Adjuvant Templating Improves On-Target/Off-Target Antibody Ratio Better than Linker Addition for M2-Derived Peptide Amphiphile Micelle Vaccines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Peptide Synthesis and Purification
2.2. Micelle Characterization
2.3. Bone-Marrow-Derived Dendritic Cell (BMDC) Studies
2.4. Vaccination Schedule
2.5. Serum Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Statistics
3. Results
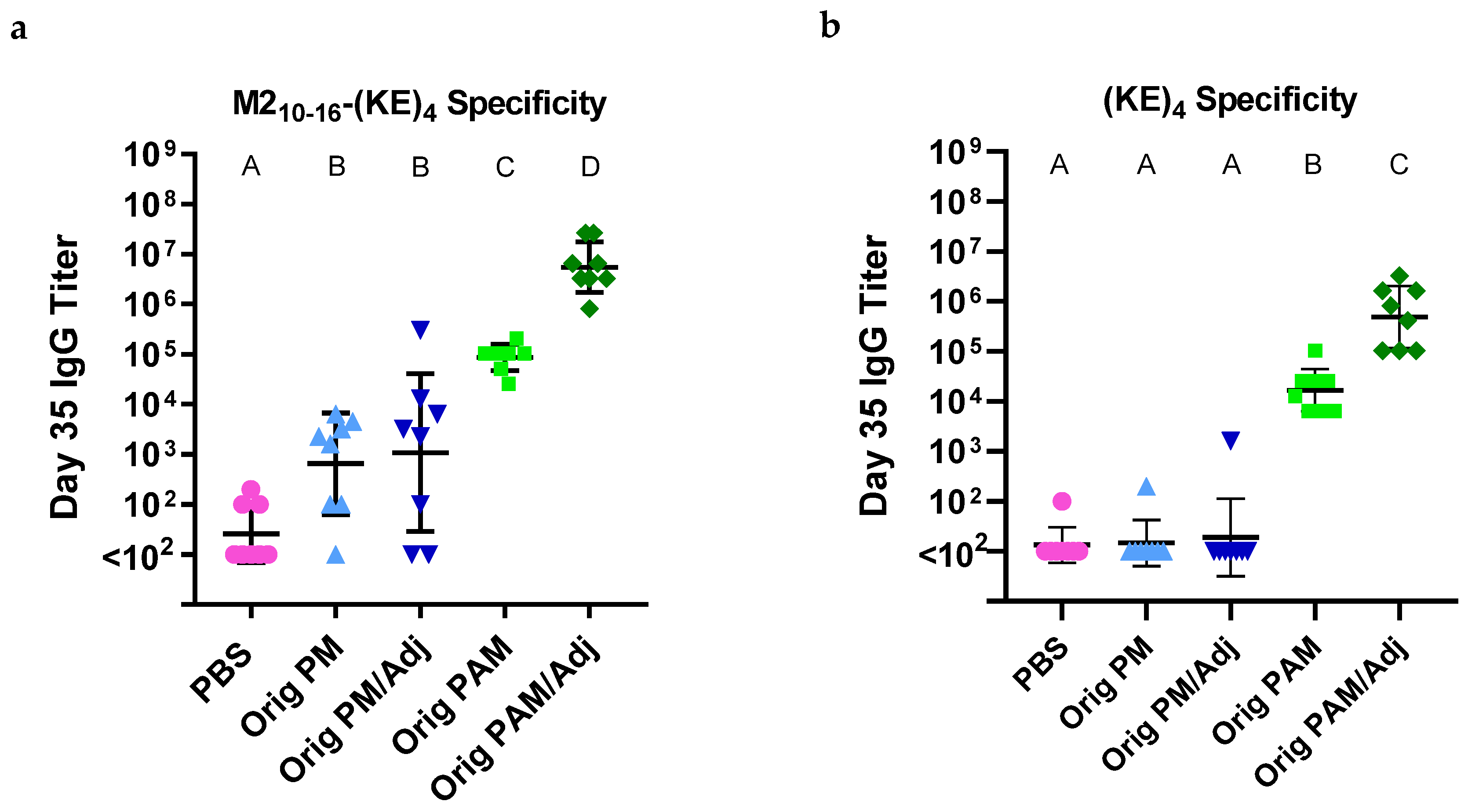
3.1. Palm2K-M22–16-(KE)4 Peptide Amphiphile Micelles Elicited Off-Target Antibody Production
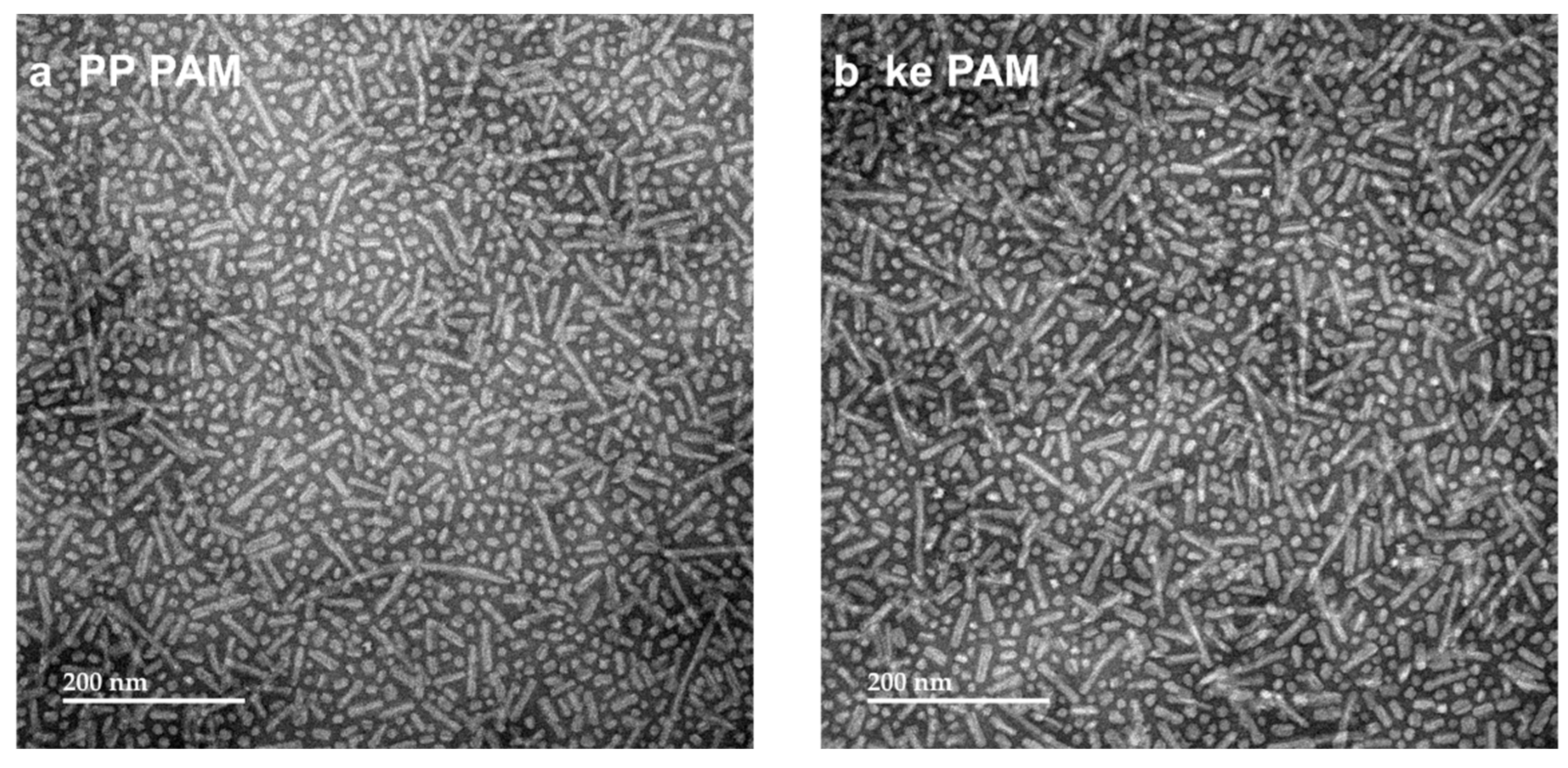
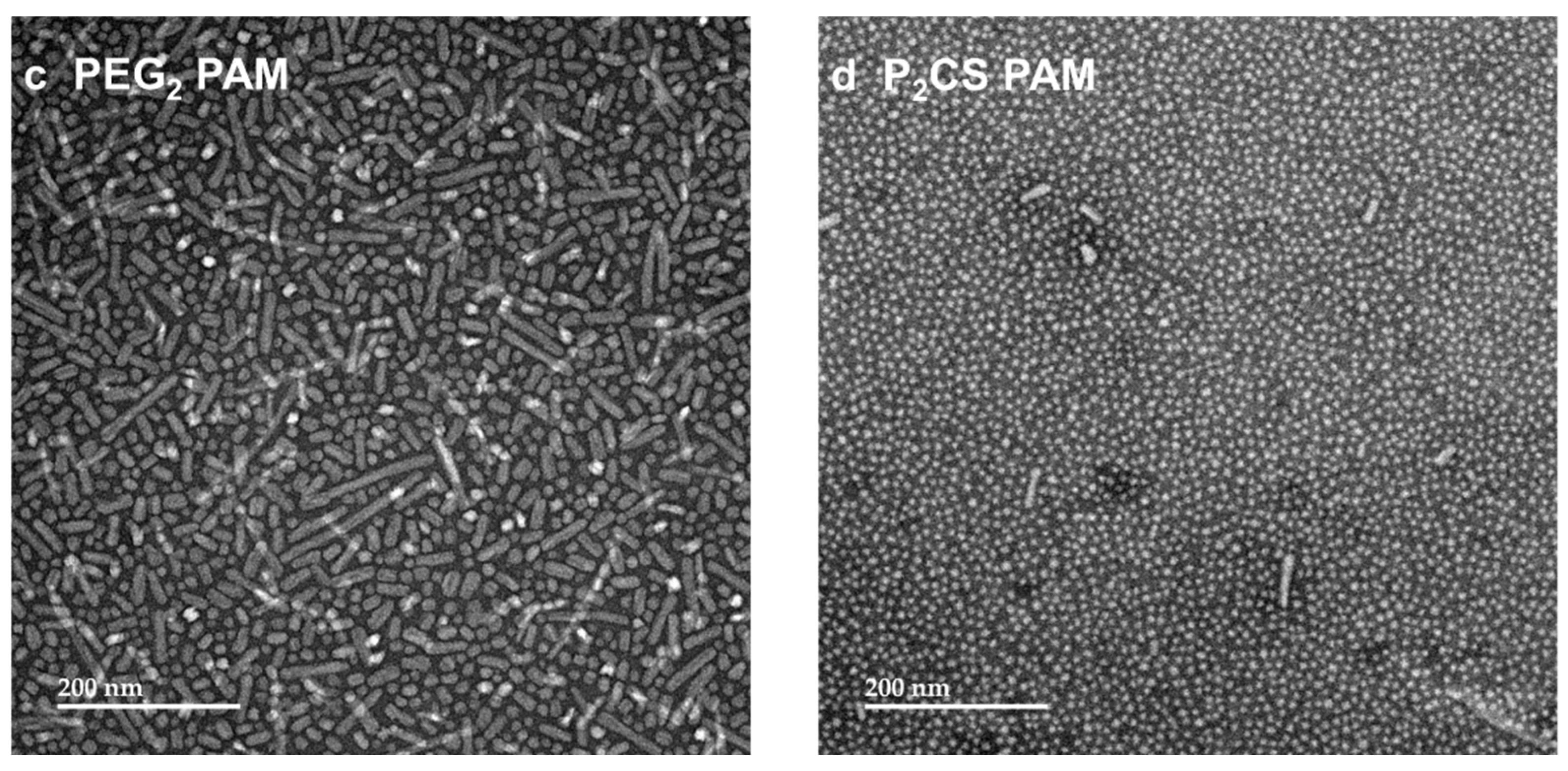
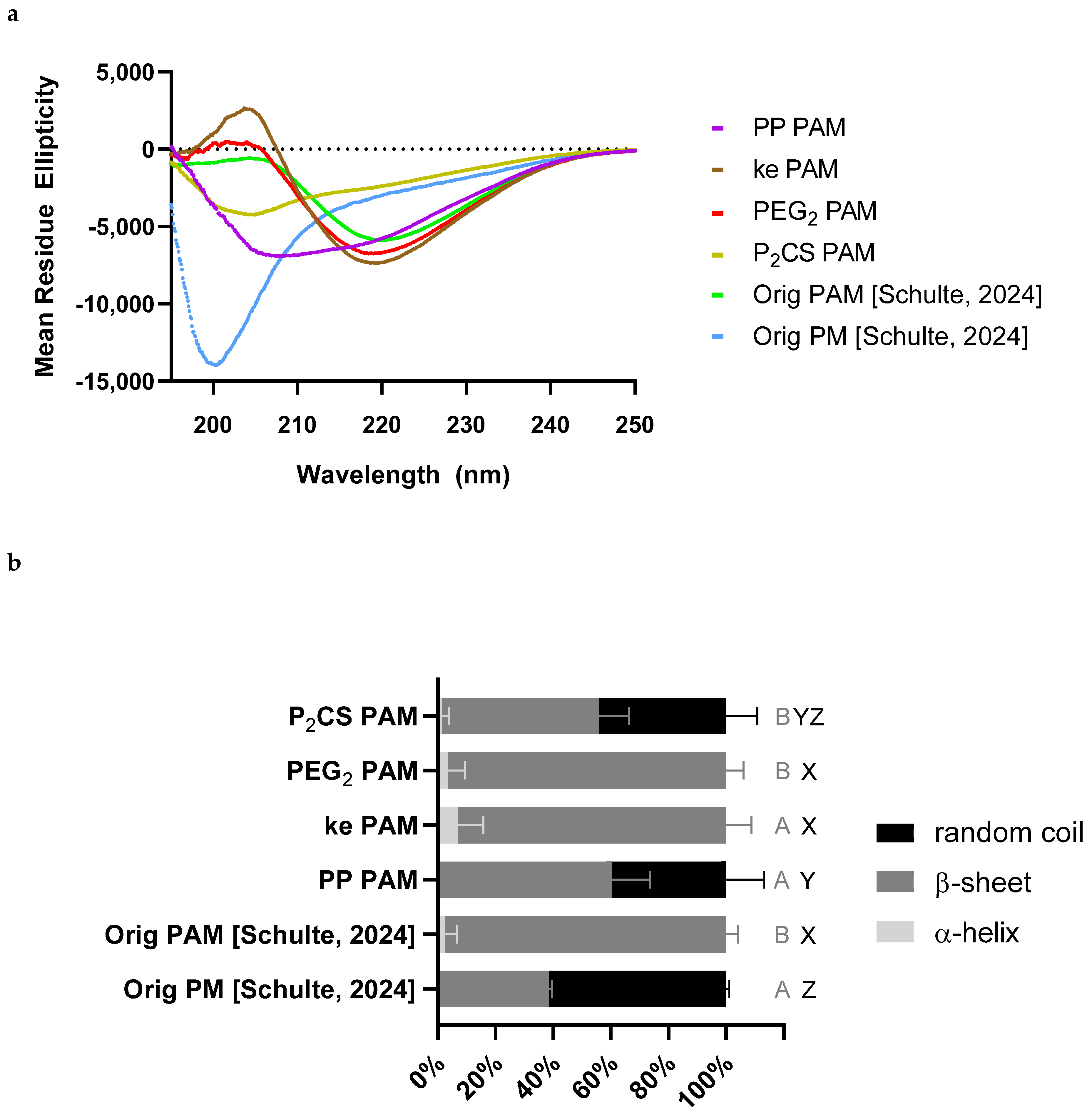
3.2. Proline–Proline and Pam2CS Moieties Induced Slight Changes to the Physical Properties of M22–16-Containing Peptide Amphiphile Micelles
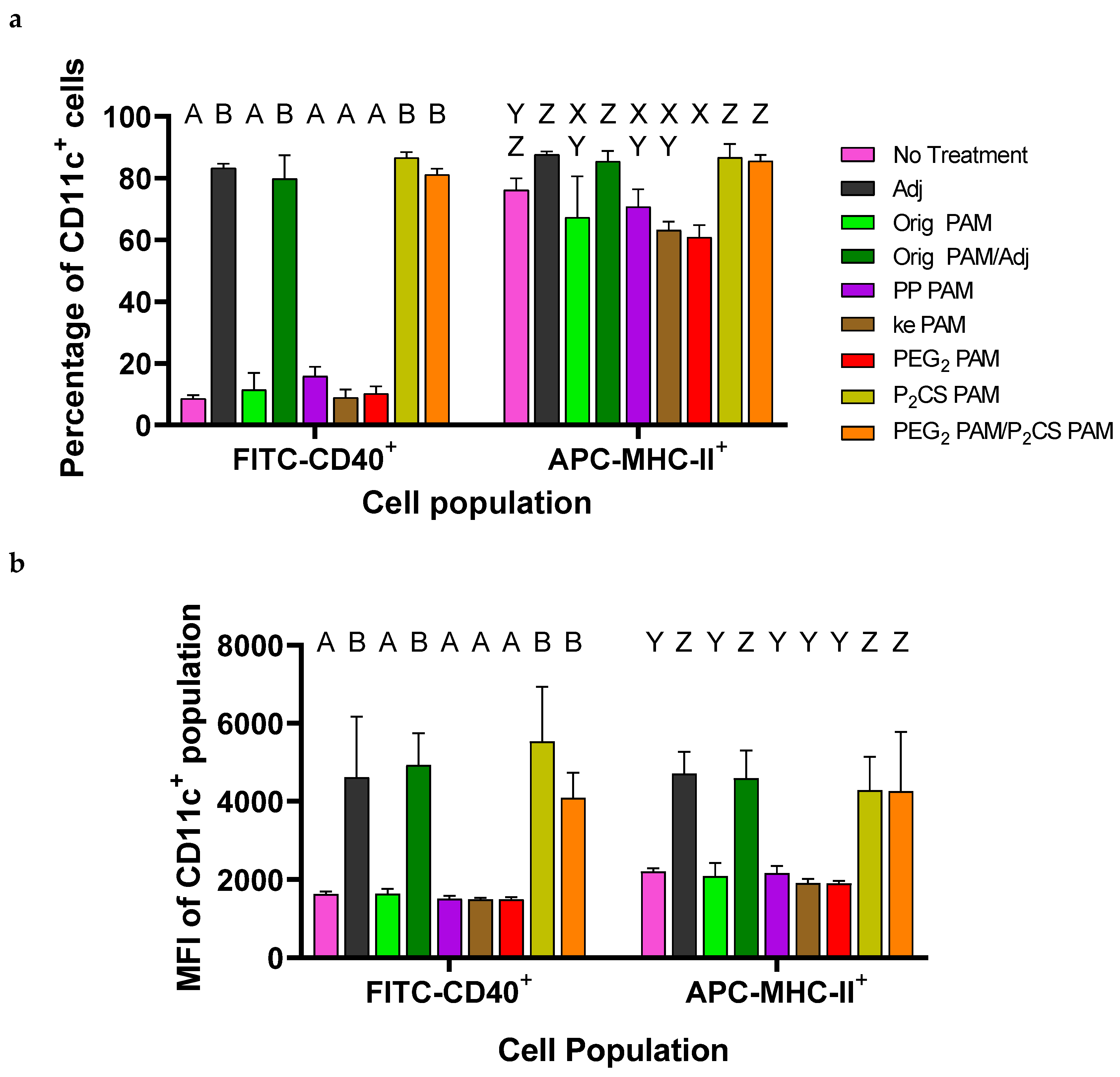
3.3. Bone-Marrow-Derived Dendritic Cells Were Activated by P2CS PAMs
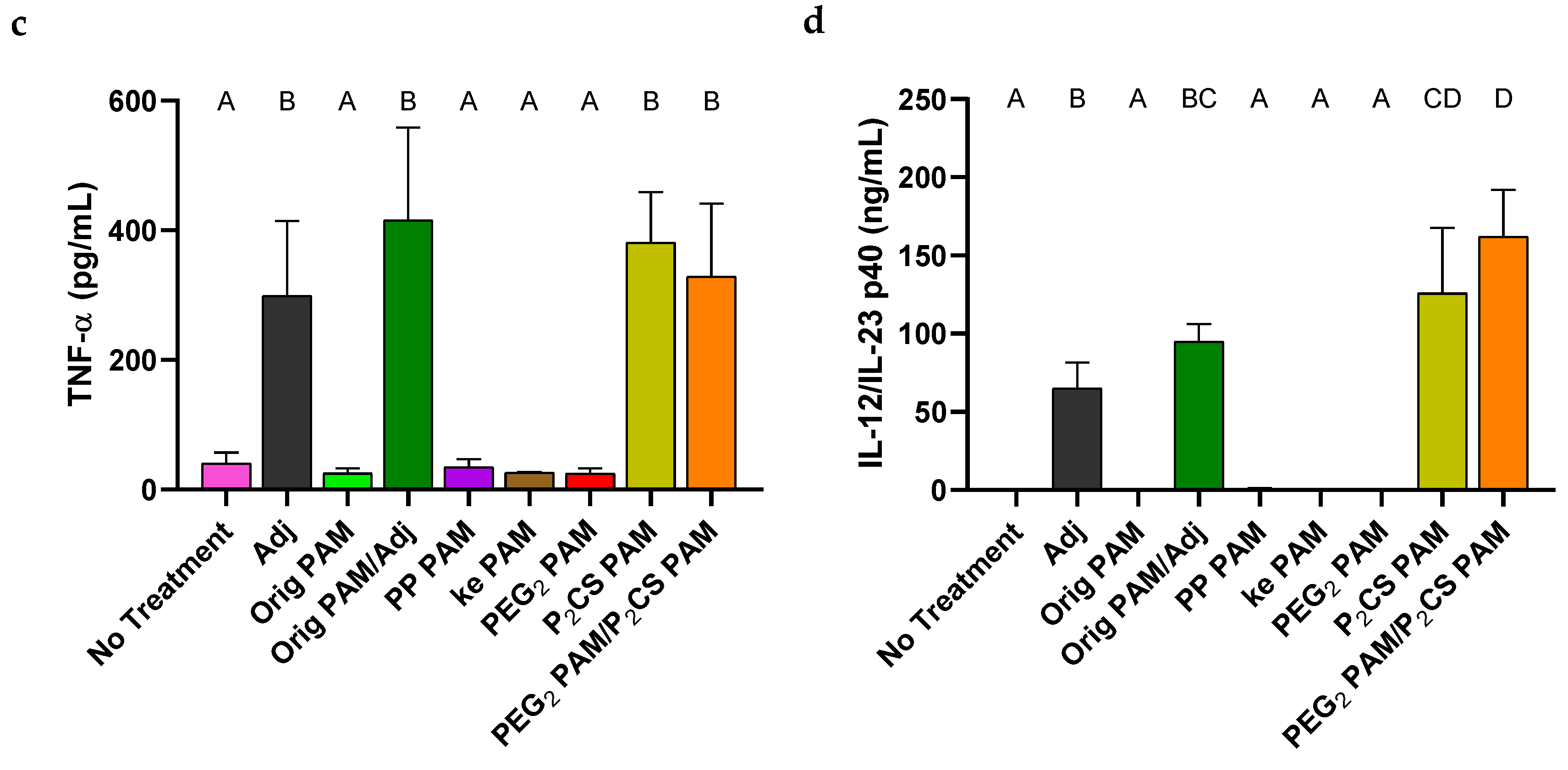
3.4. Linker-Containing Peptide Amphiphile Micelles Elicited Off-Target IgG Antibodies but P2CS PAMs Also Elicited Strong On-Target Titers
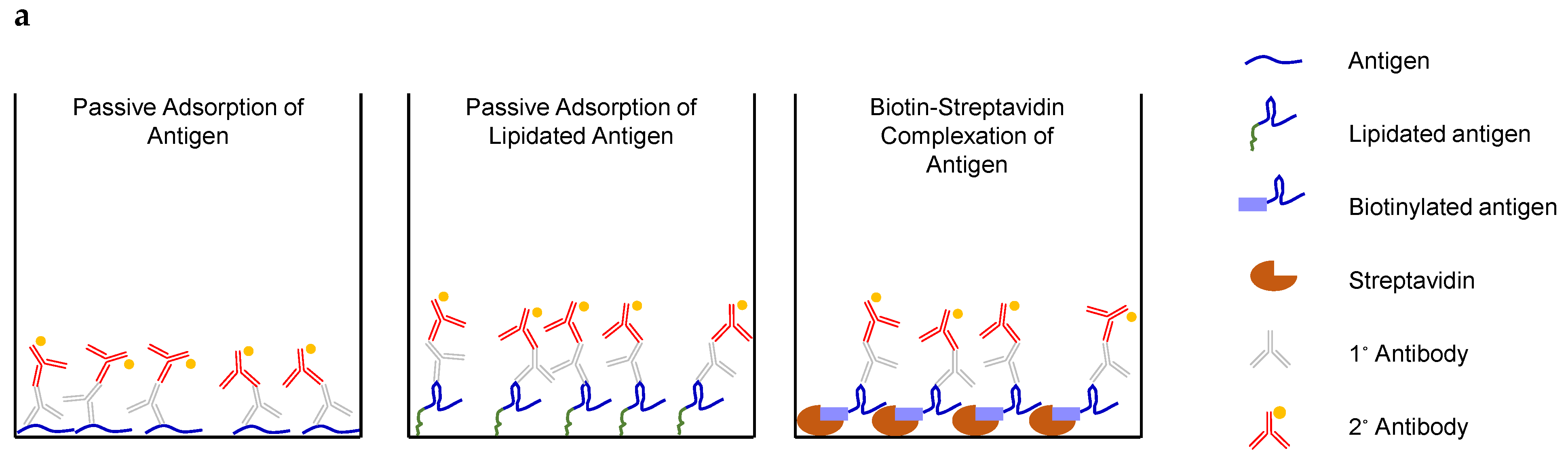
3.5. IgG Titers Were More Dependent on the Sequence than the Attachment Method of the Coating Antigen
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poon, C.; Gallo, J.; Joo, J.; Chang, T.; Bañobre-López, M.; Chung, E.J. Hybrid, metal oxide-peptide amphiphile micelles for molecular magnetic resonance imaging of atherosclerosis. J. Nanobiotechnol. 2018, 16, 92. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.J.; Cheng, Y.; Morshed, R.; Nord, K.; Han, Y.; Wegscheid, M.L.; Auffinger, B.; Wainwright, D.A.; Lesniak, M.S.; Tirrell, M.V. Fibrin-binding, peptide amphiphile micelles for targeting glioblastoma. Biomaterials 2014, 35, 1249–1256. [Google Scholar] [CrossRef]
- Smith, J.D.; Cardwell, L.N.; Porciani, D.; Nolla, A.; Cornelison, B.T.; Schulte, M.C.; Gallazzi, F.; Burke, D.H.; Daniels, M.A.; Ulery, B.D. Therapeutic peptide delivery: Via aptamer-displaying, disulfide-linked peptide amphiphile micelles. Mol. Syst. Des. Eng. 2020, 5, 269–283. [Google Scholar] [CrossRef]
- Rodrigues de Almeida, N.; Han, Y.; Perez, J.; Kirkpatrick, S.; Wang, Y.; Sheridan, M.C. Design, Synthesis, and Nanostructure-Dependent Antibacterial Activity of Cationic Peptide Amphiphiles. ACS Appl. Mater. Interfaces 2019, 11, 2790–2801. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Kramer, J.S.; Smith, J.D.; Allen, B.N.; Leeper, C.N.; Li, X.; Morton, L.D.; Gallazzi, F.; Ulery, B.D. Vaccine Adjuvant Incorporation Strategy Dictates Peptide Amphiphile Micelle Immunostimulatory Capacity. AAPS J. 2018, 20, 73. [Google Scholar] [CrossRef]
- Boato, F.; Thomas, R.M.; Ghasparian, A.; Freund-Renard, A.; Moehle, K.; Robinson, J.A. Synthetic Virus-Like Particles from Self-Assembling Coiled-Coil Lipopeptides and Their Use in Antigen Display to the Immune System. Angew. Chem. Int. Ed. 2007, 46, 9015–9018. [Google Scholar] [CrossRef]
- Lee, K.C.; Carlson, P.A.; Goldstein, A.S.; Yager, P.; Gelb, M.H. Protection of a Decapeptide from Proteolytic Cleavage by Lipidation and Self-Assembly into High-Axial-Ratio Microstructures: A Kinetic and Structural Study. Langmuir 1999, 15, 5500–5508. [Google Scholar] [CrossRef]
- Missirlis, D.; Khant, H.; Tirrell, M. Mechanisms of peptide amphiphile internalization by SJSA-1 Cells in Vitro. Biochemistry 2009, 48, 3304–3314. [Google Scholar] [CrossRef]
- Henninot, A.; Collins, J.C.; Nuss, J.M. The Current State of Peptide Drug Discovery: Back to the Future? J. Med. Chem. 2018, 61, 1382–1414. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Onyuksel, H. Peptide delivery using phospholipid micelles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012, 4, 562–574. [Google Scholar] [CrossRef]
- Hald Albertsen, C.; Kulkarni, J.A.; Witzigmann, D.; Lind, M.; Petersson, K.; Simonsen, J.B. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv. Drug Deliv. Rev. 2022, 188, 114416. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.C.; Ulery, B.D.; Trent, A.; Liang, S.; David, N.A.; Tirrell, M.V. Modular Peptide Amphiphile Micelles Improving an Antibody-Mediated Immune Response to Group A Streptococcus. ACS Biomater. Sci. Eng. 2017, 3, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Smith, J.D.; Allen, B.N.; Kramer, J.S.; Schauflinger, M.; Ulery, B.D. Peptide Amphiphile Micelle Vaccine Size and Charge Influence the Host Antibody Response. ACS Biomater. Sci. Eng. 2018, 4, 2463–2472. [Google Scholar] [CrossRef]
- Schulte, M.C.; Boll, A.C.; Barcellona, A.T.; Lopez, E.A.; Schrum, A.G.; Ulery, B.D. Peptide Antigen Modifications Influence the On-Target and Off-Target Antibody Response for an Influenza Subunit Vaccine. Vaccines 2025, 13, 51. [Google Scholar] [CrossRef] [PubMed]
- Schulte, M.C.; Barcellona, A.T.; Wang, X.; Schrum, A.G.; Ulery, B.D. M2e-Derived Peptidyl and Peptide Amphiphile Micelles as Novel Influenza Vaccines. Pharmaceuticals 2024, 17, 1503. [Google Scholar] [CrossRef]
- Tai, W.; Chen, J.; Zhao, G.; Geng, Q.; He, L.; Chen, Y.; Zhou, Y.; Li, F.; Du, L. Rational Design of Zika Virus Subunit Vaccine with Enhanced Efficacy. J. Virol. 2019, 93, e02187-18. [Google Scholar] [CrossRef]
- Duan, H.; Chen, X.; Boyington, J.C.; Cheng, C.; Zhang, Y.; Jafari, A.J.; Stephens, T.; Tsybovsky, Y.; Kalyuzhniy, O.; Zhao, P.; et al. Glycan Masking Focuses Immune Responses to the HIV-1 CD4-Binding Site and Enhances Elicitation of VRC01-Class Precursor Antibodies. Immunity 2018, 49, 301–311.e5. [Google Scholar] [CrossRef]
- Deupi, X.; Olivella, M.; Govaerts, C.; Ballesteros, J.A.; Campillo, M.; Pardo, L. Ser and Thr Residues Modulate the Conformation of Pro-Kinked Transmembrane α-Helices. Biophys. J. 2004, 86, 105–115. [Google Scholar] [CrossRef]
- Lang, E.; Otvos, L. A serine → proline change in the Alzheimer’s disease-associated epitope tau 2 results in altered secondary structure, but phosphorylation overcomes the conformational GAP. Biochem. Biophys. Res. Commun. 1992, 188, 162–169. [Google Scholar] [CrossRef]
- Abe, K.; Higashi, K.; Watabe, K.; Kobayashi, A.; Limwikrant, W.; Yamamoto, K.; Moribe, K. Effects of the PEG molecular weight of a PEG-lipid and cholesterol on PEG chain flexibility on liposome surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2015, 474, 63–70. [Google Scholar] [CrossRef]
- Uray, K.; Kajtár, J.; Vass, E.; Price, M.R.; Hollósi, M.; Hudecz, F. Effect of D-amino acid substitution in a mucin 2 epitope on mucin- specific monoclonal antibody recognition. Arch. Biochem. Biophys. 2000, 378, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Di Grazia, A.; Cappiello, F.; Cohen, H.; Casciaro, B.; Luca, V.; Pini, A.; Di, Y.P.; Shai, Y.; Mangoni, M.L. D-Amino acids incorporation in the frog skin-derived peptide esculentin-1a(1-21)NH2 is beneficial for its multiple functions. Amino Acids 2015, 47, 2505–2519. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.Y.; Oh, J.E.; Lee, K.H. Effect of D-amino acid substitution on the stability, the secondary structure, and the activity of membrane-active peptide. Biochem. Pharmacol. 1999, 58, 1775–1780. [Google Scholar] [CrossRef]
- Lee, D.L.; Powers, J.P.S.; Pflegerl, K.; Vasil, M.L.; Hancock, R.E.W.; Hodges, R.S. Effects of single d-amino acid substitutions on disruption of β-sheet structure and hydrophobicity in cyclic 14-residue antimicrobial peptide analogs related to gramicidin S. J. Pept. Res. 2004, 63, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Rothemund, S.; Beyermann, M.; Krause, E.; Krause, G.; Bienert, M.; Hodges, R.S.; Sykes, B.D.; Sönnichsen, F.D. Structure Effects of Double d-Amino Acid Replacements: A Nuclear Magnetic Resonance and Circular Dichroism Study Using Amphipathic Model Helices. Biochemistry 1995, 34, 12954–12962. [Google Scholar] [CrossRef]
- Moyle, P.M.; Dai, W.; Zhang, Y.; Batzloff, M.R.; Good, M.F.; Toth, I. Site-Specific Incorporation of Three Toll-Like Receptor 2 Targeting Adjuvants into Semisynthetic, Molecularly Defined Nanoparticles: Application to Group A Streptococcal Vaccines. Bioconjug. Chem. 2014, 25, 965–978. [Google Scholar] [CrossRef]
- Moyle, P.M.; Dai, W.; Liu, T.Y.; Hussein, W.M.; Maruthayanar, P.; Wells, J.W.; McMillan, N.A.J.; Skwarczynski, M.; Toth, I. Combined synthetic and recombinant techniques for the development of lipoprotein-based, self-adjuvanting vaccines targeting human papillomavirus type-16 associated tumors. Bioorg. Med. Chem. Lett. 2015, 25, 5570–5575. [Google Scholar] [CrossRef]
- Tan, A.C.L.; Eriksson, E.M.Y.; Kedzierska, K.; Deliyannis, G.; Valkenburg, S.A.; Zeng, W.; Jackson, D.C. Polyfunctional CD8 + T cells are associated with the vaccination-induced control of a novel recombinant influenza virus expressing an HCV epitope. Antivir. Res. 2012, 94, 168–178. [Google Scholar] [CrossRef]
- Zeng, W.; Ghosh, S.; Lau, Y.F.; Brown, L.E.; Jackson, D.C. Highly Immunogenic and Totally Synthetic Lipopeptides as Self-Adjuvanting Immunocontraceptive Vaccines1. J. Immunol. 2002, 169, 4905–4912. [Google Scholar] [CrossRef]
- Ng, W.C.; Gilbertson, B.; Lim, B.; Zeng, W.; Jackson, D.C.; Brown, L.E. Lipopeptide vaccines illustrate the potential role of subtype-crossreactive T cells in the control of highly virulent influenza. Influenza Other Respir. Viruses 2009, 3, 177–182. [Google Scholar] [CrossRef]
- Zeng, W.; Tan, A.C.L.; Horrocks, K.; Jackson, D.C. A lipidated form of the extracellular domain of influenza M2 protein as a self-adjuvanting vaccine candidate. Vaccine 2015, 33, 3526–3532. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.Y.; Nan, X.; Jin, M.S.; Youn, S.J.; Ryu, Y.H.; Mah, S.; Han, S.H.; Lee, H.; Paik, S.G.; Lee, J.O. Recognition of Lipopeptide Patterns by Toll-like Receptor 2-Toll-like Receptor 6 Heterodimer. Immunity 2009, 31, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Diehl, K.H.; Hull, R.; Morton, D.; Pfister, R.; Rabemampianina, Y.; Smith, D.; Vidal, J.M.; Vorstenbosch, C.V.D. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J. Appl. Toxicol. 2001, 21, 15–23. [Google Scholar] [CrossRef]
- Petersen, L.K.; Ramer-Tait, A.E.; Broderick, S.R.; Kong, C.S.; Ulery, B.D.; Rajan, K.; Wannemuehler, M.J.; Narasimhan, B. Activation of innate immune responses in a pathogen-mimicking manner by amphiphilic polyanhydride nanoparticle adjuvants. Biomaterials 2011, 32, 6815–6822. [Google Scholar] [CrossRef] [PubMed]
- Black, M.; Trent, A.; Kostenko, Y.; Lee, J.S.; Olive, C.; Tirrell, M. Self-assembled peptide amphiphile micelles containing a cytotoxic T-cell epitope promote a protective immune response in vivo. Adv. Mater. 2012, 24, 3845–3849. [Google Scholar] [CrossRef]
- Akazawa, T.; Ohashi, T.; Nakajima, H.; Nishizawa, Y.; Kodama, K.; Sugiura, K.; Inaba, T.; Inoue, N. Development of a dendritic cell-targeting lipopeptide as an immunoadjuvant that inhibits tumor growth without inducing local inflammation. Int. J. Cancer 2014, 135, 2847–2856. [Google Scholar] [CrossRef]
- Ansari, H.R.; Raghava, G.P. Identification of conformational B-cell Epitopes in an antigen from its primary sequence. Immunome Res. 2010, 6, 6. [Google Scholar] [CrossRef]
- Jung, Y.; Kang, H.J.; Lee, J.M.; Jung, S.O.; Yun, W.S.; Chung, S.J.; Chung, B.H. Controlled antibody immobilization onto immunoanalytical platforms by synthetic peptide. Anal. Biochem. 2008, 374, 99–105. [Google Scholar] [CrossRef]
- Danczyk, R.; Krieder, B.; North, A.; Webster, T.; HogenEsch, H.; Rundell, A. Comparison of antibody functionality using different immobilization methods. Biotechnol. Bioeng. 2003, 84, 215–223. [Google Scholar] [CrossRef]
- Fu, J.; Reinhold, J.; Woodbury, N.W. Peptide-Modified Surfaces for Enzyme Immobilization. PLoS ONE 2011, 6, e18692. [Google Scholar] [CrossRef]
- North, S.H.; Wojciechowski, J.; Chu, V.; Taitt, C.R. Surface immobilization chemistry influences peptide-based detection of lipopolysaccharide and lipoteichoic acid. J. Pept. Sci. 2012, 18, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.E.; Ni, L.; Brown, W.R.; Joshi, K.S.; Chang, J.; Rosenberg, B.; Voss, E.W. The immunochemistry of sandwich elisas—VI. Greater than 90% of monoclonal and 75% of polyclonal anti-fluorescyl capture antibodies (CAbs) are denatured by passive adsorption. Mol. Immunol. 1993, 30, 1165–1175. [Google Scholar] [CrossRef]
- Zhang, R.; Morton, L.; Smith, J.; Gallazzi, F.; White, T.; Ulery, B. Instructive Design of Triblock Peptide Amphiphiles for Structurally Complex Micelle Fabrication. ACS Biomater. Sci. Eng. 2018, 4, 2330–2339. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, R.; Lindaman, B.D.; Leeper, C.N.; Schrum, A.G.; Ulery, B.D. Vasoactive Intestinal Peptide Amphiphile Micelle Chemical Structure and Hydrophobic Domain Influence Immunomodulatory Potentiation. ACS Appl. Bio Mater. 2022, 5, 1464–1475. [Google Scholar] [CrossRef]
- Hamley, I.W.; Kirkham, S.; Dehsorkhi, A.; Castelletto, V.; Reza, M.; Ruokolainen, J. Toll-like receptor agonist lipopeptides self-assemble into distinct nanostructures. Chem. Commun. 2014, 50, 15948–15951. [Google Scholar] [CrossRef] [PubMed]
- Dharmayanti, C.; Clulow, A.J.; Gillam, T.A.; Klingler-Hoffmann, M.; Albrecht, H.; Blencowe, A. Position Matters: Pyridine Regioisomers Influence Secondary Structure and Micelle Morphology in Polymer-Homopolypeptide Micelles. Biomacromolecules 2024, 25, 4095–4109. [Google Scholar] [CrossRef]
- Azuma, M.; Sawahata, R.; Akao, Y.; Ebihara, T.; Yamazaki, S.; Matsumoto, M.; Hashimoto, M.; Fukase, K.; Fujimoto, Y.; Seya, T. The Peptide Sequence of Diacyl Lipopeptides Determines Dendritic Cell TLR2-Mediated NK Activation. PLoS ONE 2010, 5, e12550. [Google Scholar] [CrossRef]
- Takeda, Y.; Azuma, M.; Hatsugai, R.; Fujimoto, Y.; Hashimoto, M.; Fukase, K.; Matsumoto, M.; Seya, T. The second and third amino acids of Pam2 lipopeptides are key for the proliferation of cytotoxic T cells. Innate Immun. 2018, 24, 323–331. [Google Scholar] [CrossRef]
- Dearman, R.J.; Cumberbatch, M.; Maxwell, G.; Basketter, D.A.; Kimber, I. Toll-like receptor ligand activation of murine bone marrow-derived dendritic cells. Immunology 2009, 126, 475–484. [Google Scholar] [CrossRef]
- Zaman, M.; Toth, I. Immunostimulation by Synthetic Lipopeptide-Based Vaccine Candidates: Structure-Activity Relationships. Front. Immunol. 2013, 4, 318. [Google Scholar] [CrossRef]
- Buwitt-Beckmann, U.; Heine, H.; Wiesmüller, K.H.; Jung, G.; Brock, R.; Akira, S.; Ulmer, A.J. Toll-like receptor 6-independent signaling by diacylated lipopeptides. Eur. J. Immunol. 2005, 35, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Deutschmann, C.; Roggenbuck, D.; Schierack, P.; Rödiger, S. Autoantibody testing by enzyme-linked immunosorbent assay-a case in which the solid phase decides on success and failure. Heliyon 2020, 6, e03270. [Google Scholar] [CrossRef] [PubMed]
- Chamczuk, A.J.; Ursell, M.; O’Connor, P.; Jackowski, G.; Moscarello, M.A. A rapid ELISA-based serum assay for myelin basic protein in multiple sclerosis. J. Immunol. Methods 2002, 262, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Pandolfi, R.; Ramos de Almeida, D.; Alves Pinto, M.; Kreutz, L.C.; Frandoloso, R. In house ELISA based on recombinant ORF2 protein underline high prevalence of IgG anti-hepatitis E virus amongst blood donors in south Brazil. PLoS ONE 2017, 12, e0176409. [Google Scholar] [CrossRef]



| Abbreviation | Peptide/PA | % Acetonitrile at Elution |
|---|---|---|
| Orig PM | M22–16 | 35% |
| Orig PAM | Palm2K-M22–16-(KE)4 | 65% |
| M210–16-(KE)4 | 25% | |
| (KE)4 | 5% | |
| PP PAM | Palm2K-PP-M22–16-PP-(KE)4 | 65% |
| ke PAM | Palm2K-PEG2-M22–16-(ke)2(KE)2 | 65% |
| PEG2 PAM | Palm2K-PEG2-M22–16-PEG2-(KE)4 | 70% |
| P2CS PAM | Pam2CS-M22–16-PEG2-(KE)4 | 65% |
| M21–24 | 35% | |
| Biotin-PEG2-M22–16 | 30% | |
| Biotin-PEG2-M22–16-PEG2-(KE)4 | 30% | |
| Palm-RDRD-M22–16 | 40% |
| Experimental Group | Treatment |
|---|---|
| No Treatment | n/a |
| Adj | 0.2 μM Pam2CSK4 |
| Orig PAM | 1.8 μM Palm2K-M22–16-(KE)4 |
| Orig PAM/Adj | 1.8 μM Palm2K-M22–16-(KE)4 and 0.2 μM Pam2CSK4 |
| PP PAM | 1.8 μM Palm2K-PP-M22–16-PP-(KE)4 |
| ke PAM | 1.8 μM Palm2K-PEG2-M22–16-(ke)2(KE)2 |
| PEG2 PAM | 1.8 μM Palm2K-PEG2-M22–16-PEG2-(KE)4 |
| P2CS PAM | 1.8 μM Pam2CS-M22–16-PEG2-(KE)4 |
| PEG2 PAM/P2CS PAM | 1.8 μM Palm2K-PEG2-M22–16-PEG2-(KE)4 and 0.2 μM Pam2CS-M22–16-PEG2-(KE)4 |
| Experimental Group | Treatment |
|---|---|
| PBS | n/a |
| Orig PM | 20 nmol M22–16 |
| Orig PM/Adj | 20 nmol M22–16 and 2.22 nmol Pam2CSK4 |
| Orig PAM | 20 nmol Palm2K-M22–16-(KE)4 |
| Orig PAM/Adj | 20 nmol Palm2K-M22–16-(KE)4 and 2.22 nmol Pam2CSK4 |
| PP PAM | 20 nmol Palm2K-PP-M22–16-PP-(KE)4 |
| ke PAM | 20 nmol Palm2K-PEG2-M22–16-(ke)2(KE)2 |
| PEG2 PAM | 20 nmol Palm2K-PEG2-M22–16-PEG2-(KE)4 |
| P2CS PAM | 20 nmol Pam2CS-M22–16-PEG2-(KE)4 |
| PEG2 PAM/P2CS PAM | 20 nmol Palm2K-PEG2-M22–16-PEG2-(KE)4 and 2.22 nmol Pam2CS-M22–16-PEG2-(KE)4 |
| Abbreviation | Peptide/PA |
|---|---|
| Original PAM formulation: | |
| Orig PAM | Palm2K-M22–16-(KE)4 |
| New formulations: | |
| PP PAM | Palm2K-PP-M22–16-PP-(KE)4 |
| ke PAM | Palm2K-PEG2-M22–16-(ke)2(KE)2 |
| PEG2 PAM | Palm2K-PEG2-M22–16-PEG2-(KE)4 |
| P2CS PAM | Pam2CS-M22–16-PEG2-(KE)4 |
| Formulation | Average CMC (μM) ± 1 Standard Deviation |
|---|---|
| Orig PM [15] | 2.70 ± 1.60 |
| Orig PAM [15] | 0.15 ± 0.06 |
| PP PAM | 0.47 ± 0.14 |
| ke PAM | 0.07 ± 0.05 |
| PEG2 PAM | 0.14 ± 0.06 |
| P2CS PAM | 0.76 ± 0.20 |
| Formulation | Maximum Diameter (nm) | Minimum Diameter (nm) | Aspect Ratio |
|---|---|---|---|
| PP PAM | 24 ± 17 | 13 ± 7 | 2.1 ± 1.1 |
| ke PAM | 22 ± 18 | 12 ± 9 | 1.9 ± 1.0 |
| PEG2 PAM | 22 ± 19 | 12 ± 8 | 1.9 ± 0.9 |
| P2CS PAM | 11 ± 4 | 8 ± 2 | 1.3 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulte, M.C.; Boll, A.C.; Conomos, N.L.; Rezaei, F.; Barcellona, A.T.; Schrum, A.G.; Ulery, B.D. Adjuvant Templating Improves On-Target/Off-Target Antibody Ratio Better than Linker Addition for M2-Derived Peptide Amphiphile Micelle Vaccines. Vaccines 2025, 13, 422. https://doi.org/10.3390/vaccines13040422
Schulte MC, Boll AC, Conomos NL, Rezaei F, Barcellona AT, Schrum AG, Ulery BD. Adjuvant Templating Improves On-Target/Off-Target Antibody Ratio Better than Linker Addition for M2-Derived Peptide Amphiphile Micelle Vaccines. Vaccines. 2025; 13(4):422. https://doi.org/10.3390/vaccines13040422
Chicago/Turabian StyleSchulte, Megan C., Adam C. Boll, Natalie L. Conomos, Farnoushsadat Rezaei, Agustin T. Barcellona, Adam G. Schrum, and Bret D. Ulery. 2025. "Adjuvant Templating Improves On-Target/Off-Target Antibody Ratio Better than Linker Addition for M2-Derived Peptide Amphiphile Micelle Vaccines" Vaccines 13, no. 4: 422. https://doi.org/10.3390/vaccines13040422
APA StyleSchulte, M. C., Boll, A. C., Conomos, N. L., Rezaei, F., Barcellona, A. T., Schrum, A. G., & Ulery, B. D. (2025). Adjuvant Templating Improves On-Target/Off-Target Antibody Ratio Better than Linker Addition for M2-Derived Peptide Amphiphile Micelle Vaccines. Vaccines, 13(4), 422. https://doi.org/10.3390/vaccines13040422







