Next-Generation Adenoviral Vector-Based Vaccines for Severe Acute Respiratory Syndrome Coronavirus-2
Abstract
1. Introduction
2. Shortcomings of Current SARS-CoV-2 Vaccines
2.1. Decreased Vaccine Efficacy Against Emerging SARS-CoV-2 Variants
2.2. Short Durability of Protective Immune Responses
2.3. Failure to Prevent Disease Symptoms and Virus Transmission
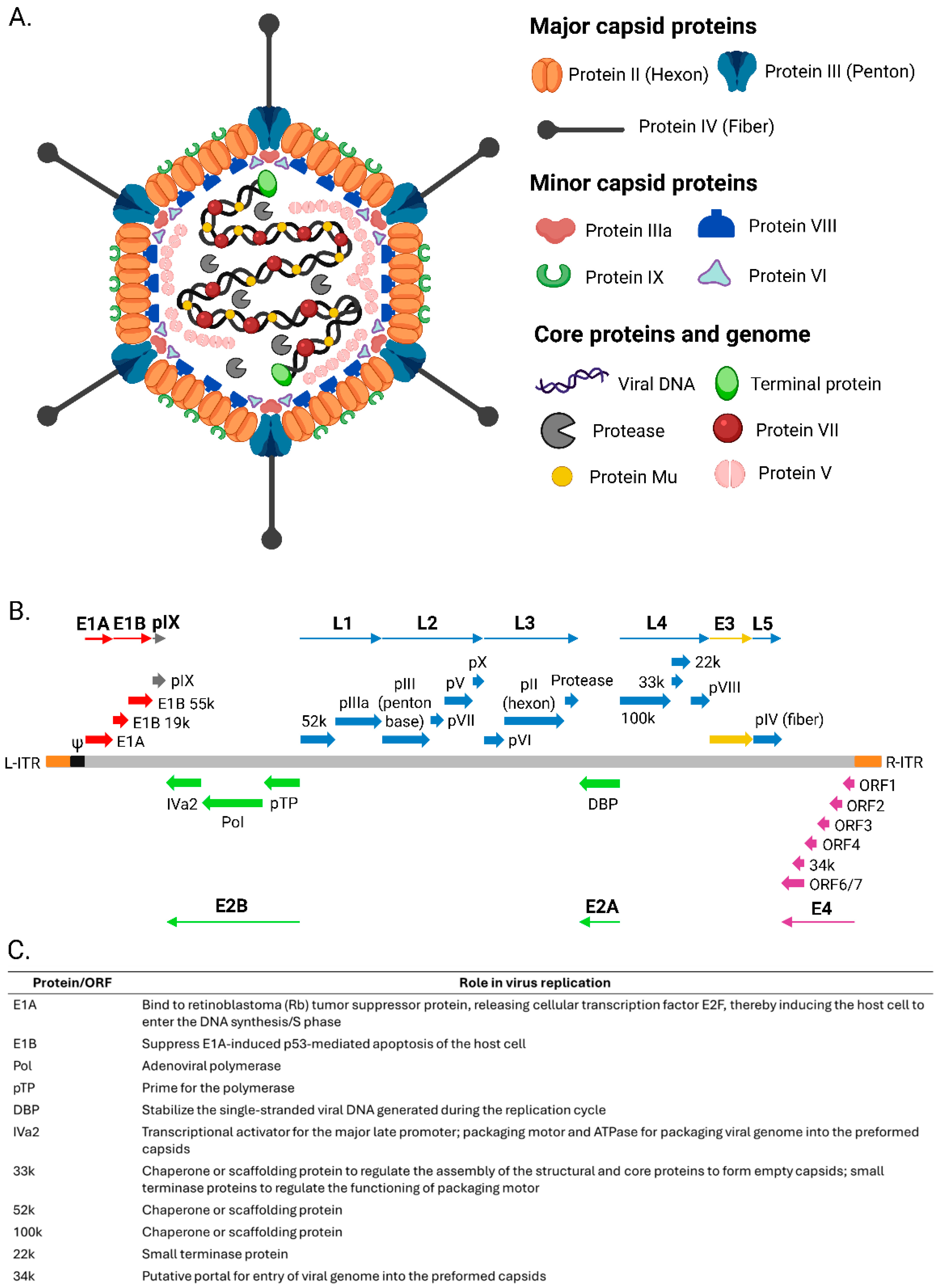
3. Ad Biology, Vector Structure, and Immune Responses
4. Licensed Ad Vector-Based SARS-CoV-2 Vaccines
4.1. AZD1222 Vaccine
4.2. Ad26.COV2.S Vaccine
4.3. Gam-COVID-Vac Vaccine
4.4. Convidecia Vaccine
| Vaccine | Vector Type | Antigen Design | Dosing Regimen | Storage Conditions | Variant Efficacy | References |
|---|---|---|---|---|---|---|
| ASTRAZENECA (AZD1222) | ChAd (ChAdOx1) | Full-length spike protein with stabilizing changes | Two doses, i.m. | −20 °C for long-term storage; 2–8 °C for short-term storage | Effective against B.1.1.7; reduced efficacy against B.1.351 | [82,99,100] |
| JANSSEN (Ad26.COV2.S) | HAd26 | Full-length spike protein with stabilizing mutations | Single dose, i.m. | 2–8 °C for up to 3 months; −20 °C for up to 2 years | Effective against B.1.1.7, B.1.351, and P.2 variants | [89,101] |
| SPUTNIK V (Gam-COVID-Vac) | HAd26 for prime, HAd5 for boost | Full-length spike protein using prime-boost strategy | Two doses, i.m. | Available in two formulations: frozen (−18 °C) and lyophilized (2–8 °C) | Effective against B.1.1.7, but neutralizing antibody response is significantly reduced against B.1.351, P.1, and B.1.1.28 variants | [102,103] |
| CONVIDECIA (Ad5-nCoV) | HAd5 | Full-length spike protein | Single dose, i.m. | Stored at 2–8 °C | Limited data available regarding efficacy against emerging variants | [94,97,104,105] |
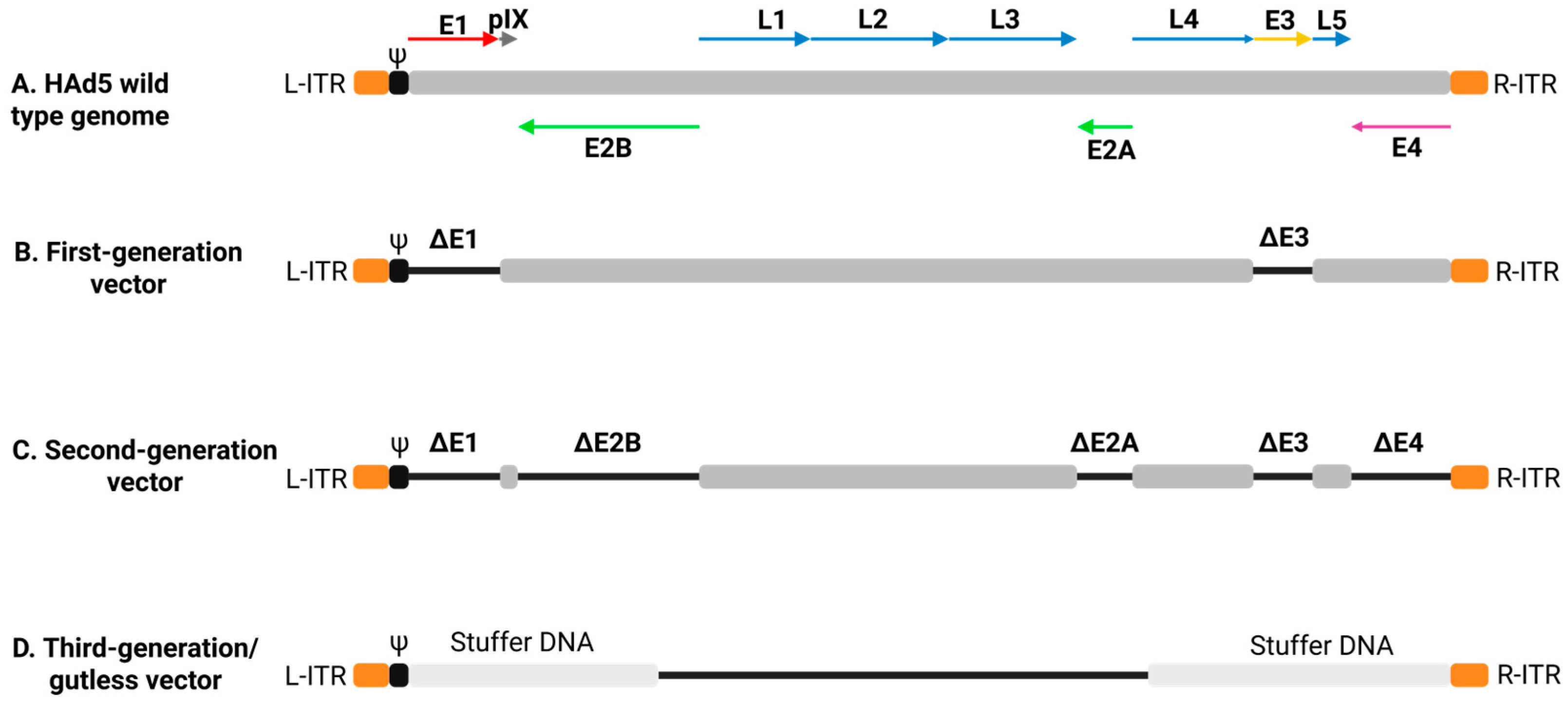
5. Challenges Associated with Ad Vector-Based Vaccines
5.1. Preexisting Ad Immunity
5.2. Vaccine-Induced Immune Thrombotic Thrombocytopenia
5.3. Guillain–Barré Syndrome
6. Strategies for Developing Next-Generation Ad Vector-Based SARS-CoV-2 Vaccines
6.1. Targeting Other SARS-CoV-2 Immunogens in Addition to S
6.2. Bivalent or Multivalent S-Based Vaccine Approach
6.3. Use of Less Prevalent Human or Nonhuman Ads as Vaccine Vectors
6.4. Different Immunization Routes for Prime and Boost with the Same Ad Vector-Based Vaccine
6.5. Enhancing Immunity with Diverse Vaccine Platforms
6.6. Enhanced Protection Against Respiratory Pathogens by Mucosal Immunization with Ad Vector-Based Vaccines
6.7. Ad Vector Modification for Improved Vaccine Efficacy
6.8. Encapsulation of Ad Vectors to Elude Vector Immunity
6.9. Use of Bispecific Adapters to Enhance Ad Vector-Mediated Immune Responses
6.10. Use of Signaling Molecules or Peptides to Increase Ad Vector-Mediated Immune Responses
7. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sadeghi Dousari, A.; Taati Moghadam, M.; Satarzadeh, N. COVID-19 (Coronavirus Disease 2019): A New Coronavirus Disease. Infect. Drug Resist. 2020, 13, 2819–2828. [Google Scholar] [CrossRef] [PubMed]
- Ravi, V.; Saxena, S.; Panda, P.S. Basic virology of SARS-CoV 2. Indian. J. Med. Microbiol. 2022, 40, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, S.A.; Lorincz, R.; Boucher, P.; Curiel, D.T. Adenoviral vector vaccine platforms in the SARS-CoV-2 pandemic. npj Vac. 2021, 6, 97. [Google Scholar] [CrossRef] [PubMed]
- Lenart, K.; Arcoverde Cerveira, R.; Hellgren, F.; Ols, S.; Sheward, D.J.; Kim, C.; Cagigi, A.; Gagne, M.; Davis, B.; Germosen, D.; et al. Three immunizations with Novavax’s protein vaccines increase antibody breadth and provide durable protection from SARS-CoV-2. npj Vac. 2024, 9, 17. [Google Scholar] [CrossRef]
- Lamb, Y.N. BNT162b2 mRNA COVID-19 Vaccine: First Approval. Drugs 2021, 81, 495–501. [Google Scholar] [CrossRef]
- Blakney, A.K.; Bekker, L.-G. DNA vaccines join the fight against COVID-19. Lancet 2022, 399, 1281–1282. [Google Scholar] [CrossRef]
- Elkashif, A.; Alhashimi, M.; Sayedahmed, E.E.; Sambhara, S.; Mittal, S.K. Adenoviral vector-based platforms for developing effective vaccines to combat respiratory viral infections. Clin. Transl. Immunol. 2021, 10, e1345. [Google Scholar] [CrossRef]
- Okuyama, R. mRNA and Adenoviral Vector Vaccine Platforms Utilized in COVID-19 Vaccines: Technologies, Ecosystem, and Future Directions. Vaccines 2023, 11, 1737. [Google Scholar] [CrossRef]
- Chi, W.-Y.; Li, Y.-D.; Huang, H.-C.; Chan, T.E.H.; Chow, S.-Y.; Su, J.-H.; Ferrall, L.; Hung, C.-F.; Wu, T.C. COVID-19 vaccine update: Vaccine effectiveness, SARS-CoV-2 variants, boosters, adverse effects, and immune correlates of protection. J. Biomed. Sci. 2022, 29, 82. [Google Scholar] [CrossRef]
- Lin, D.-Y.; Gu, Y.; Wheeler, B.; Young, H.; Holloway, S.; Sunny, S.-K.; Moore, Z.; Zeng, D. Effectiveness of COVID-19 Vaccines over a 9-Month Period in North Carolina. N. Engl. J. Med. 2022, 386, 933–941. [Google Scholar] [CrossRef]
- Chavda, V.P.; Bezbaruah, R.; Valu, D.; Patel, B.; Kumar, A.; Prasad, S.; Kakoti, B.B.; Kaushik, A.; Jesawadawala, M. Adenoviral Vector-Based Vaccine Platform for COVID-19: Current Status. Vaccines 2023, 11, 432. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-C.; Sayedahmed, E.E.; Mittal, S.K. Significance of Preexisting Vector Immunity and Activation of Innate Responses for Adenoviral Vector-Based Therapy. Viruses 2022, 14, 2727. [Google Scholar] [CrossRef]
- Mittal, S.K.; Ahi, Y.S.; Vemula, S.V. Xenogenic Adenoviral Vectors. In Adenoviral Vectors for Gene Therapy; Elsevier: Amsterdam, The Netherlands, 2016; pp. 495–528. [Google Scholar]
- Zhu, F.-C.; Li, Y.-H.; Guan, X.-H.; Hou, L.-H.; Wang, W.-J.; Li, J.-X.; Wu, S.-P.; Wang, B.-S.; Wang, Z.; Wang, L.; et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: A dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 2020, 395, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Beltran, W.F.; Lam, E.C.; St Denis, K.; Nitido, A.D.; Garcia, Z.H.; Hauser, B.M.; Feldman, J.; Pavlovic, M.N.; Gregory, D.J.; Poznansky, M.C.; et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell 2021, 184, 2372–2383.e2379. [Google Scholar] [CrossRef] [PubMed]
- Vinzón, S.E.; Lopez, M.V.; Cafferata, E.G.A.; Soto, A.S.; Berguer, P.M.; Vazquez, L.; Nusblat, L.; Pontoriero, A.V.; Belotti, E.M.; Salvetti, N.R.; et al. Cross-protection and cross-neutralization capacity of ancestral and VOC-matched SARS-CoV-2 adenoviral vector-based vaccines. npj Vac. 2023, 8, 149. [Google Scholar] [CrossRef]
- Nordström, P.; Ballin, M.; Nordström, A. Risk of infection, hospitalisation, and death up to 9 months after a second dose of COVID-19 vaccine: A retrospective, total population cohort study in Sweden. Lancet 2022, 399, 814–823. [Google Scholar] [CrossRef]
- Thompson, M.G.; Stenehjem, E.; Grannis, S.; Ball, S.W.; Naleway, A.L.; Ong, T.C.; DeSilva, M.B.; Natarajan, K.; Bozio, C.H.; Lewis, N.; et al. Effectiveness of COVID-19 Vaccines in Ambulatory and Inpatient Care Settings. N. Engl. J. Med. 2021, 385, 1355–1371. [Google Scholar] [CrossRef]
- Singanayagam, A.; Hakki, S.; Dunning, J.; Madon, K.J.; Crone, M.A.; Koycheva, A.; Derqui-Fernandez, N.; Barnett, J.L.; Whitfield, M.G.; Varro, R.; et al. Community transmission and viral load kinetics of the SARS-CoV-2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: A prospective, longitudinal, cohort study. Lancet Infect. Dis. 2022, 22, 183–195. [Google Scholar] [CrossRef]
- Franco-Paredes, C. Transmissibility of SARS-CoV-2 among fully vaccinated individuals. Lancet Infect. Dis. 2022, 22, 16. [Google Scholar] [CrossRef]
- Mongin, D.; Bürgisser, N.; Laurie, G.; Schimmel, G.; Vu, D.-L.; Cullati, S.; Covid-SMC Study Group; Courvoisier, D.S. Effect of SARS-CoV-2 prior infection and mRNA vaccination on contagiousness and susceptibility to infection. Nat. Commun. 2023, 14, 5452. [Google Scholar] [CrossRef]
- Dangi, T.; Class, J.; Palacio, N.; Richner, J.M.; Penaloza MacMaster, P. Combining spike- and nucleocapsid-based vaccines improves distal control of SARS-CoV-2. Cell Rep. 2021, 36, 109664. [Google Scholar] [CrossRef] [PubMed]
- Benkő, M.; Aoki, K.; Arnberg, N.; Davison, A.J.; Echavarría, M.; Hess, M.; Jones, M.S.; Kaján, G.L.; Kajon, A.E.; Mittal, S.K.; et al. ICTV Virus Taxonomy Profile: Adenoviridae 2022: This article is part of the ICTV Virus Taxonomy Profiles collection. J. Gen. Virol. 2022, 103, 001721. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, A.; Hage, E.; Ganzenmueller, T.; Böttcher, S.; Hofmann, J.; Hamprecht, K.; Obermeier, P.; Rath, B.; Hausmann, F.; Dobner, T.; et al. Molecular Evolution of Human Adenovirus (HAdV) Species C. Sci. Rep. 2019, 9, 1039. [Google Scholar] [CrossRef]
- Colloca, S.; Barnes, E.; Folgori, A.; Ammendola, V.; Capone, S.; Cirillo, A.; Siani, L.; Naddeo, M.; Grazioli, F.; Esposito, M.L.; et al. Vaccine Vectors Derived from a Large Collection of Simian Adenoviruses Induce Potent Cellular Immunity Across Multiple Species. Sci. Transl. Med. 2012, 4, 115. [Google Scholar] [CrossRef] [PubMed]
- Harrach, B.; Tarján, Z.L.; Benkő, M. Adenoviruses across the animal kingdom: A walk in the zoo. FEBS Lett. 2019, 593, 3660–3673. [Google Scholar] [CrossRef]
- Liu, L. Fields Virology, 6th Edition. Clin. Infect. Dis. 2014, 59, 613. [Google Scholar] [CrossRef]
- Maclachlan, N.J.; Dubovi, E.J. (Eds.) Adenoviridae. In Fenner’s Veterinary Virology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 217–227. [Google Scholar]
- San Martín, C. Latest Insights on Adenovirus Structure and Assembly. Viruses 2012, 4, 847–877. [Google Scholar] [CrossRef]
- Davison, A.J.; Benkő, M.; Harrach, B. Genetic content and evolution of adenoviruses. J. Gen. Virol. 2003, 84, 2895–2908. [Google Scholar] [CrossRef]
- Van Raaij, M.J.; Mitraki, A.; Lavigne, G.; Cusack, S. A triple β-spiral in the adenovirus fibre shaft reveals a new structural motif for a fibrous protein. Nature 1999, 401, 935–938. [Google Scholar] [CrossRef]
- Bergelson, J.M.; Cunningham, J.A.; Droguett, G.; Kurt-Jones, E.A.; Krithivas, A.; Hong, J.S.; Horwitz, M.S.; Crowell, R.L.; Finberg, R.W. Isolation of a Common Receptor for Coxsackie B Viruses and Adenoviruses 2 and 5. Science 1997, 275, 1320–1323. [Google Scholar] [CrossRef]
- Wickham, T.J.; Mathias, P.; Cheresh, D.A.; Nemerow, G.R. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell 1993, 73, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Cohen, C.J.; Xiang, Z.Q.; Gao, G.-P.; Ertl, H.C.J.; Wilson, J.M.; Bergelson, J.M. Chimpanzee adenovirus CV-68 adapted as a gene delivery vector interacts with the coxsackievirus and adenovirus receptor. J. Gen. Virol. 2002, 83, 151–155. [Google Scholar] [CrossRef]
- Tan, P.K.; Michou, A.I.; Bergelson, J.M.; Cotten, M. Defining CAR as a cellular receptor for the avian adenovirus CELO using a genetic analysis of the two viral fibre proteins. J. Gen. Virol. 2001, 82, 1465–1472. [Google Scholar] [CrossRef]
- Soudais, C.; Boutin, S.; Hong, S.S.; Chillon, M.; Danos, O.; Bergelson, J.M.; Boulanger, P.; Kremer, E.J. Canine adenovirus type 2 attachment and internalization: Coxsackievirus-adenovirus receptor, alternative receptors, and an RGD-independent pathway. J. Virol. 2000, 74, 10639–10649. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bangari, D.S.; Sharma, A.; Mittal, S.K. Bovine adenovirus serotype 3 utilizes sialic acid as a cellular receptor for virus entry. Virology 2009, 392, 162–168. [Google Scholar] [CrossRef]
- Sharma, A.; Li, X.; Bangari, D.S.; Mittal, S.K. Adenovirus receptors and their implications in gene delivery. Virus Res. 2009, 143, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Stasiak, A.C.; Stehle, T. Human adenovirus binding to host cell receptors: A structural view. Med. Microbiol. Immunol. 2020, 209, 325–333. [Google Scholar] [CrossRef]
- Kremer, E.J.; Nemerow, G.R. Adenovirus Tales: From the Cell Surface to the Nuclear Pore Complex. PLoS Pathog. 2015, 11, e1004821. [Google Scholar] [CrossRef]
- Wiethoff, C.M.; Wodrich, H.; Gerace, L.; Nemerow, G.R. Adenovirus Protein VI Mediates Membrane Disruption following Capsid Disassembly. J. Virol. 2005, 79, 1992–2000. [Google Scholar] [CrossRef]
- Bremner, K.H.; Scherer, J.; Yi, J.; Vershinin, M.; Gross, S.P.; Vallee, R.B. Adenovirus Transport via Direct Interaction of Cytoplasmic Dynein with the Viral Capsid Hexon Subunit. Cell Host Microbe 2009, 6, 523–535. [Google Scholar] [CrossRef]
- Strunze, S.; Engelke, M.F.; Wang, I.H.; Puntener, D.; Boucke, K.; Schleich, S.; Way, M.; Schoenenberger, P.; Burckhardt, C.J.; Greber, U.F. Kinesin-1-Mediated Capsid Disassembly and Disruption of the Nuclear Pore Complex Promote Virus Infection. Cell Host Microbe 2011, 10, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Acheson, N.H. Fundamentals of Molecular Virology; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Ostapchuk, P.; Hearing, P. Control of adenovirus packaging. J. Cell. Biochem. 2005, 96, 25–35. [Google Scholar] [CrossRef]
- Hong, S.S.; Szolajska, E.; Schoehn, G.; Franqueville, L.; Myhre, S.; Lindholm, L.; Ruigrok, R.W.; Boulanger, P.; Chroboczek, J. The 100K-chaperone protein from adenovirus serotype 2 (Subgroup C) assists in trimerization and nuclear localization of hexons from subgroups C and B adenoviruses. J. Mol. Biol. 2005, 352, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Ahi, Y.S.; Mittal, S.K. Components of Adenovirus Genome Packaging. Front. Microbiol. 2016, 7, 1503. [Google Scholar] [CrossRef]
- Ahi, Y.S.; Hassan, A.O.; Vemula, S.V.; Li, K.; Jiang, W.; Zhang, G.J.; Mittal, S.K. Adenoviral E4 34K protein interacts with virus packaging components and may serve as the putative portal. Sci. Rep. 2017, 7, 7582. [Google Scholar] [CrossRef] [PubMed]
- Chang, J. Adenovirus Vectors: Excellent Tools for Vaccine Development. Immune Netw. 2021, 21, e6. [Google Scholar] [CrossRef]
- Crenshaw, B.J.; Jones, L.B.; Bell, C.R.; Kumar, S.; Matthews, Q.L. Perspective on Adenoviruses: Epidemiology, Pathogenicity, and Gene Therapy. Biomedicines 2019, 7, 61. [Google Scholar] [CrossRef]
- Sayedahmed, E.E.; Kumari, R.; Mittal, S.K. Current Use of Adenovirus Vectors and Their Production Methods. In Viral Vectors for Gene Therapy; Manfredsson, F.P., Benskey, M.J., Eds.; Springer: New York, NY, USA, 2019; Volume 1937, pp. 155–175. [Google Scholar]
- Danthinne, X.; Imperiale, M.J. Production of first generation adenovirus vectors: A review. Gene Ther. 2000, 7, 1707–1714. [Google Scholar] [CrossRef]
- Russell, W.C.; Graham, F.L.; Smiley, J.; Nairn, R. Characteristics of a Human Cell Line Transformed by DNA from Human Adenovirus Type 5. J. Gen. Virol. 1977, 36, 59–72. [Google Scholar] [CrossRef]
- Wen, S.; Schneider, D.B.; Driscoll, R.M.; Vassalli, G.; Sassani, A.B.; Dichek, D.A. Second-Generation Adenoviral Vectors Do Not Prevent Rapid Loss of Transgene Expression and Vector DNA From the Arterial Wall. Atertio. Thromb. Vasc. Biol. 2000, 20, 1452–1458. [Google Scholar] [CrossRef]
- Brescia, M.; Janssen, J.M.; Liu, J.; Gonçalves, M.A.F.V. High-Capacity Adenoviral Vectors Permit Robust and Versatile Testing of DMD Gene Repair Tools and Strategies in Human Cells. Cells 2020, 9, 869. [Google Scholar] [CrossRef] [PubMed]
- Parks, R.J.; Chen, L.; Anton, M.; Sankar, U.; Rudnicki, M.A.; Graham, F.L. A helper-dependent adenovirus vector system: Removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc. Natl. Acad. Sci. USA 1996, 93, 13565–13570. [Google Scholar] [CrossRef] [PubMed]
- Sargent, K.; Ng, P.; Evelegh, C.; Graham, F.; Parks, R. Development of a size-restricted pIX-deleted helper virus for amplification of helper-dependent adenovirus vectors. Gene Ther. 2004, 11, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Bangari, D.S.; Tandon, M.; Hogenesch, H.; Mittal, S.K. Evaluation of innate immunity and vector toxicity following inoculation of bovine, porcine or human adenoviral vectors in a mouse model. Virus Res. 2010, 153, 134–142. [Google Scholar] [CrossRef]
- Rhee, E.G.; Blattman, J.N.; Kasturi, S.P.; Kelley, R.P.; Kaufman, D.R.; Lynch, D.M.; La Porte, A.; Simmons, N.L.; Clark, S.L.; Pulendran, B.; et al. Multiple Innate Immune Pathways Contribute to the Immunogenicity of Recombinant Adenovirus Vaccine Vectors. J. Virol. 2011, 85, 315–323. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-Like Receptor Signaling Pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Lam, E.; Stein, S.; Falck-Pedersen, E. Adenovirus Detection by the cGAS/STING/TBK1 DNA Sensing Cascade. J. Virol. 2014, 88, 974–981. [Google Scholar] [CrossRef]
- Suzuki, M.; Cela, R.; Bertin, T.K.; Sule, G.; Cerullo, V.; Rodgers, J.R.; Lee, B. NOD2 Signaling Contributes to the Innate Immune Response Against Helper-Dependent Adenovirus Vectors Independently of MyD88 In Vivo. Hum. Gene Ther. 2011, 22, 1071–1082. [Google Scholar] [CrossRef]
- Reyes-Sandoval, A.; Fitzgerald, J.C.; Grant, R.; Roy, S.; Xiang, Z.Q.; Li, Y.; Gao, G.P.; Wilson, J.M.; Ertl, H.C. Human immunodeficiency virus type 1-specific immune responses in primates upon sequential immunization with adenoviral vaccine carriers of human and simian serotypes. J. Virol. 2004, 78, 7392–7399. [Google Scholar] [CrossRef][Green Version]
- Hensley, S.E.; Cun, A.S.; Giles-Davis, W.; Li, Y.; Xiang, Z.; Lasaro, M.O.; Williams, B.R.; Silverman, R.H.; Ertl, H.C. Type I interferon inhibits antibody responses induced by a chimpanzee adenovirus vector. Mol. Ther. 2007, 15, 393–403. [Google Scholar]
- Barouch, D.H.; Pau, M.G.; Custers, J.H.; Koudstaal, W.; Kostense, S.; Havenga, M.J.; Truitt, D.M.; Sumida, S.M.; Kishko, M.G.; Arthur, J.C. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J. Immunol. 2004, 172, 6290–6297. [Google Scholar]
- Van Der Lubbe, J.E.M.; Rosendahl Huber, S.K.; Vijayan, A.; Dekking, L.; Van Huizen, E.; Vreugdenhil, J.; Choi, Y.; Baert, M.R.M.; Feddes-de Boer, K.; Izquierdo Gil, A.; et al. Ad26.COV2.S protects Syrian hamsters against G614 spike variant SARS-CoV-2 and does not enhance respiratory disease. npj Vac. 2021, 6, 39. [Google Scholar] [CrossRef]
- Doronin, K.; Flatt, J.W.; Di Paolo, N.C.; Khare, R.; Kalyuzhniy, O.; Acchione, M.; Sumida, J.P.; Ohto, U.; Shimizu, T.; Akashi-Takamura, S.; et al. Coagulation Factor X Activates Innate Immunity to Human Species C Adenovirus. Science 2012, 338, 795–798. [Google Scholar] [CrossRef]
- Muruve, D.A. The Innate Immune Response to Adenovirus Vectors. Hum. Gene Ther. 2004, 15, 1157–1166. [Google Scholar] [CrossRef]
- Nociari, M.; Ocheretina, O.; Schoggins, J.W.; Falck-Pedersen, E. Sensing Infection by Adenovirus: Toll-Like Receptor-Independent Viral DNA Recognition Signals Activation of the Interferon Regulatory Factor 3 Master Regulator. J. Virol. 2007, 81, 4145–4157. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Tandon, M.; Ahi, Y.S.; Bangari, D.S.; Vemulapalli, R.; Mittal, S.K. Evaluation of cross-reactive cell-mediated immune responses among human, bovine and porcine adenoviruses. Gene Ther. 2010, 17, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Atasheva, S.; Shayakhmetov, D.M. Cytokine Responses to Adenovirus and Adenovirus Vectors. Viruses 2022, 14, 888. [Google Scholar] [CrossRef]
- Sayedahmed, E.E.; Elshafie, N.O.; Zhang, G.; Mohammed, S.I.; Sambhara, S.; Mittal, S.K. Enhancement of mucosal innate and adaptive immunity following intranasal immunization of mice with a bovine adenoviral vector. Front. Immunol. 2023, 14, 1305937. [Google Scholar] [CrossRef]
- Liu, Q.; Muruve, D.A. Molecular basis of the inflammatory response to adenovirus vectors. Gene Ther. 2003, 10, 935–940. [Google Scholar] [CrossRef]
- Ewer, K.J.; Barrett, J.R.; Belij-Rammerstorfer, S.; Sharpe, H.; Makinson, R.; Morter, R.; Flaxman, A.; Wright, D.; Bellamy, D.; Bittaye, M.; et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat. Med. 2021, 27, 270–278. [Google Scholar] [CrossRef]
- Bos, R.; Rutten, L.; Van Der Lubbe, J.E.M.; Bakkers, M.J.G.; Hardenberg, G.; Wegmann, F.; Zuijdgeest, D.; De Wilde, A.H.; Koornneef, A.; Verwilligen, A.; et al. Ad26 vector-based COVID-19 vaccine encoding a prefusion-stabilized SARS-CoV-2 Spike immunogen induces potent humoral and cellular immune responses. npj Vac. 2020, 5, 91. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, Q. Safety and Efficacy of the Common Vaccines against COVID-19. Vaccines 2022, 10, 513. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.M.; Pandi-Perumal, S.R.; Trakht, I.; Thyagarajan, S.P. Strategy for COVID-19 vaccination in India: The country with the second highest population and number of cases. npj Vac. 2021, 6, 60. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Lambe, T.; Spencer, A.; Belij-Rammerstorfer, S.; Purushotham, J.N.; Port, J.R.; Avanzato, V.A.; Bushmaker, T.; Flaxman, A.; Ulaszewska, M.; et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature 2020, 586, 578–582. [Google Scholar] [CrossRef]
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet 2021, 396, 1979–1993. [Google Scholar] [CrossRef]
- Falsey, A.R.; Sobieszczyk, M.E.; Hirsch, I.; Sproule, S.; Robb, M.L.; Corey, L.; Neuzil, K.M.; Hahn, W.; Hunt, J.; Mulligan, M.J.; et al. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 nCoV-19) COVID-19 Vaccine. N. Engl. J. Med. 2021, 385, 2348–2360. [Google Scholar] [CrossRef]
- Hamaluba, M.; Sang, S.; Orindi, B.; Njau, I.; Karanja, H.; Kamau, N.; Gitonga, J.N.; Mugo, D.; Wright, D.; Nyagwange, J.; et al. Safety and immunogenicity of ChAdOx1 nCoV-19 (AZD1222) vaccine in adults in Kenya: A phase 1/2 single-blind, randomised controlled trial. Wellcome Open Res. 2023, 8, 182. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- van der Lubbe, J.E.; Rosendahl Huber, S.K.; Vijayan, A.; Dekking, L.; van Huizen, E.; Vreugdenhil, J.; Choi, Y.; Baert, M.R.; Feddes-de Boer, K.; Gil, A.I.; et al. Ad26. COV2. S-elicited immunity protects against G614 spike variant SARS-CoV-2 infection in Syrian hamsters and does not enhance respiratory disease in challenged animals with breakthrough infection after sub-optimal vaccine dosing. bioRxiv 2021. Available online: https://www.biorxiv.org/content/10.1101/2021.01.08.425915v1 (accessed on 7 April 2025).
- He, X.; Chandrashekar, A.; Zahn, R.; Wegmann, F.; Yu, J.; Mercado, N.B.; McMahan, K.; Martinot, A.J.; Piedra-Mora, C.; Beecy, S. Low-dose Ad26. COV2. S protection against SARS-CoV-2 challenge in rhesus macaques. Cell 2021, 184, 3467–3473.e3411. [Google Scholar]
- Solforosi, L.; Kuipers, H.; Huber, S.K.R.; van der Lubbe, J.E.; Dekking, L.; Czapska-Casey, D.N.; Gil, A.I.; Baert, M.R.; Drijver, J.; Vaneman, J.; et al. Immunogenicity and protective efficacy of one-and two-dose regimens of the Ad26. COV2. S COVID-19 vaccine candidate in adult and aged rhesus macaques. bioRxiv 2021. Available online: https://www.biorxiv.org/content/10.1101/2020.11.17.368258v2 (accessed on 7 April 2025).
- Sadoff, J.; Le Gars, M.; Shukarev, G.; Heerwegh, D.; Truyers, C.; de Groot, A.M.; Stoop, J.; Tete, S.; Van Damme, W.; Leroux-Roels, I. Interim results of a phase 1–2a trial of Ad26. COV2. S COVID-19 vaccine. N. Engl. J. Med. 2021, 384, 1824–1835. [Google Scholar]
- Tsuchiya, Y.; Tamura, H.; Fujii, K.; Numaguchi, H.; Toyoizumi, K.; Liu, T.; Le Gars, M.; Cárdenas, V.; Eto, T. Safety, reactogenicity, and immunogenicity of Ad26.COV2.S: Results of a phase 1, randomized, double-blind, placebo-controlled COVID-19 vaccine trial in Japan. Vaccine 2023, 41, 1602–1610. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, V.; Le Gars, M.; Truyers, C.; Ruiz-Guiñazú, J.; Struyf, F.; Colfer, A.; Bonten, M.; Borobia, A.; Reisinger, E.C.; Kamerling, I.M.C.; et al. Safety and immunogenicity of Ad26.COV2.S in adults: A randomised, double-blind, placebo-controlled Phase 2a dose-finding study. Vaccine 2024, 42, 3536–3546. [Google Scholar] [CrossRef] [PubMed]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B. Safety and efficacy of single-dose Ad26. COV2. S vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar]
- Hardt, K.; Vandebosch, A.; Sadoff, J.; Le Gars, M.; Truyers, C.; Lowson, D.; Van Dromme, I.; Vingerhoets, J.; Kamphuis, T.; Scheper, G.; et al. Efficacy, safety, and immunogenicity of a booster regimen of Ad26.COV2.S vaccine against COVID-19 (ENSEMBLE2): Results of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Infect. Dis. 2022, 22, 1703–1715. [Google Scholar] [CrossRef]
- Balakrishnan, V.S. The arrival of Sputnik V. Lancet Infect. Dis. 2020, 20, 1128. [Google Scholar] [CrossRef] [PubMed]
- Logunov, D.Y.; Dolzhikova, I.V.; Zubkova, O.V.; Tukhvatulin, A.I.; Shcheblyakov, D.V.; Dzharullaeva, A.S.; Grousova, D.M.; Erokhova, A.S.; Kovyrshina, A.V.; Botikov, A.G.; et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: Two open, non-randomised phase 1/2 studies from Russia. Lancet 2020, 396, 887–897. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Zhu, Y.; Tang, R.; Li, X.; Chen, X.; Wang, X.; Wang, Y.; Wang, R.; Zhu, F.; Li, J. Vaccination with Adenovirus Type 5 Vector-Based COVID-19 Vaccine as the Primary Series in Adults: A Randomized, Double-Blind, Placebo-Controlled Phase 1/2 Clinical Trial. Vaccines 2024, 12, 292. [Google Scholar] [CrossRef]
- Wu, S.; Zhong, G.; Zhang, J.; Shuai, L.; Zhang, Z.; Wen, Z.; Wang, B.; Zhao, Z.; Song, X.; Chen, Y.; et al. A single dose of an adenovirus-vectored vaccine provides protection against SARS-CoV-2 challenge. Nat. Commun. 2020, 11, 4081. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Wang, Q.; Shan, C.; Yang, C.; Feng, Y.; Wu, J.; Liu, X.; Zhou, Y.; Jiang, R.; Hu, P.; et al. An adenovirus-vectored COVID-19 vaccine confers protection from SARS-CoV-2 challenge in rhesus macaques. Nat. Commun. 2020, 11, 4207. [Google Scholar] [CrossRef]
- Zhu, F.-C.; Guan, X.-H.; Li, Y.-H.; Huang, J.-Y.; Jiang, T.; Hou, L.-H.; Li, J.-X.; Yang, B.-F.; Wang, L.; Wang, W.-J.; et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020, 396, 479–488. [Google Scholar] [CrossRef]
- Halperin, S.A.; Ye, L.; MacKinnon-Cameron, D.; Smith, B.; Cahn, P.E.; Ruiz-Palacios, G.M.; Ikram, A.; Lanas, F.; Lourdes Guerrero, M.; Muñoz Navarro, S.R.; et al. Final efficacy analysis, interim safety analysis, and immunogenicity of a single dose of recombinant novel coronavirus vaccine (adenovirus type 5 vector) in adults 18 years and older: An international, multicentre, randomised, double-blinded, placebo-controlled phase 3 trial. Lancet 2022, 399, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Emary, K.R.W.; Golubchik, T.; Aley, P.K.; Ariani, C.V.; Angus, B.; Bibi, S.; Blane, B.; Bonsall, D.; Cicconi, P.; Charlton, S.; et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): An exploratory analysis of a randomised controlled trial. Lancet 2021, 397, 1351–1362. [Google Scholar] [CrossRef]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S.E.; Wallace, M.; See, I.; Mbaeyi, S.; Godfrey, M.; Hadler, S.C.; Jatlaoui, T.C.; Twentyman, E.; Hughes, M.M.; Rao, A.K.; et al. Use of the Janssen (Johnson & Johnson) COVID-19 Vaccine: Updated Interim Recommendations from the Advisory Committee on Immunization Practices-United States, December 2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 90–95. [Google Scholar] [CrossRef]
- Ikegame, S.; Siddiquey, M.N.A.; Hung, C.T.; Haas, G.; Brambilla, L.; Oguntuyo, K.Y.; Kowdle, S.; Vilardo, A.E.; Edelstein, A.; Perandones, C.; et al. Neutralizing activity of Sputnik V vaccine sera against SARS-CoV-2 variants. medRxiv Prepr. Serv. Health Sci. 2021. [Google Scholar] [CrossRef]
- Fiolet, T.; Kherabi, Y.; MacDonald, C.J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. 2022, 28, 202–221. [Google Scholar] [CrossRef]
- Huang, Z.; Xu, S.; Liu, J.; Wu, L.; Qiu, J.; Wang, N.; Ren, J.; Li, Z.; Guo, X.; Tao, F.; et al. Effectiveness of inactivated and Ad5-nCoV COVID-19 vaccines against SARS-CoV-2 Omicron BA. 2 variant infection, severe illness, and death. BMC Med. 2022, 20, 400. [Google Scholar] [CrossRef]
- Richardson, V.L.; Camacho Franco, M.A.; Bautista Márquez, A.; Martínez Valdez, L.; Castro Ceronio, L.E.; Cruz Cruz, V.; Gharpure, R.; Lafond, K.E.; Yau, T.S.; Azziz-Baumgartner, E.; et al. Vaccine Effectiveness of CanSino (Adv5-nCoV) Coronavirus Disease 2019 (COVID-19) Vaccine Among Childcare Workers-Mexico, March-December 2021. Clin. Infect. Dis. 2022, 75, S167–S173. [Google Scholar] [CrossRef]
- Mennechet, F.J.D.; Paris, O.; Ouoba, A.R.; Salazar Arenas, S.; Sirima, S.B.; Takoudjou Dzomo, G.R.; Diarra, A.; Traore, I.T.; Kania, D.; Eichholz, K.; et al. A review of 65 years of human adenovirus seroprevalence. Expert. Rev. Vaccines 2019, 18, 597–613. [Google Scholar] [CrossRef] [PubMed]
- Schilham, M.W.; Claas, E.C.; Van Zaane, W.; Heemskerk, B.; Vossen, J.M.; Lankester, A.C.; Toes, R.E.; Echavarria, M.; Kroes, A.C.; Van Tol, M.J. High Levels of Adenovirus DNA in Serum Correlate with Fatal Outcome of Adenovirus Infection in Children after Allogeneic Stem-Cell Transplantation. Clin. Infect. Dis. 2002, 35, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Fausther-Bovendo, H.; Kobinger, G.P. Pre-existing immunity against Ad vectors: Humoral, cellular, and innate response, what’s important? Hum. Vaccin. Immunother. 2014, 10, 2875–2884. [Google Scholar] [CrossRef]
- Zhang, Q.; Seto, D. Chimpanzee Adenovirus Vector Ebola Vaccine--Preliminary Report. N. Engl. J. Med. 2015, 373, 775–776. [Google Scholar] [CrossRef]
- Sayedahmed, E.E.; Kumari, R.; Shukla, S.; Hassan, A.O.; Mohammed, S.I.; York, I.A.; Gangappa, S.; Sambhara, S.; Mittal, S.K. Longevity of adenovirus vector immunity in mice and its implications for vaccine efficacy. Vaccine 2018, 36, 6744–6751. [Google Scholar] [CrossRef]
- Kanack, A.J.; Padmanabhan, A. Vaccine-induced immune thrombotic thrombocytopenia. Best. Pract. Res. Clin. Haematol. 2022, 35, 101381. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A.; Selleng, K.; Warkentin, T.E. Autoimmune heparin-induced thrombocytopenia. J. Thromb. Haemost. 2017, 15, 2099–2114. [Google Scholar] [CrossRef]
- Baker, A.T.; Boyd, R.J.; Sarkar, D.; Teijeira-Crespo, A.; Chan, C.K.; Bates, E.; Waraich, K.; Vant, J.; Wilson, E.; Truong, C.D.; et al. ChAdOx1 interacts with CAR and PF4 with implications for thrombosis with thrombocytopenia syndrome. Sci. Adv. 2021, 7, eabl8213. [Google Scholar] [CrossRef]
- Kanack, A.J.; Bayas, A.; George, G.; Abou-Ismail, M.Y.; Singh, B.; Kohlhagen, M.C.; Splinter, N.P.; Christ, M.; Naumann, M.; Moser, K.A.; et al. Monoclonal and oligoclonal anti-platelet factor 4 antibodies mediate VITT. Blood 2022, 140, 73–77. [Google Scholar] [CrossRef]
- Padmanabhan, A.; Jones, C.G.; Bougie, D.W.; Curtis, B.R.; McFarland, J.G.; Wang, D.; Aster, R.H. Heparin-independent, PF4-dependent binding of HIT antibodies to platelets: Implications for HIT pathogenesis. Blood 2015, 125, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Craven, B.; Lester, W.; Boyce, S.; Thomas, W.; Kanny, A.; Davies, C.; Pavord, S.; Hermans, J.; Makris, M.; Bart-Smith, E.; et al. Natural history of PF4 antibodies in vaccine-induced immune thrombocytopenia and thrombosis. Blood 2022, 139, 2553–2560. [Google Scholar] [CrossRef] [PubMed]
- Gabarin, N.; Arnold, D.M.; Nazy, I.; Warkentin, T.E. Treatment of vaccine-induced immune thrombotic thrombocytopenia (VITT). Semin. Hematol. 2022, 59, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Kanack, A.J.; Singh, B.; George, G.; Gundabolu, K.; Koepsell, S.A.; Abou-Ismail, M.Y.; Moser, K.A.; Smock, K.J.; Green, D.; Major, A.; et al. Persistence of Ad26.COV2.S-associated vaccine-induced immune thrombotic thrombocytopenia (VITT) and specific detection of VITT antibodies. Am. J. Hematol. 2022, 97, 519–526. [Google Scholar] [CrossRef]
- Warkentin, T.E.; Kelton, J.G. Temporal aspects of heparin-induced thrombocytopenia. N. Engl. J. Med. 2001, 344, 1286–1292. [Google Scholar] [CrossRef]
- See, I.; Lale, A.; Marquez, P.; Streiff, M.B.; Wheeler, A.P.; Tepper, N.K.; Woo, E.J.; Broder, K.R.; Edwards, K.M.; Gallego, R.; et al. Case Series of Thrombosis With Thrombocytopenia Syndrome After COVID-19 Vaccination—United States, December 2020 to August 2021. Ann. Intern. Med. 2022, 175, 513–522. [Google Scholar] [CrossRef]
- Andrews, N.J.; Stowe, J.; Ramsay, M.E.; Miller, E. Risk of venous thrombotic events and thrombocytopenia in sequential time periods after ChAdOx1 and BNT162b2 COVID-19 vaccines: A national cohort study in England. Lancet Reg. Health. Eur. 2022, 13, 100260. [Google Scholar] [CrossRef]
- Buoninfante, A.; Andeweg, A.; Baker, A.T.; Borad, M.; Crawford, N.; Dogné, J.M.; Garcia-Azorin, D.; Greinacher, A.; Helfand, R.; Hviid, A.; et al. Understanding thrombosis with thrombocytopenia syndrome after COVID-19 vaccination. npj Vac. 2022, 7, 141. [Google Scholar] [CrossRef]
- Herrera-Comoglio, R.; Lane, S. Vaccine-Induced Immune Thrombocytopenia and Thrombosis after the Sputnik V Vaccine. N. Engl. J. Med. 2022, 387, 1431–1432. [Google Scholar] [CrossRef]
- Van De Munckhof, A.; Krzywicka, K.; Aguiar De Sousa, D.; Sánchez Van Kammen, M.; Heldner, M.R.; Jood, K.; Lindgren, E.; Tatlisumak, T.; Putaala, J.; Kremer Hovinga, J.A.; et al. Declining mortality of cerebral venous sinus thrombosis with thrombocytopenia after SARS-CoV-2 vaccination. Eur. J. Neurol. 2022, 29, 339–344. [Google Scholar] [CrossRef]
- Thiele, T.; Weisser, K.; Schönborn, L.; Funk, M.B.; Weber, G.; Greinacher, A.; Keller-Stanislawski, B. Laboratory confirmed vaccine-induced immune thrombotic thrombocytopenia: Retrospective analysis of reported cases after vaccination with ChAdOx-1 nCoV-19 in Germany. Lancet Reg. Health—Eur. 2022, 12, 100270. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, R.; Barary, M.; Mehdinezhad, H.; Sio, T.T.; Langer, F.; Khosravi, S. Thrombotic thrombocytopenia After Sinopharm BBIBP-CorV COVID-19 vaccination. Res. Pract. Thromb. Haemost. 2022, 6, e12750. [Google Scholar] [CrossRef]
- Roytenberg, R.; García-Sastre, A.; Li, W. Vaccine-induced immune thrombotic thrombocytopenia: What do we know hitherto? Front. Med. 2023, 10, 1155727. [Google Scholar] [CrossRef]
- Kim, A.Y.; Woo, W.; Yon, D.K.; Lee, S.W.; Yang, J.W.; Kim, J.H.; Park, S.; Koyanagi, A.; Kim, M.S.; Lee, S.; et al. Thrombosis patterns and clinical outcome of COVID-19 vaccine-induced immune thrombotic thrombocytopenia: A Systematic Review and Meta-Analysis. Int. J. Infect. Dis. 2022, 119, 130–139. [Google Scholar] [CrossRef]
- Abrignani, M.G.; Murrone, A.; De Luca, L.; Roncon, L.; Di Lenarda, A.; Valente, S.; Caldarola, P.; Riccio, C.; Oliva, F.; Gulizia, M.M.; et al. COVID-19, Vaccines, and Thrombotic Events: A Narrative Review. JCM 2022, 11, 948. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.A.C.; Cornblath, D.R. Guillain-Barré syndrome. Lancet 2005, 366, 1653–1666. [Google Scholar] [CrossRef]
- Van Den Berg, B.; Walgaard, C.; Drenthen, J.; Fokke, C.; Jacobs, B.C.; Van Doorn, P.A. Guillain–Barré syndrome: Pathogenesis, diagnosis, treatment and prognosis. Nat. Rev. Neurol. 2014, 10, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P. Guillain-Barré syndrome and COVID-19 vaccines: Focus on adenoviral vectors. Front. Immunol. 2023, 14, 1183258. [Google Scholar] [CrossRef] [PubMed]
- Patone, M.; Handunnetthi, L.; Saatci, D.; Pan, J.; Katikireddi, S.V.; Razvi, S.; Hunt, D.; Mei, X.W.; Dixon, S.; Zaccardi, F.; et al. Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection. Nat. Med. 2021, 27, 2144–2153. [Google Scholar] [CrossRef]
- Maramattom, B.V.; Krishnan, P.; Paul, R.; Padmanabhan, S.; Cherukudal Vishnu Nampoothiri, S.; Syed, A.A.; Mangat, H.S. Guillain-Barré Syndrome following ChAdOx1-S/nCoV-19 Vaccine. Ann. Neurol. 2021, 90, 312–314. [Google Scholar] [CrossRef]
- Osowicki, J.; Morgan, H.; Harris, A.; Crawford, N.W.; Buttery, J.P.; Kiers, L. Guillain-Barré Syndrome in an Australian State Using Both mRNA and Adenovirus-Vector SARS-CoV-2 Vaccines. Ann. Neurol. 2021, 90, 856–858. [Google Scholar] [CrossRef] [PubMed]
- Ogunjimi, O.B.; Tsalamandris, G.; Paladini, A.; Varrassi, G.; Zis, P. Guillain-Barré Syndrome Induced by Vaccination Against COVID-19: A Systematic Review and Meta-Analysis. Cureus 2023, 15, e37578. [Google Scholar] [CrossRef] [PubMed]
- Callaway, E. The next generation of coronavirus vaccines: A graphical guide. Nature 2023, 614, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Deng, Y.; Huang, B.; Han, D.; Wang, W.; Huang, M.; Zhai, C.; Zhao, Z.; Yang, R.; Zhao, Y.; et al. DNA Vaccines Expressing the Envelope and Membrane Proteins Provide Partial Protection Against SARS-CoV-2 in Mice. Front. Immunol. 2022, 13, 827605. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Kurtesi, A.; Budylowski, P.; Potts, K.G.; Menon, H.; Tan, Y.; Samaan, P.; Liu, X.; Wang, Y.; et al. A bivalent COVID-19 mRNA vaccine elicited broad immune responses and protection against Omicron subvariants infection. npj Vac. 2025, 10, 4. [Google Scholar] [CrossRef]
- Shrestha, L.B.; Tungatt, K.; Aggarwal, A.; Stubis, A.; Fewings, N.L.; Fichter, C.; Akerman, A.; Rodrigo, C.; Tedla, N.; Lee, S.; et al. Bivalent Omicron BA.1 vaccine booster increases memory B cell breadth and neutralising antibodies against emerging SARS-CoV-2 variants. eBioMedicine 2024, 110, 105461. [Google Scholar] [CrossRef]
- Ying, B.; Darling, T.L.; Desai, P.; Liang, C.-Y.; Dmitriev, I.P.; Soudani, N.; Bricker, T.; Kashentseva, E.A.; Harastani, H.; Raju, S.; et al. Mucosal vaccine-induced cross-reactive CD8+ T cells protect against SARS-CoV-2 XBB.1.5 respiratory tract infection. Nat. Immunol. 2024, 25, 537–551. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Shin, K.-S.; Park, B.; Park, S.; Shin, J.; Park, H.; Jung, I.K.; Kim, J.H.; Bae, S.E.; Kim, J.-O.; et al. Strategy to develop broadly effective multivalent COVID-19 vaccines against emerging variants based on Ad5/35 platform. Proc. Natl. Acad. Sci. USA 2024, 121, e2313681121. [Google Scholar] [CrossRef]
- Wang, R.; Huang, H.; Yu, C.; Sun, C.; Ma, J.; Kong, D.; Lin, Y.; Zhao, D.; Zhou, S.; Lu, J.; et al. A spike-trimer protein-based tetravalent COVID-19 vaccine elicits enhanced breadth of neutralization against SARS-CoV-2 Omicron subvariants and other variants. Sci. China. Life Sci. 2023, 66, 1818–1830. [Google Scholar] [CrossRef]
- Hannawi, S.; Yan, L.; Saf Eldin, L.; Abuquta, A.; Alamadi, A.; Mahmoud, S.A.; Hassan, A.; Zhang, M.; Gao, C.; Chen, Y.; et al. Safety and immunogenicity of multivalent SARS-CoV-2 protein vaccines: A randomized phase 3 trial. eClinicalMedicine 2023, 64, 102195. [Google Scholar] [CrossRef]
- Abbink, P.; Lemckert, A.A.C.; Ewald, B.A.; Lynch, D.M.; Denholtz, M.; Smits, S.; Holterman, L.; Damen, I.; Vogels, R.; Thorner, A.R.; et al. Comparative Seroprevalence and Immunogenicity of Six Rare Serotype Recombinant Adenovirus Vaccine Vectors from Subgroups B and D. J. Virol. 2007, 81, 4654–4663. [Google Scholar] [CrossRef] [PubMed]
- Seshidhar Reddy, P.; Ganesh, S.; Limbach, M.P.; Brann, T.; Pinkstaff, A.; Kaloss, M.; Kaleko, M.; Connelly, S. Development of adenovirus serotype 35 as a gene transfer vector. Virology 2003, 311, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Stone, D.; Ni, S.; Li, Z.-Y.; Gaggar, A.; DiPaolo, N.; Feng, Q.; Sandig, V.; Lieber, A. Development and assessment of human adenovirus type 11 as a gene transfer vector. J. Virol. 2005, 79, 5090–5104. [Google Scholar] [CrossRef]
- Baden, L.R.; Walsh, S.R.; Seaman, M.S.; Tucker, R.P.; Krause, K.H.; Patel, A.; Johnson, J.A.; Kleinjan, J.; Yanosick, K.E.; Perry, J.; et al. First-in-Human Evaluation of the Safety and Immunogenicity of a Recombinant Adenovirus Serotype 26 HIV-1 Env Vaccine (IPCAVD 001). J. Infect. Dis. 2013, 207, 240–247. [Google Scholar] [CrossRef]
- Keefer, M.C.; Gilmour, J.; Hayes, P.; Gill, D.; Kopycinski, J.; Cheeseman, H.; Cashin-Cox, M.; Naarding, M.; Clark, L.; Fernandez, N.; et al. A Phase I Double Blind, Placebo-Controlled, Randomized Study of a Multigenic HIV-1 Adenovirus Subtype 35 Vector Vaccine in Healthy Uninfected Adults. PLoS ONE 2012, 7, e41936. [Google Scholar] [CrossRef]
- Ouédraogo, A.; Tiono, A.B.; Kargougou, D.; Yaro, J.B.; Ouédraogo, E.; Kaboré, Y.; Kangoye, D.; Bougouma, E.C.; Gansane, A.; Henri, N.; et al. A Phase 1b Randomized, Controlled, Double-Blinded Dosage-Escalation Trial to Evaluate the Safety, Reactogenicity and Immunogenicity of an Adenovirus Type 35 Based Circumsporozoite Malaria Vaccine in Burkinabe Healthy Adults 18 to 45 Years of Age. PLoS ONE 2013, 8, e78679. [Google Scholar] [CrossRef]
- Tameris, M.; Hokey, D.A.; Nduba, V.; Sacarlal, J.; Laher, F.; Kiringa, G.; Gondo, K.; Lazarus, E.M.; Gray, G.E.; Nachman, S.; et al. A double-blind, randomised, placebo-controlled, dose-finding trial of the novel tuberculosis vaccine AERAS-402, an adenovirus-vectored fusion protein, in healthy, BCG-vaccinated infants. Vaccine 2015, 33, 2944–2954. [Google Scholar] [CrossRef]
- Matz, K.M.; Marzi, A.; Feldmann, H. Ebola vaccine trials: Progress in vaccine safety and immunogenicity. Expert. Rev. Vaccines 2019, 18, 1229–1242. [Google Scholar] [CrossRef] [PubMed]
- Alhashimi, M.; Elkashif, A.; Sayedahmed, E.E.; Mittal, S.K. Nonhuman Adenoviral Vector-Based Platforms and Their Utility in Designing Next Generation of Vaccines for Infectious Diseases. Viruses 2021, 13, 1493. [Google Scholar] [CrossRef]
- Xiang, Z.; Li, Y.; Cun, A.; Yang, W.; Ellenberg, S.; Switzer, W.M.; Kalish, M.L.; Ertl, H.C.J. Chimpanzee adenovirus antibodies in humans, sub-Saharan Africa. Emerg. Infect. Dis. 2006, 12, 1596–1599. [Google Scholar] [CrossRef]
- Fitzgerald, J.C.; Gao, G.-P.; Reyes-Sandoval, A.; Pavlakis, G.N.; Xiang, Z.Q.; Wlazlo, A.P.; Giles-Davis, W.; Wilson, J.M.; Ertl, H.C.J. A Simian Replication-Defective Adenoviral Recombinant Vaccine to HIV-1 Gag. J. Immunol. 2003, 170, 1416–1422. [Google Scholar] [CrossRef] [PubMed]
- Ledgerwood, J.E.; DeZure, A.D.; Stanley, D.A.; Coates, E.E.; Novik, L.; Enama, M.E.; Berkowitz, N.M.; Hu, Z.; Joshi, G.; Ploquin, A.; et al. Chimpanzee Adenovirus Vector Ebola Vaccine. N. Engl. J. Med. 2017, 376, 928–938. [Google Scholar] [CrossRef]
- Quinn, K.M.; Da Costa, A.; Yamamoto, A.; Berry, D.; Lindsay, R.W.B.; Darrah, P.A.; Wang, L.; Cheng, C.; Kong, W.-P.; Gall, J.G.D.; et al. Comparative Analysis of the Magnitude, Quality, Phenotype, and Protective Capacity of Simian Immunodeficiency Virus Gag-Specific CD8+ T Cells following Human-, Simian-, and Chimpanzee-Derived Recombinant Adenoviral Vector Immunization. J. Immunol. 2013, 190, 2720–2735. [Google Scholar] [CrossRef]
- Tapia, M.D.; Sow, S.O.; Lyke, K.E.; Haidara, F.C.; Diallo, F.; Doumbia, M.; Traore, A.; Coulibaly, F.; Kodio, M.; Onwuchekwa, U.; et al. Use of ChAd3-EBO-Z Ebola virus vaccine in Malian and US adults, and boosting of Malian adults with MVA-BN-Filo: A phase 1, single-blind, randomised trial, a phase 1b, open-label and double-blind, dose-escalation trial, and a nested, randomised, double-blind, placebo-controlled trial. Lancet Infect. Dis. 2016, 16, 31–42. [Google Scholar] [CrossRef]
- Rampling, T.; Ewer, K.J.; Bowyer, G.; Bliss, C.M.; Edwards, N.J.; Wright, D.; Payne, R.O.; Venkatraman, N.; De Barra, E.; Snudden, C.M.; et al. Safety and High Level Efficacy of the Combination Malaria Vaccine Regimen of RTS,S/AS01 B with Chimpanzee Adenovirus 63 and Modified Vaccinia Ankara Vectored Vaccines Expressing ME-TRAP. J. Infect. Dis. 2016, 214, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Capone, S.; Reyes-Sandoval, A.; Naddeo, M.; Siani, L.; Ammendola, V.; Rollier, C.S.; Nicosia, A.; Colloca, S.; Cortese, R.; Folgori, A.; et al. Immune responses against a liver-stage malaria antigen induced by simian adenoviral vector AdCh63 and MVA prime–boost immunisation in non-human primates. Vaccine 2010, 29, 256–265. [Google Scholar] [CrossRef]
- Barnes, E.; Folgori, A.; Capone, S.; Swadling, L.; Aston, S.; Kurioka, A.; Meyer, J.; Huddart, R.; Smith, K.; Townsend, R.; et al. Novel Adenovirus-Based Vaccines Induce Broad and Sustained T Cell Responses to HCV in Man. Sci. Transl. Med. 2012, 4, 115ra1. [Google Scholar] [CrossRef] [PubMed]
- Antrobus, R.D.; Coughlan, L.; Berthoud, T.K.; Dicks, M.D.; Hill, A.V.; Lambe, T.; Gilbert, S.C. Clinical Assessment of a Novel Recombinant Simian Adenovirus ChAdOx1 as a Vectored Vaccine Expressing Conserved Influenza A Antigens. Mol. Ther. 2014, 22, 668–674. [Google Scholar] [CrossRef]
- Von Delft, A.; Donnison, T.A.; Lourenço, J.; Hutchings, C.; Mullarkey, C.E.; Brown, A.; Pybus, O.G.; Klenerman, P.; Chinnakannan, S.; Barnes, E. The generation of a simian adenoviral vectored HCV vaccine encoding genetically conserved gene segments to target multiple HCV genotypes. Vaccine 2018, 36, 313–321. [Google Scholar] [CrossRef]
- Wilkie, M.; Satti, I.; Minhinnick, A.; Harris, S.; Riste, M.; Ramon, R.L.; Sheehan, S.; Thomas, Z.-R.M.; Wright, D.; Stockdale, L.; et al. A phase I trial evaluating the safety and immunogenicity of a candidate tuberculosis vaccination regimen, ChAdOx1 85A prime–MVA85A boost in healthy UK adults. Vaccine 2020, 38, 779–789. [Google Scholar] [CrossRef]
- Tandon, M.; Sharma, A.; Vemula, S.V.; Bangari, D.S.; Mittal, S.K. Sequential administration of bovine and human adenovirus vectors to overcome vector immunity in an immunocompetent mouse model of breast cancer. Virus Res. 2012, 163, 202–211. [Google Scholar] [CrossRef]
- Moffatt, S.; Hays, J.; HogenEsch, H.; Mittal, S.K. Circumvention of vector-specific neutralizing antibody response by alternating use of human and non-human adenoviruses: Implications in gene therapy. Virology 2000, 272, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Bangari, D.S.; Sharma, A.; Mittal, S.K. Bovine adenovirus type 3 internalization is independent of primary receptors of human adenovirus type 5 and porcine adenovirus type 3. Biochem. Biophys. Res. Commun. 2005, 331, 1478–1484. [Google Scholar] [CrossRef]
- Sayedahmed, E.E.; Hassan, A.O.; Kumari, R.; Cao, W.; Gangappa, S.; York, I.; Sambhara, S.; Mittal, S.K. A Bovine Adenoviral Vector-Based H5N1 Influenza -Vaccine Provides Enhanced Immunogenicity and Protection at a Significantly Low Dose. Mol. Ther. Methods Clin. Dev. 2018, 10, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Bouet-Cararo, C.; Contreras, V.; Fournier, A.; Jallet, C.; Guibert, J.M.; Dubois, E.; Thiery, R.; Bréard, E.; Tordo, N.; Richardson, J.; et al. Canine adenoviruses elicit both humoral and cell-mediated immune responses against rabies following immunisation of sheep. Vaccine 2011, 29, 1304–1310. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.L.; Liu, Y.; Zhang, S.F.; Zhang, F.; Fooks, A.R. Experimental immunization of cats with a recombinant rabies-canine adenovirus vaccine elicits a long-lasting neutralizing antibody response against rabies. Vaccine 2007, 25, 5301–5307. [Google Scholar] [CrossRef]
- Xiang, Z.Q.; Gao, G.P.; Reyes-Sandoval, A.; Li, Y.; Wilson, J.M.; Ertl, H.C.J. Oral Vaccination of Mice with Adenoviral Vectors Is Not Impaired by Preexisting Immunity to the Vaccine Carrier. J. Virol. 2003, 77, 10780–10789. [Google Scholar] [CrossRef]
- Pandey, A.; Singh, N.; Vemula, S.V.; Couëtil, L.; Katz, J.M.; Donis, R.; Sambhara, S.; Mittal, S.K. Impact of Preexisting Adenovirus Vector Immunity on Immunogenicity and Protection Conferred with an Adenovirus-Based H5N1 Influenza Vaccine. PLoS ONE 2012, 7, e33428. [Google Scholar] [CrossRef]
- Xing, M.; Wang, Y.; Wang, X.; Liu, J.; Dai, W.; Hu, G.; He, F.; Zhao, Q.; Li, Y.; Sun, L.; et al. Broad-spectrum vaccine via combined immunization routes triggers potent immunity to SARS-CoV-2 and its variants. J. Virol. 2023, 97, e00723–e00724. [Google Scholar] [CrossRef]
- Barros-Martins, J.; Hammerschmidt, S.I.; Cossmann, A.; Odak, I.; Stankov, M.V.; Morillas Ramos, G.; Dopfer-Jablonka, A.; Heidemann, A.; Ritter, C.; Friedrichsen, M.; et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat. Med. 2021, 27, 1525–1529. [Google Scholar] [CrossRef]
- Schmidt, T.; Klemis, V.; Schub, D.; Mihm, J.; Hielscher, F.; Marx, S.; Abu-Omar, A.; Ziegler, L.; Guckelmus, C.; Urschel, R.; et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat. Med. 2021, 27, 1530–1535. [Google Scholar] [CrossRef] [PubMed]
- Kant, R.; Dwivedi, G.; Zaman, K.; Sahay, R.R.; Sapkal, G.; Kaushal, H.; Nyayanit, D.A.; Yadav, P.D.; Deshpande, G.; Singh, R.; et al. Serendipitous COVID-19 Vaccine-Mix in Uttar Pradesh, India: Safety and Immunogenicity Assessment of a Heterologous Regime. medRxiv Prepr. Serv. Health Sci. 2021. [Google Scholar] [CrossRef]
- Santosuosso, M.; McCormick, S.; Xing, Z. Adenoviral Vectors for Mucosal Vaccination Against Infectious Diseases. Viral Immunol. 2005, 18, 283–291. [Google Scholar] [CrossRef]
- Freitag, T.L.; Fagerlund, R.; Karam, N.L.; Leppänen, V.-M.; Ugurlu, H.; Kant, R.; Mäkinen, P.; Tawfek, A.; Jha, S.K.; Strandin, T.; et al. Intranasal administration of adenoviral vaccines expressing SARS-CoV-2 spike protein improves vaccine immunity in mouse models. Vaccine 2023, 41, 3233–3246. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.E.; Ku, K.B.; Kang, B.H.; Park, J.H.; Kim, H.C.; Kim, K.-D.; Lee, H.K. Intranasal delivery of an adenovirus-vector vaccine co-expressing a modified spike protein and a genetic adjuvant confers lasting mucosal immunity against SARS-CoV-2. Antivir. Res. 2023, 216, 105656. [Google Scholar] [CrossRef]
- Hassan, A.O.; Kafai, N.M.; Dmitriev, I.P.; Fox, J.M.; Smith, B.K.; Harvey, I.B.; Chen, R.E.; Winkler, E.S.; Wessel, A.W.; Case, J.B.; et al. A Single-Dose Intranasal ChAd Vaccine Protects Upper and Lower Respiratory Tracts against SARS-CoV-2. Cell 2020, 183, 169–184.e13. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.M.; Nanda, A.; Havenga, M.J.E.; Abbink, P.; Lynch, D.M.; Ewald, B.A.; Liu, J.; Thorner, A.R.; Swanson, P.E.; Gorgone, D.A.; et al. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature 2006, 441, 239–243. [Google Scholar] [CrossRef]
- Ma, J.; Duffy, M.R.; Deng, L.; Dakin, R.S.; Uil, T.; Custers, J.; Kelly, S.M.; McVey, J.H.; Nicklin, S.A.; Baker, A.H. Manipulating Adenovirus Hexon Hypervariable Loops Dictates Immune Neutralisation and Coagulation Factor X-dependent Cell Interaction In Vitro and In Vivo. PLoS Path. 2015, 11, e1004673. [Google Scholar] [CrossRef]
- Särkioja, M.; Pesonen, S.; Raki, M.; Hakkarainen, T.; Salo, J.; Ahonen, M.T.; Kanerva, A.; Hemminki, A. Changing the adenovirus fiber for retaining gene delivery efficacy in the presence of neutralizing antibodies. Gene Ther. 2008, 15, 921–929. [Google Scholar] [CrossRef]
- Rogée, S.; Grellier, E.; Bernard, C.; Jouy, N.; Loyens, A.; Beauvillain, J.C.; Fender, P.; Corjon, S.; Hong, S.S.; Boulanger, P.; et al. Influence of chimeric human-bovine fibers on adenoviral uptake by liver cells and the antiviral immune response. Gene Ther. 2010, 17, 880–891. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Anguiano-Zarate, S.S.; Matchett, W.E.; Barry, M.E.; Barry, M.A. Retargeted and detargeted adenovirus for gene delivery to the muscle. Virology 2018, 514, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Nicklin, S.A.; Von Seggern, D.J.; Work, L.M.; Pek, D.C.K.; Dominiczak, A.F.; Nemerow, G.R.; Baker, A.H. Ablating Adenovirus Type 5 Fiber–CAR Binding and HI Loop Insertion of the SIGYPLP Peptide Generate an Endothelial Cell-Selective Adenovirus. Mol. Ther. 2001, 4, 534–542. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, C.R.; Lachapelle, A.; Delgado, C.; Parkes, V.; Wadsworth, S.C.; Smith, A.E.; Francis, G.E. PEGylation of Adenovirus with Retention of Infectivity and Protection from Neutralizing Antibody in Vitro and in Vivo. Hum. Gene Ther. 1999, 10, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Croyle, M.A.; Le, H.T.; Linse, K.D.; Cerullo, V.; Toietta, G.; Beaudet, A.; Pastore, L. PEGylated helper-dependent adenoviral vectors: Highly efficient vectors with an enhanced safety profile. Gene Ther. 2005, 12, 579–587. [Google Scholar] [CrossRef]
- Dicks, M.D.J.; Rose, L.M.; Russell, R.A.; Bowman, L.A.H.; Graham, C.; Jimenez-Guardeño, J.M.; Doores, K.J.; Malim, M.H.; Draper, S.J.; Howarth, M.; et al. Modular capsid decoration boosts adenovirus vaccine-induced humoral immunity against SARS-CoV-2. Mol. Ther. 2022, 30, 3639–3657. [Google Scholar] [CrossRef]
- Luo, S.; Zhang, P.; Zou, P.; Wang, C.; Liu, B.; Wu, C.; Li, T.; Zhang, L.; Zhang, Y.; Li, C. A Self-Biomineralized Novel Adenovirus Vectored COVID-19 Vaccine for Boosting Immunization of Mice. Virol. Sin. 2021, 36, 1113–1123. [Google Scholar] [CrossRef]
- Mendez, N.; Herrera, V.; Zhang, L.; Hedjran, F.; Feuer, R.; Blair, S.L.; Trogler, W.C.; Reid, T.R.; Kummel, A.C. Encapsulation of adenovirus serotype 5 in anionic lecithin liposomes using a bead-based immunoprecipitation technique enhances transfection efficiency. Biomaterials 2014, 35, 9554–9561. [Google Scholar] [CrossRef]
- Yotnda, P.; Chen, D.-H.; Chiu, W.; Piedra, P.A.; Davis, A.; Templeton, N.S.; Brenner, M.K. Bilamellar Cationic Liposomes Protect Adenovectors from Preexisting Humoral Immune Responses. Mol. Ther. 2002, 5, 233–241. [Google Scholar] [CrossRef]
- Vupputuri, S.; Tayebi, L.; Hikkaduwa Koralege, R.S.; Nigatu, A.; Mozafari, M.; Mishra, A.; Liu, L.; Ramsey, J.D. Polyethylene glycol–modified DOTAP:cholesterol/adenovirus hybrid vectors have improved transduction efficiency and reduced immunogenicity. J. Nanopart. Res. 2021, 23, 37. [Google Scholar] [CrossRef]
- Gonzalez-Pastor, R.; Hernandez, Y.; Gimeno, M.; De Martino, A.; Man, Y.K.S.; Hallden, G.; Quintanilla, M.; De La Fuente, J.M.; Martin-Duque, P. Coating an adenovirus with functionalized gold nanoparticles favors uptake, intracellular trafficking and anti-cancer therapeutic efficacy. Acta Biomater. 2021, 134, 593–604. [Google Scholar] [CrossRef]
- Khalil, I.R.; Khechara, M.P.; Kurusamy, S.; Armesilla, A.L.; Gupta, A.; Mendrek, B.; Khalaf, T.; Scandola, M.; Focarete, M.L.; Kowalczuk, M.; et al. Poly-Gamma-Glutamic Acid (γ-PGA)-Based Encapsulation of Adenovirus to Evade Neutralizing Antibodies. Molecules 2018, 23, 2565. [Google Scholar] [CrossRef] [PubMed]
- Lameiro, M.H.; Malpique, R.; Silva, A.C.; Alves, P.M.; Melo, E. Encapsulation of adenoviral vectors into chitosan–bile salt microparticles for mucosal vaccination. J. Biotechnol. 2006, 126, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Sailaja, G.; HogenEsch, H.; North, A.; Hays, J.; Mittal, S.K. Encapsulation of recombinant adenovirus into alginate microspheres circumvents vector-specific immune response. Gene Ther. 2002, 9, 1722–1729. [Google Scholar] [CrossRef]
- Pereboev, A.V.; Nagle, J.M.; Shakhmatov, M.A.; Triozzi, P.L.; Matthews, Q.L.; Kawakami, Y.; Curiel, D.T.; Blackwell, J.L. Enhanced Gene Transfer to Mouse Dendritic Cells Using Adenoviral Vectors Coated with a Novel Adapter Molecule. Mol. Ther. 2004, 9, 712–720. [Google Scholar] [CrossRef]
- Khan, A.; Sayedahmed, E.E.; Singh, V.K.; Mishra, A.; Dorta-Estremera, S.; Nookala, S.; Canaday, D.H.; Chen, M.; Wang, J.; Sastry, K.J.; et al. A recombinant bovine adenoviral mucosal vaccine expressing mycobacterial antigen-85B generates robust protection against tuberculosis in mice. Cell Rep. Med. 2021, 2, 100372. [Google Scholar] [CrossRef]
- Sayedahmed, E.E.; Araújo, M.V.; Silva-Pereira, T.T.; Chothe, S.K.; Elkashif, A.; Alhashimi, M.; Wang, W.-C.; Santos, A.P.; Nair, M.S.; Gontu, A.; et al. Impact of an autophagy-inducing peptide on immunogenicity and protection efficacy of an adenovirus-vectored SARS-CoV-2 vaccine. Mol. Ther. Methods Clin. Dev. 2023, 30, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Sayedahmed, E.E.; Elshafie, N.O.; Dos Santos, A.P.; Jagannath, C.; Sambhara, S.; Mittal, S.K. Development of NP-Based Universal Vaccine for Influenza A Viruses. Vaccines 2024, 12, 157. [Google Scholar] [CrossRef]
- Hegde, R.S.; Bernstein, H.D. The surprising complexity of signal sequences. Trends Biochem. Sci. 2006, 31, 563–571. [Google Scholar] [CrossRef]
- Fonseca, J.A.; McCaffery, J.N.; Caceres, J.; Kashentseva, E.; Singh, B.; Dmitriev, I.P.; Curiel, D.T.; Moreno, A. Inclusion of the murine IgGκ signal peptide increases the cellular immunogenicity of a simian adenoviral vectored Plasmodium vivax multistage vaccine. Vaccine 2018, 36, 2799–2808. [Google Scholar] [CrossRef]
- Rollier, C.S.; Spencer, A.J.; Sogaard, K.C.; Honeycutt, J.; Furze, J.; Bregu, M.; Gilbert, S.C.; Wyllie, D.; Hill, A.V.S. Modification of Adenovirus vaccine vector-induced immune responses by expression of a signalling molecule. Sci. Rep. 2020, 10, 5716. [Google Scholar] [CrossRef]
- Szvetnik, A.; Tubak, V. The Production of Complement Inhibitor Proteins in Mammalian Cell Lines—Light at the End of the Tunnel? Biomedicines 2024, 12, 646. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Saribas, A.S.; Liu, J.; Lin, Y.; Bodnar, B.; Zhao, R.; Guo, Q.; Ting, J.; Wei, Z.; Ellis, A.; et al. Protein expression/secretion boost by a novel unique 21-mer cis-regulatory motif (Exin21) via mRNA stabilization. Mol. Ther. 2023, 31, 1136–1158. [Google Scholar] [CrossRef] [PubMed]
- Rethi-Nagy, Z.; Abraham, E.; Udvardy, K.; Klement, E.; Darula, Z.; Pal, M.; Katona, R.L.; Tubak, V.; Pali, T.; Kota, Z.; et al. STABILON, a Novel Sequence Motif That Enhances the Expression and Accumulation of Intracellular and Secreted Proteins. Int. J. Mol. Sci. 2022, 23, 8168. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murala, M.S.T.; Gairola, V.; Sayedahmed, E.E.; Mittal, S.K. Next-Generation Adenoviral Vector-Based Vaccines for Severe Acute Respiratory Syndrome Coronavirus-2. Vaccines 2025, 13, 406. https://doi.org/10.3390/vaccines13040406
Murala MST, Gairola V, Sayedahmed EE, Mittal SK. Next-Generation Adenoviral Vector-Based Vaccines for Severe Acute Respiratory Syndrome Coronavirus-2. Vaccines. 2025; 13(4):406. https://doi.org/10.3390/vaccines13040406
Chicago/Turabian StyleMurala, Muralimanohara S. T., Vivek Gairola, Ekramy E. Sayedahmed, and Suresh K. Mittal. 2025. "Next-Generation Adenoviral Vector-Based Vaccines for Severe Acute Respiratory Syndrome Coronavirus-2" Vaccines 13, no. 4: 406. https://doi.org/10.3390/vaccines13040406
APA StyleMurala, M. S. T., Gairola, V., Sayedahmed, E. E., & Mittal, S. K. (2025). Next-Generation Adenoviral Vector-Based Vaccines for Severe Acute Respiratory Syndrome Coronavirus-2. Vaccines, 13(4), 406. https://doi.org/10.3390/vaccines13040406








