040405








