Prevention of Cardiovascular Diseases with Standard-Dose Quadrivalent Influenza Vaccine in People Aged ≥50 Years in Australia During the 2017 A/H3N2 Epidemic
Abstract
:1. Introduction
2. Methods
2.1. Study Setting and Design
2.2. Data Collection and Linkage
2.3. Data Transfer and Security
2.4. Exposure and Outcome Measures
2.5. Data Analysis
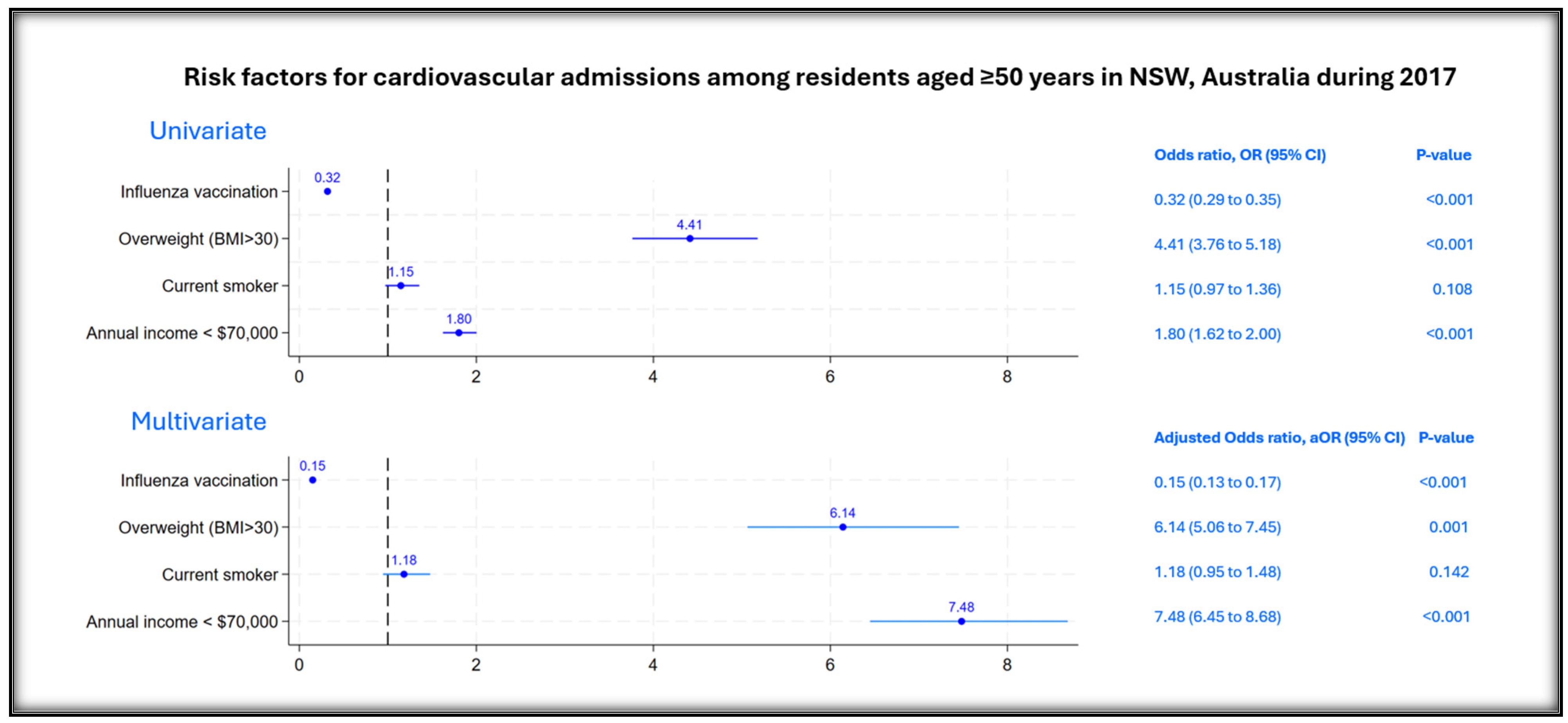
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Group, S.W. Background Paper on Influenza Vaccines and Immunization; World Health Organization: Geneva, Switzerland, 2012; p. 463. [Google Scholar]
- Davidson, J.A.; Banerjee, A.; Smeeth, L.; McDonald, H.I.; Grint, D.; Herrett, E.; Forbes, H.; Pebody, R.; Warren-Gash, C. Risk of acute respiratory infection and acute cardiovascular events following acute respiratory infection among adults with increased cardiovascular risk in England between 2008 and 2018: A retrospective, population-based cohort study. Lancet Digit. Health 2021, 3, e773–e783. [Google Scholar] [CrossRef] [PubMed]
- Bin Seo, Y.; Suk Choi, W.; Hyeon Baek, J.; Lee, J.; Young Song, J.; Soo Lee, J.; Cheong, H.J.; Kim, W.J. Effectiveness of the influenza vaccine at preventing hospitalization due to acute exacerbation of cardiopulmonary disease in Korea from 2011 to 2012. Hum. Vaccines Immunother. 2014, 10, 423–427. [Google Scholar] [CrossRef]
- Barnes, M.; Heywood, A.E.; Mahimbo, A.; Rahman, B.; Newall, A.T.; Macintyre, C.R. Acute myocardial infarction and influenza: A meta-analysis of case–control studies. Heart 2015, 101, 1738–1747. [Google Scholar] [CrossRef] [PubMed]
- Kwong, J.C.; Schwartz, K.L.; Campitelli, M.A.; Chung, H.; Crowcroft, N.S.; Karnauchow, T.; Katz, K.; Ko, D.T.; McGeer, A.J.; McNally, D.; et al. Acute Myocardial Infarction after Laboratory-Confirmed Influenza Infection. N. Engl. J. Med. 2018, 378, 345–353. [Google Scholar] [CrossRef]
- Song, J.Y.; Noh, J.Y.; Lee, J.S.; Wie, S.-H.; Kim, Y.K.; Lee, J.; Jeong, H.W.; Kim, S.W.; Lee, S.H.; Park, K.-H.; et al. Effectiveness of influenza and pneumococcal polysaccharide vaccines against influenza-related outcomes including pneumonia and acute exacerbation of cardiopulmonary diseases: Analysis by dominant viral subtype and vaccine matching. PLoS ONE 2018, 13, e0207918. [Google Scholar] [CrossRef]
- Kytömaa, S.; Hegde, S.; Claggett, B.; Udell, J.A.; Rosamond, W.; Temte, J.; Nichol, K.; Wright, J.D.; Solomon, S.D.; Vardeny, O. Association of Influenza-like Illness Activity With Hospitalizations for Heart Failure: The Atherosclerosis Risk in Communities Study. JAMA Cardiol. 2019, 4, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Kinlay, S.; Ganz, P. Role of endothelial dysfunction in coronary artery disease and implications for therapy. Am. J. Cardiol. 1997, 80, 11I–16I. [Google Scholar] [CrossRef]
- Chan, N.N.; Colhoun, H.M.; Vallance, P. Cardiovascular risk factors as determinants of endothelium-dependent and endothelium-independent vascular reactivity in the general population. J. Am. Coll. Cardiol. 2001, 38, 1814–1820. [Google Scholar] [CrossRef]
- Davies, M. The composition of coronary-artery plaques. N. Engl. J. Med. 1997, 336, 1312–1314. [Google Scholar] [CrossRef]
- Zhou, Y.; Wanishswad, C.; Epstein, S. Chlamydia pneumoniae-induced transactivation of cytomegalovirus: Potential synergy of infectious agents in the pathogenesis of atherosclerosis. J. Am. Coll. Cardiol. 1999, 33 (Suppl. A), 260A. [Google Scholar]
- Richard Conti, C. Vascular events responsible for thrombotic occlusion of a blood vessel. Clin. Cardiol. 1993, 16, 761–763. [Google Scholar] [CrossRef] [PubMed]
- Lundman, P.; Eriksson, M.; Schenck-Gustafsson, K.; Karpe, F.; Tornvall, P. Transient triglyceridemia decreases vascular reactivity in young, healthy men without risk factors for coronary heart disease. Circulation 1997, 96, 3266–3268. [Google Scholar] [CrossRef] [PubMed]
- Kawano, H.; Motoyama, T.; Hirashima, O.; Hirai, N.; Miyao, Y.; Sakamoto, T.; Miura, K.; Mine, T.; Tanaka, Y.; Mitsumata, M.; et al. Hyperglycemia rapidly suppresses flow-mediated endothelium-dependent vasodilation of brachial artery. J. Am. Coll. Cardiol. 1999, 34, 146–154. [Google Scholar] [CrossRef]
- Ang, L.W.; Yap, J.; Lee, V.; Chng, W.Q.; Jaufeerally, F.R.; Lam, C.S.P.; Cutter, J.; Yeo, K.K.; Ma, S. Influenza-Associated Hospitalizations for Cardiovascular Diseases in the Tropics. Am. J. Epidemiol. 2017, 186, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.J.; Rolfes, M.A.; O’Halloran, A.; Anderson, E.J.; Bennett, N.M.; Billing, L.; Chai, S.; Dufort, E.; Herlihy, R.; Kim, S.; et al. Acute cardiovascular events associated with influenza in hospitalized adults: A cross-sectional study. Ann. Intern. Med. 2020, 173, 605–613. [Google Scholar] [CrossRef]
- Moa, A.M.; Menzies, R.I.; Yin, J.K.; MacIntyre, C.R. Modelling the influenza disease burden in people aged 50–64 and ≥65 years in Australia. Influenza Other Respir. Viruses 2022, 16, 132–141. [Google Scholar] [CrossRef]
- Sullivan, S.G.; Chilver, M.B.; Carville, K.S.; Deng, Y.-M.; Grant, K.A.; Higgins, G.; Komadina, N.; Leung, V.K.; Minney-Smith, C.A.; Teng, D.; et al. Low interim influenza vaccine effectiveness, Australia, 1 May to 24 September 2017. Eurosurveillance 2017, 22, 17-00707. [Google Scholar] [CrossRef]
- Grant, K.; Carville, K.S.; Sullivan, S.G.; Strachan, J.; Druce, J.; Fielding, J.E. A severe 2017 influenza season dominated by influenza A (H3N2), Victoria, Australia. West. Pac. Surveill. Response J. WPSAR 2018, 9, 18. [Google Scholar] [CrossRef]
- Department of Health, Australia Government. Influenza Season in Australia, A Summary from the National Influenza Surveillance Committee 2017. 2017. Available online: https://web.archive.org.au/awa/20190510054958mp_/http://www.health.gov.au/internet/main/publishing.nsf/Content/097F15A91C05FBE7CA2581E20017F09E/$File/2017-season-summary-22112017.pdf (accessed on 3 April 2025).
- Kelly, H.; Grant, K. Interim analysis of pandemic influenza (H1N1) 2009 in Australia: Surveillance trends, age of infection and effectiveness of seasonal vaccination. Eurosurveillance 2009, 14, 19288. [Google Scholar] [CrossRef]
- Nolte, K.B.; Alakija, P.; Oty, G.; Shaw, M.W.; Subbarao, K.; Guarner, J.; Shieh, W.-J.; Dawson, J.E.; Morken, T.; Cox, N.J.; et al. Influenza A Virus Infection Complicated by Fatal Myocarditis. Am. J. Forensic Med. Pathol. 2000, 21. [Google Scholar] [CrossRef]
- Hollowed, J.; Nsair, A. Influenza A (H3N2) Induced Fulminant Myocarditis Requiring Mechanical Circulatory Support. JACC Case Rep. 2019, 1, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Phrommintikul, A.; Kuanprasert, S.; Wongcharoen, W.; Kanjanavanit, R.; Chaiwarith, R.; Sukonthasarn, A. Influenza vaccination reduces cardiovascular events in patients with acute coronary syndrome. Eur. Heart J. 2011, 32, 1730–1735. [Google Scholar] [CrossRef]
- Udell, J.A.; Zawi, R.; Bhatt, D.L.; Keshtkar-Jahromi, M.; Gaughran, F.; Phrommintikul, A.; Ciszewski, A.; Vakili, H.; Hoffman, E.B.; Farkouh, M.E.; et al. Association Between Influenza Vaccination and Cardiovascular Outcomes in High-Risk Patients: A Meta-analysis. JAMA 2013, 310, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Clar, C.; Oseni, Z.; Flowers, N.; Keshtkar-Jahromi, M.; Rees, K. Influenza vaccines for preventing cardiovascular disease. Cochrane Database Syst. Rev. 2015, 2015, CD005050. [Google Scholar] [PubMed]
- MacIntyre, C.R.; Mahimbo, A.; Moa, A.M.; Barnes, M. Influenza vaccine as a coronary intervention for prevention of myocardial infarction. Heart 2016, 102, 1953–1956. [Google Scholar] [CrossRef]
- Fröbert, O.; Götberg, M.; Erlinge, D.; Akhtar, Z.; Christiansen, E.H.; MacIntyre, C.R.; Oldroyd, K.G.; Motovska, Z.; Erglis, A.; Moer, R.; et al. Influenza Vaccination After Myocardial Infarction: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. Circulation 2021, 144, 1476–1484. [Google Scholar] [CrossRef]
- Valeri, M.; Durrani, S.; Tran, C.; Chiu, C.; Macartney, K.K.; Giles, M.L.; Crawford, N.W. ATAGI 2023 Annual Statement on Immunisation. Commun. Dis. Intell. 2023, 47. [Google Scholar] [CrossRef]
- Department of Health and Aged Care, Australia Government. ATAGI Statement on the Administration of Seasonal Influenza Vaccines in 2024. 2024. Available online: https://www.health.gov.au/sites/default/files/2024-02/atagi-statement-on-the-administration-of-seasonal-influenza-vaccines-in-2024.pdf (accessed on 1 April 2025).
- Department of Health, Australia Government. Statement on the Transition from Quadrivalent to Trivalent Seasonal Influenza Vaccines in Australia 2024. Available online: https://www.health.gov.au/sites/default/files/2024-08/atagi-statement-on-the-transition-from-quadrivalent-to-trivalent-seasonal-influenza-vaccines-in-australia.pdf (accessed on 1 April 2025).
- World Health Organization. Recommended Composition of Influenza Virus Vaccines for Use in the 2017 Southern Hemisphere Influenza Season [Internet]; World Health Organization: Geneva, Switzerland, 2016; Available online: https://cdn.who.int/media/docs/default-source/influenza/who-influenza-recommendations/vcm-southern-hemisphere-recommendation-2017/201609_recommendation.pdf (accessed on 10 April 2025).
- Liang, Y.; Jing-Xia, G.; Ma, L.; Ni, L.; Chaolie, R.; Zhou, J.; Guo-Yang, L. Immunogenicity and safety levels of inactivated quadrivalent influenza vaccine in healthy adults via meta-analysis. Hum. Vaccines Immunother. 2021, 17, 3652–3661. [Google Scholar] [CrossRef]
- WHO. Recommended Composition of Influenza Virus Vaccines for Use in the 2024–2025 Northern Hemisphere Influenza Season 2024. Available online: https://www.who.int/publications/m/item/recommended-composition-of-influenza-virus-vaccines-for-use-in-the-2024-2025-northern-hemisphere-influenza-season (accessed on 1 April 2025).
- Department of Health, Australia Government. Statement on the Administration of Seasonal Influenza Vaccines in 2018. 2018. Available online: https://www.health.gov.au/sites/default/files/atagi_clinical_advice_factsheet_-_2018_influenza_statement.pdf (accessed on 1 April 2025).
- Statistics, A.D.; Force, L.; Australia, D.; Done, C.W. Australian Bureau of Statistics. Population 2023, 26, 30. [Google Scholar]
- 45 and Up Study Collaborators. Cohort Profile: The 45 and Up Study. Int. J. Epidemiol. 2008, 37, 941–947. [Google Scholar] [CrossRef]
- Lawrence, G.; Dinh, I.; Taylor, L. The Centre for Health Record Linkage: A new resource for health services research and evaluation. Health Inf. Manag. J. 2008, 37, 60–62. [Google Scholar] [CrossRef]
- Irvine, K.A.; Moore, E.A. Linkage of routinely collected data in practice: The Centre for Health Record Linkage. Public Health Res. Pract. 2015, 25, e2541548. [Google Scholar] [CrossRef] [PubMed]
- Moore, H.C.; Guiver, T.; Woollacott, A.; De Klerk, N.; Gidding, H.F. Establishing a process for conducting cross-jurisdictional record linkage in Australia. Aust. N. Z. J. Public health 2016, 40, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Iwagami, M.; Shinozaki, T. Introduction to matching in case-control and cohort studies. Ann. Clin. Epidemiol. 2022, 4, 33–40. [Google Scholar] [CrossRef] [PubMed]
- VanderWeele, T.J.; Ding, P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann. Intern. Med. 2017, 167, 268–274. [Google Scholar] [CrossRef]
- Ding, P.; VanderWeele, T.J. Sensitivity analysis without assumptions. Epidemiology 2016, 27, 368–377. [Google Scholar] [CrossRef]
- Hull, B.; Hendry, A.; Dey, A.; Macartney, K.; McIntyre, P.; Beard, F. Exploratory Analysis of the First 2 Years of Adult Vaccination Data Recorded on AIR; National Centre for Immunisation Research and Surveillance: Sydney, NSW, Australia, 2019. [Google Scholar]
- Puig-Barberà, J.; Díez-Domingo, J.; Varea, Á.B.; Chavarri, G.S.; Rodrigo, J.A.L.; Hoyos, S.P.; Vidal, D.G. Effectiveness of MF59™-adjuvanted subunit influenza vaccine in preventing hospitalisations for cardiovascular disease, cerebrovascular disease and pneumonia in the elderly. Vaccine 2007, 25, 7313–7321. [Google Scholar] [CrossRef]
- Tippett, A.; Ess, G.; Hussaini, L.; Reese, O.; Salazar, L.; Kelly, M.; Taylor, M.; Ciric, C.; Keane, A.; Cheng, A.; et al. Influenza Vaccine Effectiveness Pre-pandemic Among Adults Hospitalized With Congestive Heart Failure or Chronic Obstructive Pulmonary Disease and Older Adults. Clin. Infect. Diseases 2023, 78, 1065–1072. [Google Scholar] [CrossRef]
- Skowronski, D.M.; Chambers, C.; Sabaiduc, S.; De Serres, G.; Winter, A.-L.; Dickinson, J.A.; Krajden, M.; Gubbay, J.B.; Drews, S.J.; Martineau, C.; et al. A perfect storm: Impact of genomic variation and serial vaccination on low influenza vaccine effectiveness during the 2014–2015 season. Clin. Infect. Diseases 2016, 63, 21–32. [Google Scholar] [CrossRef]
- Flannery, B.; Kondor, R.J.G.; Chung, J.R.; Gaglani, M.; Reis, M.; Zimmerman, R.K.; Nowalk, M.P.; Jackson, M.L.; A Jackson, L.; Monto, A.S.; et al. Spread of antigenically drifted influenza A (H3N2) viruses and vaccine effectiveness in the United States during the 2018–2019 season. J. Infect. Diseases 2020, 221, 8–15. [Google Scholar] [CrossRef]
- Gostic, K.M.; Bridge, R.; Brady, S.; Viboud, C.; Worobey, M.; Lloyd-Smith, J.O. Childhood immune imprinting to influenza A shapes birth year-specific risk during seasonal H1N1 and H3N2 epidemics. PLoS Pathog. 2019, 15, e1008109. [Google Scholar] [CrossRef] [PubMed]
- McLean, H.Q.; Belongia, E.A. Influenza vaccine effectiveness: New insights and challenges. Cold Spring Harb. Perspect. Med. 2021, 11, a038315. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, Z.; Götberg, M.; Erlinge, D.; Christiansen, E.H.; Oldroyd, K.G.; Motovska, Z.; Erglis, A.; Hlinomaz, O.; Jakobsen, L.; Engstrøm, T.; et al. Optimal timing of influenza vaccination among patients with acute myocardial infarction–Findings from the IAMI trial. Vaccine 2023, 41, 7159–7165. [Google Scholar] [CrossRef]
- Wu, N.; Joyal-Desmarais, K.; Ribeiro, P.A.B.; Vieira, A.M.; Stojanovic, J.; Sanuade, C.; Yip, D.; Bacon, S.L. Long-term effectiveness of COVID-19 vaccines against infections, hospitalisations, and mortality in adults: Findings from a rapid living systematic evidence synthesis and meta-analysis up to December, 2022. Lancet Respir. Med. 2023, 11, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Antunes, L.; Mazagatos, C.; Martínez-Baz, I.; Naesens, R.; Borg, M.-L.; Petrović, G.; Fatukasi, T.; Jancoriene, L.; Machado, A.; Oroszi, B.; et al. Early COVID-19 XBB.1.5 Vaccine Effectiveness Against Hospitalisation Among Adults Targeted for Vaccination, VEBIS Hospital Network, Europe, October 2023–January 2024. Influenza Other Respir. Viruses 2024, 18, e13360. [Google Scholar] [CrossRef]
- Sukik, L.; Chemaitelly, H.; Ayoub, H.H.; Coyle, P.; Tang, P.; Yassine, H.M.; Al Thani, A.A.; Hasan, M.R.; Al-Kanaani, Z.; Al-Kuwari, E.; et al. Effectiveness of two and three doses of COVID-19 mRNA vaccines against infection, symptoms, and severity in the pre-omicron era: A time-dependent gradient. Vaccine 2024, 42, 3307–3320. [Google Scholar] [CrossRef]
- Siriwardena, A.N.; Gwini, S.M.; Coupland, C.A.C. Influenza vaccination, pneumococcal vaccination and risk of acute myocardial infarction: Matched case–control study. Can. Med. Assoc. J. 2010, 182, 1617–1623. [Google Scholar] [CrossRef]
- Davidson, J.A.; Banerjee, A.; Douglas, I.; Leyrat, C.; Pebody, R.; McDonald, H.I.; Herrett, E.; Forbes, H.; Smeeth, L.; Warren-Gash, C. Primary prevention of acute cardiovascular events by influenza vaccination: An observational study. Eur. Heart J. 2022, 44, 610–620. [Google Scholar] [CrossRef]
- Hjelholt, A.J.; Bergh, C.; Bhatt, D.L.; Fröbert, O.; Kjolby, M.F. Pleiotropic Effects of Influenza Vaccination. Vaccines 2023, 11, 1419. [Google Scholar] [CrossRef]
- MacIntyre, C.R.; Akhtar, Z.; Moa, A. Influenza Vaccine—Low-Hanging Fruit for Prevention of Myocardial Infarction. NEJM Evid. 2024, 3, EVIDe2400178. [Google Scholar] [CrossRef]
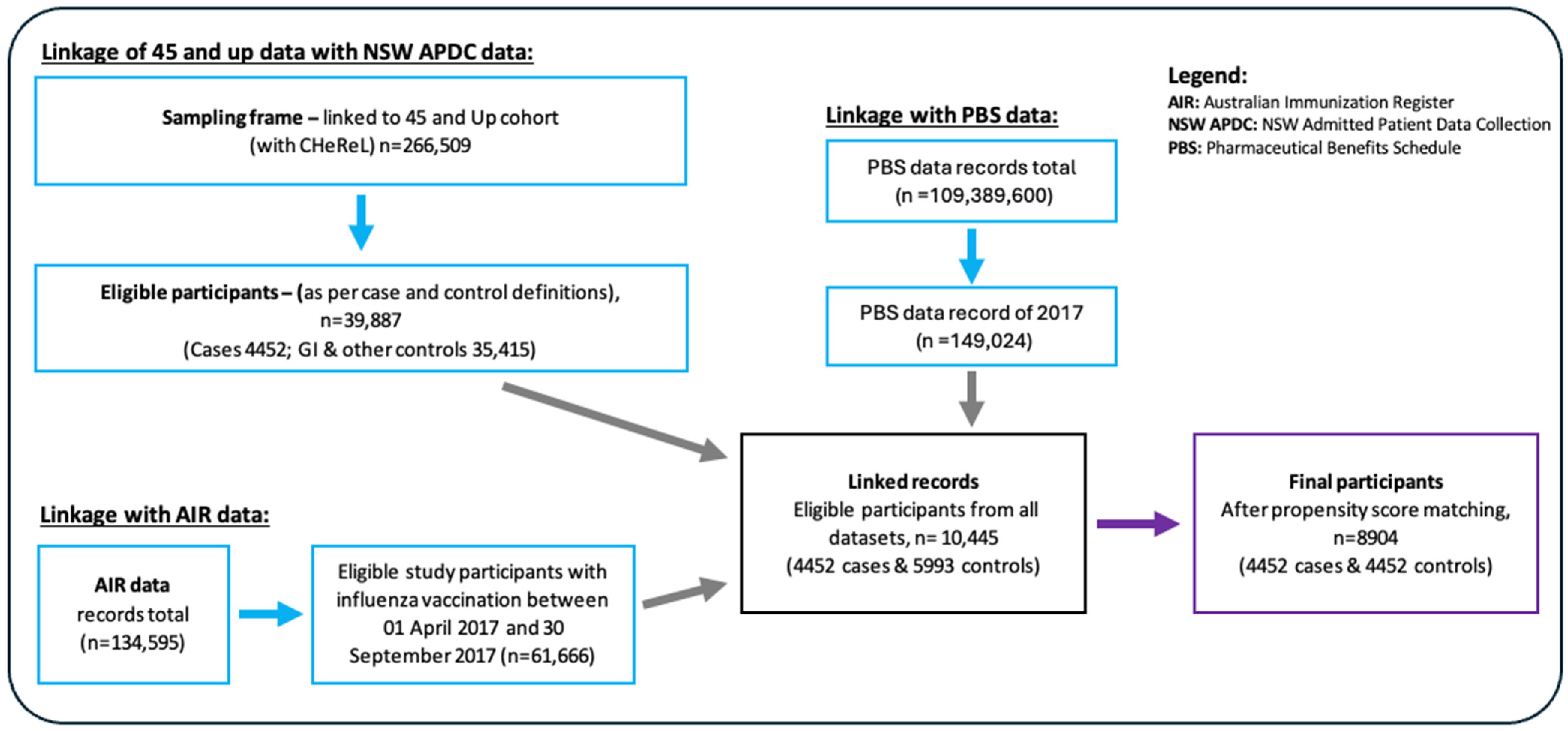
| Characteristics | Cases (n = 4452) | Controls (n = 4452) | Total (n = 8904) | p Value |
|---|---|---|---|---|
| Median years (IQR, ±SD) | 78 (70–85, ±10.2) | 76 (65–89, ±10.6) | 76 (68–86, ±10.4) | <0.001 |
| Age groups, years | ||||
| 50–64 | 582 (13.1) | 582 (13.1) | 1164 (13.1) | 1.000 |
| 65–74 | 1167 (26.2) | 1167 (26.2) | 2334 (26.2) | |
| 75–84 | 1494 (33.6) | 1494 (33.6) | 2988 (33.6) | |
| ≥85 | 1209 (27.2) | 1209 (27.2) | 2418 (27.2) | |
| Sex | ||||
| Male | 2596 (58.3) | 2596 (58.3) | 5192 (58.3) | 1.000 |
| Female | 1856 (41.7) | 1856 (41.7) | 3712 (41.7) | |
| Body mass index, BMI | ||||
| Below 25 | 1197 (26.9) | 887 (19.9) | 2084 (23.4) | <0.001 |
| 25–30 (overweight) | 1766 (39.7) | 3315 (74.5) | 5081 (57.1) | |
| Above 30 (obese) | 1489 (33.5) | 250 (5.6) | 1739 (19.5) | |
| Country of birth * | ||||
| Australia | 1076 (24.2) | 901 (20.2) | 1977 (22.2) | <0.001 |
| Others | 3326 (74.7) | 2897 (65.1) | 6223 (69.9) | |
| Aboriginal or Torres Strait Islander * | ||||
| Yes | 35 (0.8) | 0 (0) | 35 (0.4) | <0.001 |
| No | 4305 (96.7) | 4452 (100.0) | 8757 (98.4) | |
| Education * | ||||
| Certificate or lower | 3541 (79.5) | 4202 (94.4) | 7743 (87.0) | <0.001 |
| University or higher | 824 (18.5) | 250 (5.6) | 1074 (12.1) | |
| Income in Australian dollars * | ||||
| <70,000 | 2761 (62.0) | 2616 (58.8) | 5377 (60.4) | <0.001 |
| >70,000 | 742 (16.7) | 1267 (28.5) | 2009 (22.6) | |
| Prefer not to answer | 717 (16.1) | 569 (12.8) | 1286 (14.4) | |
| Smoking status * | ||||
| Never smoker | 2338 (52.5) | 1894 (42.5) | 4232 (47.5) | <0.001 |
| Former smoker | 1801 (40.5) | 2226 (50.0) | 4027 (45.2) | |
| Current smoker | 308 (6.9) | 332 (7.5) | 640 (7.2) | |
| History of past cardiovascular-related admission | ||||
| No | 2044 (45.9) | 3565 (80.1) | 5609 (63.0) | <0.001 |
| Yes | 2408 (54.1) | 887 (19.9) | 3295 (37.0) | |
| Health insurance | ||||
| No private insurance | 1669 (37.5) | 2466 (55.4) | 4135 (46.4) | <0.001 |
| Private insurance | 2783 (62.5) | 1986 (44.6) | 4769 (53.6) | |
| Chronic diseases ᶷ | ||||
| Cardiovascular diseases ˠ | 2911 (65.4) | 3195 (71.8) | 6106 (68.6) | <0.001 |
| Hypertension | 1638 (36.8) | 1589 (35.7) | 3227 (36.2) | <0.001 |
| Diabetes | 669 (15.0) | 569 (12.8) | 1238 (13.9) | 0.002 |
| Asthma or hay fever | 559 (12.6) | 0 (0) | 559 (6.3) | <0.001 |
| Anxiety and depression | 679 (15.3) | 482 (10.8) | 1161 (13.0) | <0.001 |
| Skin cancer | 296 (6.7) | 1383 (31.1) | 1679 (18.9) | <0.001 |
| Other cancers | 601 (13.5) | 654 (14.7) | 1255 (14.1) | <0.001 |
| Medications ᶷ | ||||
| ASA | 1386 (31.1) | 654 (14.7) | 2040 (22.9) | <0.001 |
| Beta blockers | 333 (7.5) | 0 (0) | 333 (3.7) | <0.001 |
| ACEI or ARB | 1338 (30.1) | 1209 (27.2) | 2547 (28.6) | 0.646 |
| Statins | 1537 (34.5) | 1254 (28.2) | 2791 (31.3) | <0.001 |
| Influenza vaccination | ||||
| Not vaccinated | 2985 (67.0) | 1749 (39.3) | 4734 (53.2) | <0.001 |
| Vaccinated | 1467 (33.0) | 2703 (60.7) | 4170 (46.8) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhtar, Z.; Moa, A.M.; Tan, T.C.; Fröbert, O.; Menzies, R.; MacIntyre, C.R. Prevention of Cardiovascular Diseases with Standard-Dose Quadrivalent Influenza Vaccine in People Aged ≥50 Years in Australia During the 2017 A/H3N2 Epidemic. Vaccines 2025, 13, 407. https://doi.org/10.3390/vaccines13040407
Akhtar Z, Moa AM, Tan TC, Fröbert O, Menzies R, MacIntyre CR. Prevention of Cardiovascular Diseases with Standard-Dose Quadrivalent Influenza Vaccine in People Aged ≥50 Years in Australia During the 2017 A/H3N2 Epidemic. Vaccines. 2025; 13(4):407. https://doi.org/10.3390/vaccines13040407
Chicago/Turabian StyleAkhtar, Zubair, Aye M. Moa, Timothy C. Tan, Ole Fröbert, Robert Menzies, and C. Raina MacIntyre. 2025. "Prevention of Cardiovascular Diseases with Standard-Dose Quadrivalent Influenza Vaccine in People Aged ≥50 Years in Australia During the 2017 A/H3N2 Epidemic" Vaccines 13, no. 4: 407. https://doi.org/10.3390/vaccines13040407
APA StyleAkhtar, Z., Moa, A. M., Tan, T. C., Fröbert, O., Menzies, R., & MacIntyre, C. R. (2025). Prevention of Cardiovascular Diseases with Standard-Dose Quadrivalent Influenza Vaccine in People Aged ≥50 Years in Australia During the 2017 A/H3N2 Epidemic. Vaccines, 13(4), 407. https://doi.org/10.3390/vaccines13040407





