Oral Delivery of Lactococcus lactis Expressing Full-Length S Protein via Alginate–Chitosan Capsules Induces Immune Protection Against PEDV Infection in Mice
Abstract
:1. Introduction
2. Materials and Methods
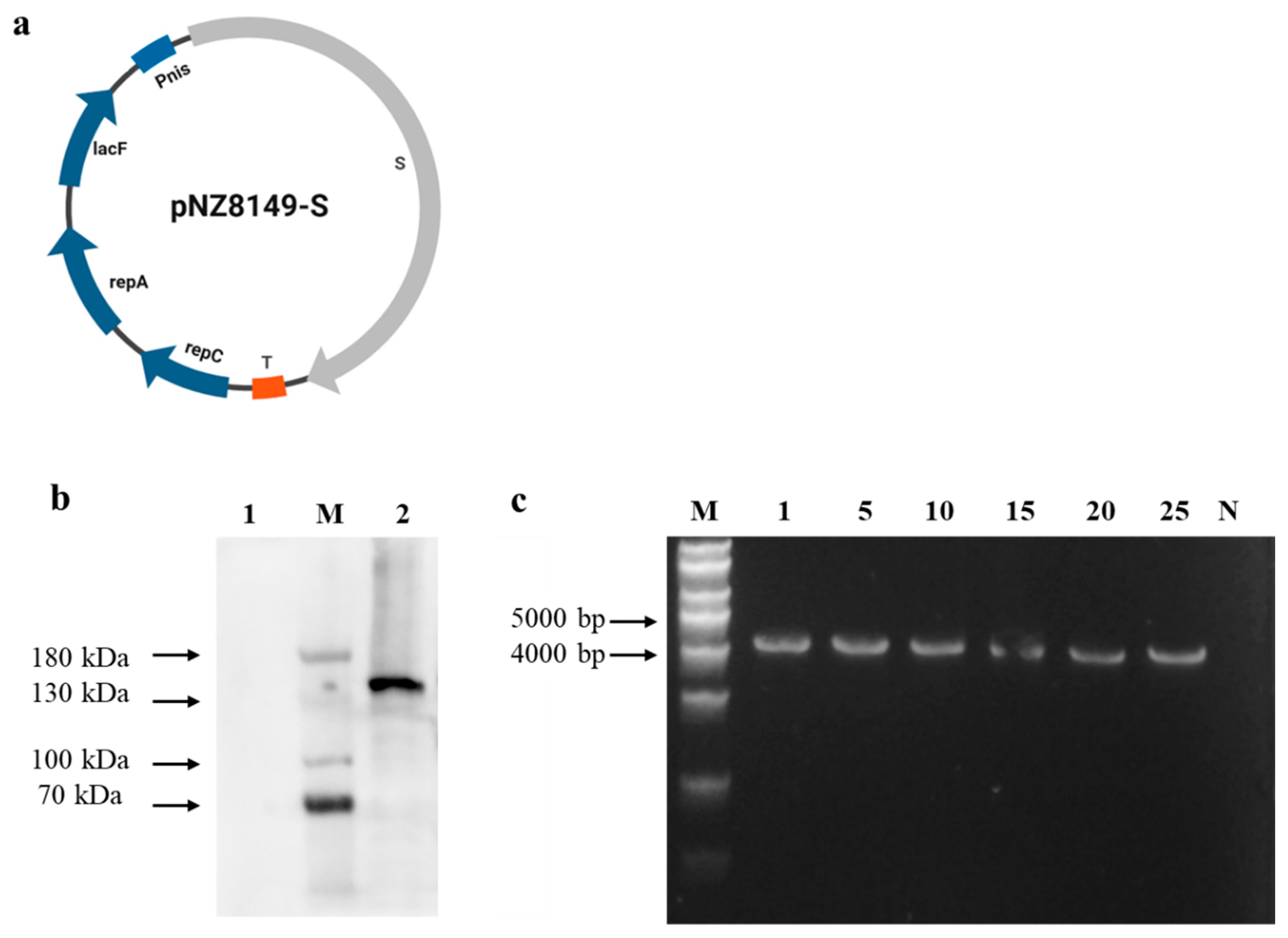
2.1. Construction of Recombinant L. lactis Strains
2.2. Preparation of Alginate–Chitosan Encapsulated L. lactis
2.3. Western Blotting
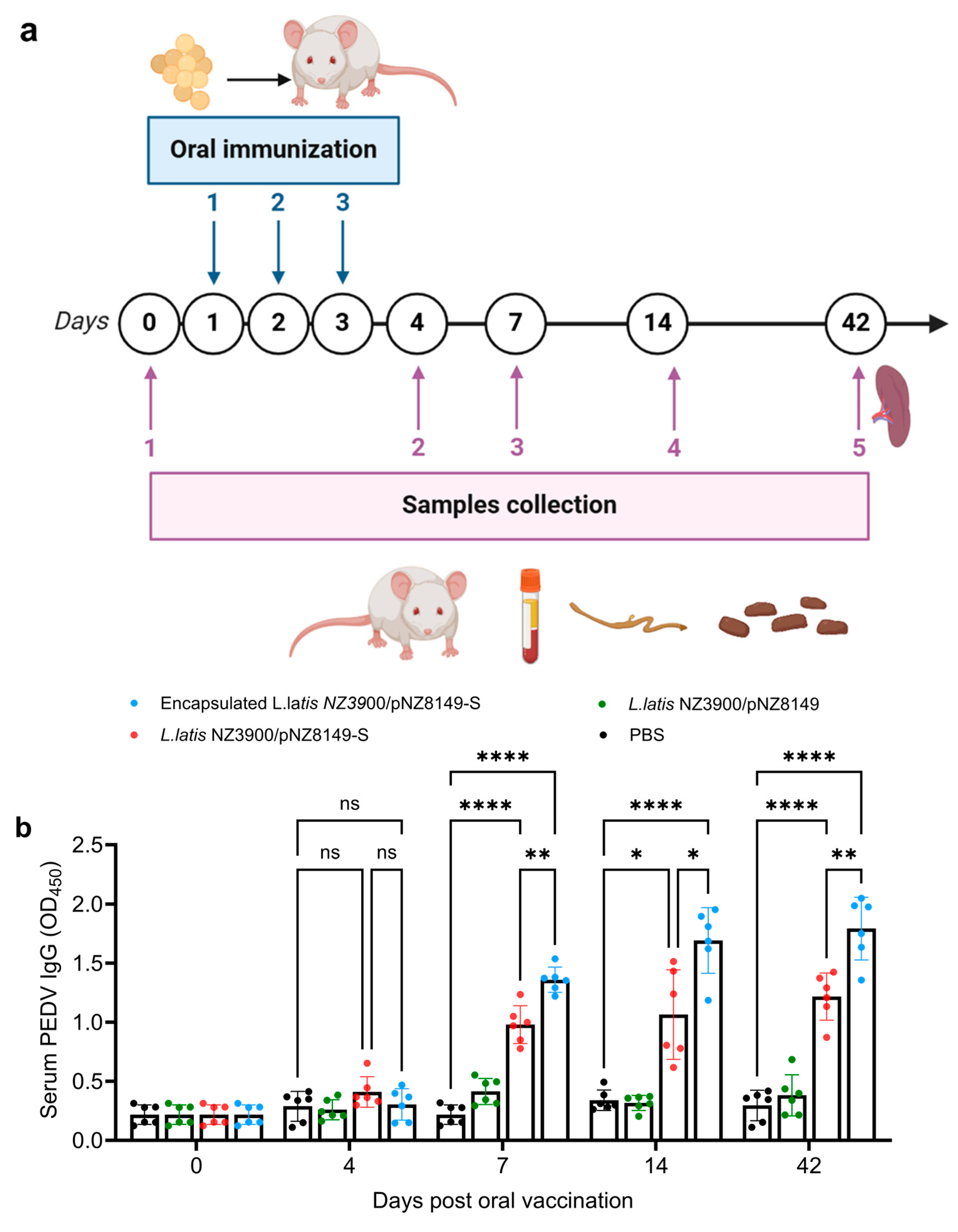
2.4. Immunization Schedule and Samples Collection
2.5. Antibody and Cytokine Assay by Indirect Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Lymphocyte Proliferation Assay
2.7. Statistical Analysis
3. Results
3.1. Identification of Recombinant L. lactis NZ3900
3.2. Characterization and Performance of Capsule Vaccine in Digestive Environments
3.3. Alginate–Chitosan L. lactis NZ3900/pNZ8149-S Vaccine Induced Higher PEDV-Specific IgG and sIgA Antibodies in Mice
3.4. Alginate–Chitosan L. lactis NZ3900/pNZ8149-S Vaccine Induced Significantly Increased IFN-r, IL-4, and IL-10 Cytokine in Vaccinated Mice
3.5. The Lymphocytes Proliferation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jung, K.; Saif, L.J. Porcine epidemic diarrhea virus infection: Etiology, epidemiology, pathogenesis and immunoprophylaxis. Vet. J. 2015, 204, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Saif, L.J.; Wang, Q. Porcine epidemic diarrhea virus (PEDV): An update on etiology, transmission, pathogenesis, and prevention and control. Virus Res. 2020, 286, 198045. [Google Scholar] [CrossRef]
- Langel, S.N.; Paim, F.C.; Lager, K.M.; Vlasova, A.N.; Saif, L.J. Lactogenic immunity and vaccines for porcine epidemic diarrhea virus (PEDV): Historical and current concepts. Virus Res. 2016, 226, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-M.; Saif, L.J.; Marthaler, D.; Wang, Q. Evolution, antigenicity and pathogenicity of global porcine epidemic diarrhea virus strains. Virus Res. 2016, 226, 20–39. [Google Scholar] [CrossRef] [PubMed]
- Lee, C. Porcine epidemic diarrhea virus: An emerging and re-emerging epizootic swine virus. Virol. J. 2015, 12, 193. [Google Scholar] [CrossRef]
- Boniotti, M.B.; Papetti, A.; Bertasio, C.; Giacomini, E.; Lazzaro, M.; Cerioli, M.; Faccini, S.; Bonilauri, P.; Vezzoli, F.; Lavazza, A.; et al. Porcine Epidemic Diarrhoea Virus in Italy: Disease spread and the role of transportation. Transbound. Emerg. Dis. 2018, 65, 1935–1942. [Google Scholar] [CrossRef]
- He, W.-T.; Bollen, N.; Xu, Y.; Zhao, J.; Dellicour, S.; Yan, Z.; Gong, W.; Zhang, C.; Zhang, L.; Lu, M.; et al. Phylogeography reveals association between swine trade and the spread of porcine epidemic diarrhea virus in china and across the world. Mol. Biol. Evol. 2021, 39, msab364. [Google Scholar] [CrossRef]
- Hulswit, R.J.G.; de Haan, C.A.M.; Bosch, B.-J. Coronavirus spike protein and tropism changes. Adv. Virus Res. 2016, 96, 29–57. [Google Scholar]
- Li, W.; van Kuppeveld, F.J.; He, Q.; Rottier, P.J.; Bosch, B.J. Cellular entry of the porcine epidemic diarrhea virus. Virus Res. 2016, 226, 117–127. [Google Scholar] [CrossRef]
- White, J.M.; Whittaker, G.R. Fusion of enveloped viruses in endosomes. Traffic 2016, 17, 593–614. [Google Scholar] [CrossRef]
- Shi, D.; Shi, H.; Wang, X.; Chen, J.; Liu, J.; Ye, D.; Jing, Z.; Liu, Q.; Fan, Q.; Li, M.; et al. A porcine epidemic diarrhea virus strain with distinct characteristics of four amino acid insertion in the COE region of spike protein. Vet. Microbiol. 2021, 253, 108955. [Google Scholar] [CrossRef]
- Yan, S.; Luo, Y.; Zhan, N.; Xu, H.; Yao, Y.; Liu, X.; Dong, X.; Kang, L.; Zhang, G.; Liu, P. Intranasal delivery of a recombinant adenovirus vaccine encoding the PEDV COE elicits potent mucosal and systemic antibody responses in mice. Microbiol. Spectr. 2024, 12, e0069224. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Wang, C.; Song, X.; Xu, H.; Zhao, S.; Gu, J.; Zou, Z.; Li, J.; Qian, J.; Zhang, X.; et al. Immunogenicity and protective efficacy of a trimeric full-length S protein subunit vaccine for porcine epidemic diarrhea virus. Vaccine 2024, 42, 828–839. [Google Scholar] [CrossRef]
- Zhao, Y.; Fan, B.; Song, X.; Gao, J.; Guo, R.; Yi, C.; He, Z.; Hu, H.; Jiang, J.; Zhao, L.; et al. PEDV-spike-protein-expressing mRNA vaccine protects piglets against PEDV challenge. mBio 2024, 15, e0295823. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, M.C.; Lam, H.C.; Zhang, Y.; Wang, L.; Hesse, R.A.; Hause, B.M.; Vlasova, A.; Wang, Q.; Zhang, J.; Nelson, M.I.; et al. Genomic and evolutionary inferences between American and global strains of porcine epidemic diarrhea virus. Prev. Vet. Med. 2016, 123, 175–184. [Google Scholar] [CrossRef]
- Song, D.; Moon, H.; Kang, B. Porcine epidemic diarrhea: A review of current epidemiology and available vaccines. Clin. Exp. Vaccine Res. 2015, 4, 166–176. [Google Scholar] [CrossRef]
- Zhang, E.; Wang, J.; Li, Y.; Huang, L.; Wang, Y.; Yang, Q. Comparison of oral and nasal immunization with inactivated porcine epidemic diarrhea virus on intestinal immunity in piglets. Exp. Ther. Med. 2020, 20, 1596–1606. [Google Scholar] [CrossRef]
- Wang, J.; Huang, L.; Mou, C.; Zhang, E.; Wang, Y.; Cao, Y.; Yang, Q. Mucosal immune responses induced by oral administration recombinant Bacillus subtilis expressing the COE antigen of PEDV in newborn piglets. Biosci. Rep. 2019, 39, BSR20182028. [Google Scholar] [CrossRef]
- Ramirez, J.E.V.; Sharpe, L.A.; Peppas, N.A. Current state and challenges in developing oral vaccines. Adv. Drug Del. Rev. 2017, 114, 116–131. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Du, G.; Chen, X.; Sun, X. Advanced oral vaccine delivery strategies for improving the immunity. Adv. Drug Deliv. Rev. 2021, 177, 113928. [Google Scholar] [CrossRef]
- Graham, K.; Stack, H.; Rea, R. Safety, beneficial and technological properties of enterococci for use in functional food applications–A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 3836–3861. [Google Scholar] [CrossRef]
- Riglar, D.T.; Silver, P.A. Silver Engineering bacteria for diagnostic and therapeutic applications. Nat. Rev. Microbiol. 2018, 16, 214–225. [Google Scholar] [CrossRef]
- Chamcha, V.; Jones, A.; Quigley, B.R.; Scott, J.R.; Amara, R.R. Oral immunization with a recombinant Lactococcus lactis–Expressing HIV-1 antigen on group A Streptococcus pilus induces strong mucosal immunity in the gut. J. Immunol. 2015, 195, 5025–5034. [Google Scholar] [CrossRef] [PubMed]
- Chatel, J.-M.; Langella, P.; Adel-Patient, K.; Commissaire, J.; Wal, J.-M.; Corthier, G. Induction of mucosal immune response after intranasal or oral inoculation of mice with Lactococcus lactis producing bovine beta-lactoglobulin. Clin. Diagn. Lab. Immunol. 2001, 8, 545–551. [Google Scholar] [CrossRef]
- Cho, H.-J.; Shin, H.-J.; Han, I.-K.; Jung, W.-W.; Kim, Y.B.; Sul, D.; Oh, Y.-K. Induction of mucosal and systemic immune responses following oral immunization of mice with Lactococcus lactis expressing human papillomavirus type 16 L1. Vaccine 2007, 25, 8049–8057. [Google Scholar] [CrossRef]
- Ding, C.; Ma, J.; Dong, Q.; Liu, Q. Live bacterial vaccine vector and delivery strategies of heterologous antigen: A review. Immunol. Lett. 2018, 197, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-J.; Li, J.; Razzak, A.; Eom, G.-D.; Yoon, K.-W.; Mao, J.; Chu, K.-B.; Jin, H.; Choi, S.S.; Quan, F.-S. Chitosan-Alginate Polymeric Nanocomposites as a Potential Oral Vaccine Carrier Against Influenza Virus Infection. ACS Appl. Mater. Interfaces 2023, 15, 50889–50897. [Google Scholar] [CrossRef]
- Najafi, A.; Ghazvini, K.; Sankian, M.; Gholami, L.; Amini, Y.; Zare, S.; Khademi, F.; Tafaghodi, M. T helper type 1 biased immune responses by PPE17 loaded core-shell alginate-chitosan nanoparticles after subcutaneous and intranasal administration. Life Sci. 2021, 282, 119806. [Google Scholar] [CrossRef] [PubMed]
- Rhee, J.H. Current and new approaches for mucosal vaccine delivery. Mucosal Vaccines 2020, 25, 325–356. [Google Scholar]
- Yu, X.; Wen, T.; Cao, P.; Shan, L.; Li, L. Alginate-chitosan coated layered double hydroxide nanocomposites for enhanced oral vaccine delivery. J. Colloid Interface Sci. 2019, 556, 258–265. [Google Scholar] [CrossRef]
- Kothale, D.; Verma, U.; Dewangan, N.; Jana, P.; Jain, A.; Jain, D. Alginate as promising natural polymer for pharmaceutical, food, and biomedical applications. Curr. Drug Deliv. 2020, 17, 755–775. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; Da Silva, C.F.; Andrade, L.N.; de Lima Oliveira, D.; Campos, J.; Souto, E.B. Alginate nanoparticles for drug delivery and targeting. Curr. Pharm. Des. 2019, 25, 1312–1334. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Alamry, K.A. Recent advances of emerging green chitosan-based biomaterials with potential biomedical applications: A review. Carbohydr. Res. 2021, 506, 108368. [Google Scholar] [CrossRef]
- Dmour, I.; Islam, N. Recent advances on chitosan as an adjuvant for vaccine delivery. Int. J. Biol. Macromol. 2022, 200, 498–519. [Google Scholar] [CrossRef]
- Wani, S.U.D.; Ali, M.; Mehdi, S.; Masoodi, M.H.; Zargar, M.I.; Shakeel, F. A review on chitosan and alginate-based microcapsules: Mechanism and applications in drug delivery systems. Int. J. Biol. Macromol. 2023, 248, 125875. [Google Scholar] [CrossRef]
- Li, X.; Kong, X.; Shi, S.; Zheng, X.; Guo, G.; Wei, Y.; Qian, Z. Preparation of alginate coated chitosan microparticles for vaccine delivery. BMC Biotechnol. 2008, 8, 89. [Google Scholar] [CrossRef]
- Akerele, G.; Ramadan, N.; Renu, S.; Renukaradhya, G.J.; Shanmugasundaram, R.; Selvaraj, R.K. In Vitro characterization and immunogenicity of chitosan nanoparticles loaded with native and inactivated extracellular proteins from a field strain of Clostridium perfringens associated with necrotic enteritis. Vet. Immunol. Immunopathol. 2020, 224, 110059. [Google Scholar] [CrossRef] [PubMed]
- Vasiliev, Y.M. Chitosan-based vaccine adjuvants: Incomplete characterization complicates preclinical and clinical evaluation. Expert Rev. Vaccines 2014, 14, 37–53. [Google Scholar] [CrossRef]
- Niculescu, A.G.; Grumezescu, A.M. Applications of Chitosan-Alginate-Based Nanoparticles—An Up-to-Date Review. Nanomaterials 2022, 12, 186. [Google Scholar] [CrossRef]
- Zhao, B.; Alonso, N.F.; Miras, J.; Vílchez, S.; García-Celma, M.J.; Morral, G.; Esquena, J. Triggered protein release from calcium alginate/chitosan gastro-resistant capsules. Colloids Surf. Physicochem. Eng. Asp. 2024, 693, 133998. [Google Scholar] [CrossRef]
- Jiang, T.; Singh, B.; Maharjan, S.; Li, H.-S.; Kang, S.-K.; Bok, J.-D.; Cho, C.-S.; Choi, Y.-J. Oral delivery of probiotic expressing M cell homing peptide conjugated BmpB vaccine encapsulated into alginate/chitosan/alginate microcapsules. Eur. J. Pharm. Biopharm 2014, 88, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Hariyadi, D.M.; Islam, N. Current Status of Alginate in Drug Delivery. Adv. Pharmacol. Pharm. Sci. 2020, 2020, 8886095. [Google Scholar] [CrossRef]
- Saraf, S.; Jain, S.; Sahoo, R.N.; Mallick, S. Lipopolysaccharide derived alginate coated Hepatitis B antigen loaded chitosan nanoparticles for oral mucosal immunization. Int. J. Biol. Macromol 2020, 154, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Jabbal-Gill, I.; Watts, P.; Smith, A. Chitosan-based delivery systems for mucosal vaccines. Expert Opin. Drug Deliv. 2012, 9, 1051–1067. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.M.; Wilson, P.W.; Le Page, R.W.F. Improved cloning vectors and transformation procedure for Lactococcus lactis. J. Appl. Microbiol. 1993, 74, 629–636. [Google Scholar] [CrossRef]
- Rocha, C.E.; Silva, M.F.; Guedes, A.C.; Carvalho, T.P.; Eckstein, C.; Ribeiro, N.Q.; Santos, D.A.; Melo, M.M.; Araújo, M.S.; Martins-Filho, O.A.; et al. Alginate-chitosan microcapsules improve vaccine potential of gamma-irradiated Listeria monocytogenes against listeriosis in murine model. Int. J. Biol. Macromol. 2021, 176, 567–577. [Google Scholar] [CrossRef]
- Liu, D.; Wang, X.; Ge, J.; Liu, S.; Li, Y. Comparison of the immune responses induced by oral immunization of mice with Lactobacillus casei—Expressing porcine parvovirus VP2 and VP2 fused to Escherichia coli heat-labile enterotoxin B subunit protein. Comp. Immunol. Microbiol. Infect. Dis. 2011, 34, 73–81. [Google Scholar] [CrossRef]
- Guo, M.; Yi, S.; Guo, Y.; Zhang, S.; Niu, J.; Wang, K.; Hu, G. Construction of a recombinant Lactococcus lactis strain expressing a variant porcine epidemic diarrhea virus S1 gene and its immunogenicity analysis in mice. Viral Immunol. 2019, 32, 144–150. [Google Scholar] [CrossRef]
- Cook, M.T.; Tzortzis, G.; Charalampopoulos, D.; Khutoryanskiy, V.V. Production and evaluation of dry alginate-chitosan microcapsules as an enteric delivery vehicle for probiotic bacteria. Biomacromolecules 2011, 12, 2834–2840. [Google Scholar] [CrossRef]
- Uyanga, V.A.; Ejeromedoghene, O.; Lambo, M.T.; Alowakennu, M.; Alli, Y.A.; Ere-Richard, A.A.; Min, L.; Zhao, J.; Wang, X.; Jiao, H.; et al. Chitosan and chitosan-based composites as beneficial compounds for animal health: Impact on gastrointestinal functions and biocarrier application. J. Funct. Foods 2023, 104, 105520. [Google Scholar] [CrossRef]
- Brandtzaeg, P. Mucosal immunity: Induction, dissemination, and effector functions. Scand. J. Immunol. 2009, 70, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wang, J.; Wang, Y.; Zhang, E.; Li, Y.; Yu, Q.; Yang, Q. Upregulation of CD4+ CD8+ memory cells in the piglet intestine following oral administration of Bacillus subtilis spores combined with PEDV whole inactivated virus. Vet. Microbiol. 2019, 235, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Chen, G.; Zhang, T.; Huang, X.; Lu, Y.; Li, M.; Li, S.; Wang, C.; Li, B.; Zhang, Y.; et al. PEDV promotes the differentiation of CD4+ T cells towards Th1, Tfh, and Treg cells via CD103+ DCs. Virology 2023, 587, 109880. [Google Scholar] [CrossRef]
- Yuan, C.; Jin, Y.; Li, Y.; Zhang, E.; Zhang, P.; Yang, Q. PEDV infection in neonatal piglets through the nasal cavity is mediated by subepithelial CD3+ T cells. Vet. Res. 2021, 52, 26. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, C.; Chen, Y.; Liu, Z.; Zheng, J.; Wang, T.; Luo, H.; Liu, Y.; Shan, Y.; Fang, W.; et al. Effect of route of inoculation on innate and adaptive immune responses to porcine epidemic diarrhea virus infection in suckling pigs. Vet. Microbiol. 2019, 228, 83–92. [Google Scholar] [CrossRef]
- Wang, D.; Fang, L.; Xiao, S. Porcine epidemic diarrhea in China. Virus Res. 2016, 226, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.B.; Feng, L.; Shi, H.Y.; Chen, J.F.; Liu, S.W.; Chen, H.Y.; Wang, Y.F. Spike protein region (aa 636789) of porcine epidemic diarrhea virus is essential for induction of neutralizing antibodies. Acta Virol. 2007, 51, 149–156. [Google Scholar]
- Bonam, S.R.; Partidos, C.D.; Halmuthur, S.K.M.; Muller, S. An overview of novel adjuvants designed for improving vaccine efficacy. Trends Pharmacol. Sci. 2017, 38, 771–793. [Google Scholar] [CrossRef]
- Patra, P.; Upadhyay, T.K.; Alshammari, N.; Saeed, M.; Kesari, K.K. Alginate-Chitosan Biodegradable and Biocompatible Based Hydrogel for Breast Cancer Immunotherapy and Diagnosis: A Comprehensive Review. ACS Appl. Bio Mater. 2024, 7, 3515–3534. [Google Scholar] [CrossRef]
- Li, X.; Xing, R.; Xu, C.; Liu, S.; Qin, Y.; Li, K.; Yu, H.; Li, P. Immunostimulatory effect of chitosan and quaternary chitosan: A review of potential vaccine adjuvants. Carbohydr. Polym. 2021, 264, 118050. [Google Scholar] [CrossRef]
- Di-Qiu, L.; Jun-Wei, G.; Xin-Yuan, Q.; Yan-Ping, J.; Song-Mei, L.; Yi-Jing, L. High-level mucosal and systemic immune responses induced by oral administration with Lactobacillus-expressed porcine epidemic diarrhea virus (PEDV) S1 region combined with Lactobacillus-expressed N protein. Appl. Microbiol. Biotechnol. 2011, 93, 2437–2446. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Huang, X.; Ma, S.; Yu, M.; Shi, W.; Qiao, X.; Tang, L.; Xu, Y.; Li, Y. Oral delivery of probiotics expressing dendritic cell-targeting peptide fused with porcine epidemic diarrhea virus COE antigen: A promising vaccine strategy against PEDV. Viruses 2017, 9, 312. [Google Scholar] [CrossRef] [PubMed]
- Qiao, N.; Du, G.; Zhong, X.; Sun, X. Recombinant lactic acid bacteria as promising vectors for mucosal vaccination. Exploration 2021, 1, 20210026. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.; Zhang, Y.; Chen, P.; Yang, X.; Hou, H. Chitosan interaction with stomach mucin layer to enhances gastric retention and mucoadhesive properties. Carbohydr. Polym. 2024, 333, 121926. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Zhang, M.; Zhu, H.; Du, X.; Wang, J. Mucosal vaccine delivery: A focus on the breakthrough of specific barriers. Acta Pharm. Sin. B 2022, 12, 3456–3474. [Google Scholar] [CrossRef]
- das Neves, J.; Arzi, R.S.; Sosnik, A. Molecular and cellular cues governing nanomaterial–mucosae interactions: From nanomedicine to nanotoxicology. Chem. Soc. Rev. 2020, 49, 5058–5100. [Google Scholar] [CrossRef]
- Lang, X.; Wang, T.; Sun, M.; Chen, X.; Liu, Y. Advances and applications of chitosan-based nanomaterials as oral delivery carriers: A review. Int. J. Biol. Macromol. 2020, 154, 433–445. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Eini, M.; Rastegari, A.; Tehrani, M.R. Chitosan as a machine for biomolecule delivery: A review. Carbohydr. Polym. 2021, 256, 117414. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, G.M.; Ali, Y.; Boehm, B.O.; Huang, Y.Y.; Venkatraman, S. Layer-by-layer coated nanoliposomes for oral delivery of insulin. Nanoscale 2020, 13, 776–789. [Google Scholar] [CrossRef]
- Bekhit, M.; Sánchez-González, L.; Ben Messaoud, G.; Desobry, S. Encapsulation of Lactococcus lactis subsp. lactis on alginate/pectin composite microbeads: Effect of matrix composition on bacterial survival and nisin release. J. Food Eng. 2016, 180, 1–9. [Google Scholar] [CrossRef]
- Maleki, G.; Woltering, E.J.; Mozafari, M.R. Applications of chitosan-based carrier as an encapsulating agent in food industry. Trends Food Sci. Technol. 2022, 120, 88–99. [Google Scholar] [CrossRef]
- Muyzer, G.; De WaalA, E.C. Uitterlinden, Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 1993, 59, 695–700. [Google Scholar] [CrossRef]
- Desfossés-Foucault, É.; Dussault-Lepage, V.; Le Boucher, C.; Savard, P.; LapointeD, G. Roy, Assessment of probiotic viability during Cheddar cheese manufacture and ripening using propidium monoazide-PCR quantification. Front. Microbiol. 2012, 3, 350. [Google Scholar] [CrossRef]



| Group | Stimulation Index (SI) | |
|---|---|---|
| S | ConA | |
| Encapsulated L. lactis NZ3900/pNZ8149-S | 2.40 ± 0.22 c | 2.79 ± 0.19 c |
| L. lactis NZ3900/pNZ8149-S | 1.92 ± 0.23 b | 2.46 ± 0.31 b |
| L. lactis NZ3900/pNZ8149 | 1.12 ± 0.14 a | 1.08 ± 0.12 a |
| PBS | 1.06 ± 0.09 a | 0.97 ± 0.13 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Xie, D.; Ji, W.; Zhu, S.J.; Zhou, Y. Oral Delivery of Lactococcus lactis Expressing Full-Length S Protein via Alginate–Chitosan Capsules Induces Immune Protection Against PEDV Infection in Mice. Vaccines 2025, 13, 421. https://doi.org/10.3390/vaccines13040421
Yang M, Xie D, Ji W, Zhu SJ, Zhou Y. Oral Delivery of Lactococcus lactis Expressing Full-Length S Protein via Alginate–Chitosan Capsules Induces Immune Protection Against PEDV Infection in Mice. Vaccines. 2025; 13(4):421. https://doi.org/10.3390/vaccines13040421
Chicago/Turabian StyleYang, Miaoyan, Denglong Xie, Wei Ji, Shu Jeffrey Zhu, and Yongqi Zhou. 2025. "Oral Delivery of Lactococcus lactis Expressing Full-Length S Protein via Alginate–Chitosan Capsules Induces Immune Protection Against PEDV Infection in Mice" Vaccines 13, no. 4: 421. https://doi.org/10.3390/vaccines13040421
APA StyleYang, M., Xie, D., Ji, W., Zhu, S. J., & Zhou, Y. (2025). Oral Delivery of Lactococcus lactis Expressing Full-Length S Protein via Alginate–Chitosan Capsules Induces Immune Protection Against PEDV Infection in Mice. Vaccines, 13(4), 421. https://doi.org/10.3390/vaccines13040421





