Viral Infections in Elderly Individuals: A Comprehensive Overview of SARS-CoV-2 and Influenza Susceptibility, Pathogenesis, and Clinical Treatment Strategies
Abstract
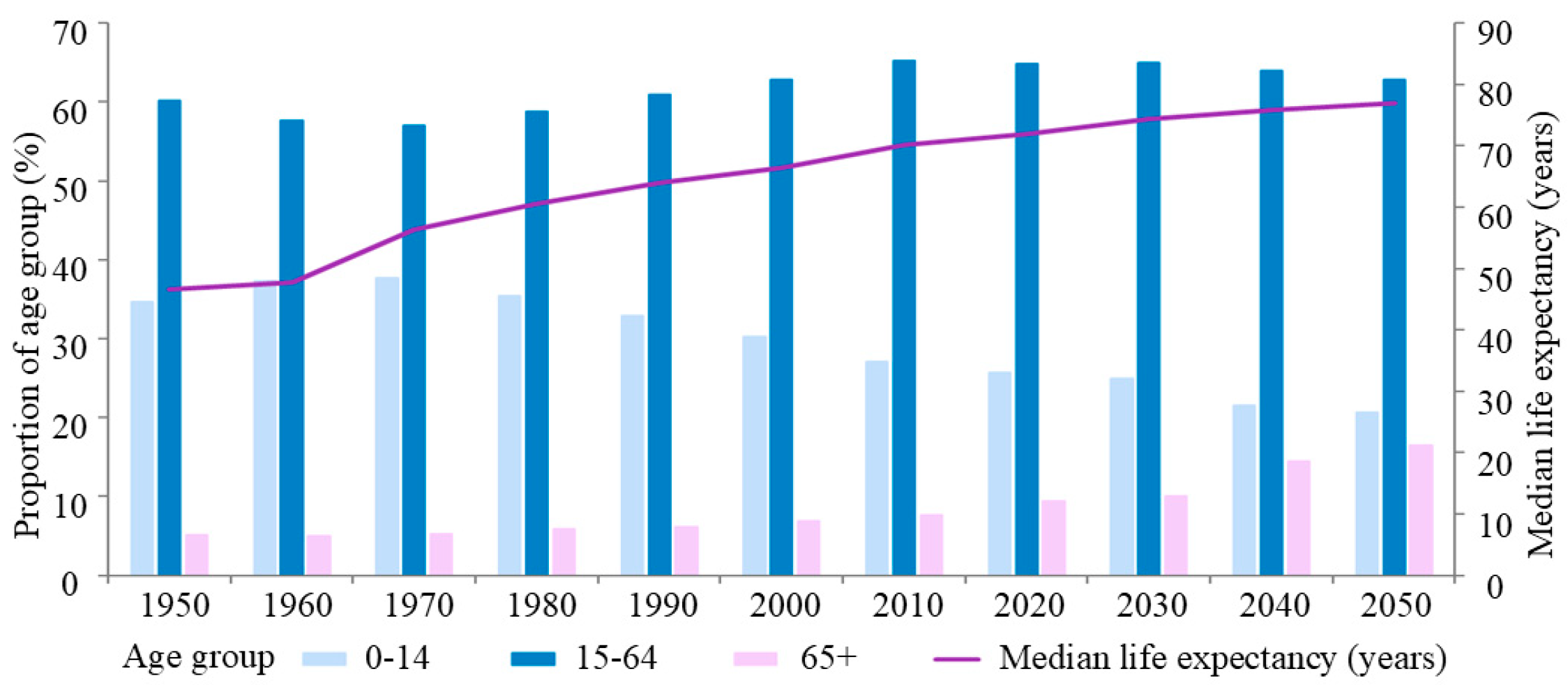
:1. Introduction
2. Results
2.1. Research Strategy
2.2. Eligibility Criteria
2.3. Study Selection
3. Epidemiological Features and Coinfection Mechanisms of SARS-CoV-2 and Influenza
3.1. Influenza Virus
Epidemiological Features of Influenza Viruses
3.2. SARS-CoV-2
Epidemiological Features of SARS-CoV-2
3.3. Coinfection with SARS-CoV-2 and Influenza Virus
4. Mechanisms That Increase the Susceptibility to and Severity of Viral Diseases in Older Adults
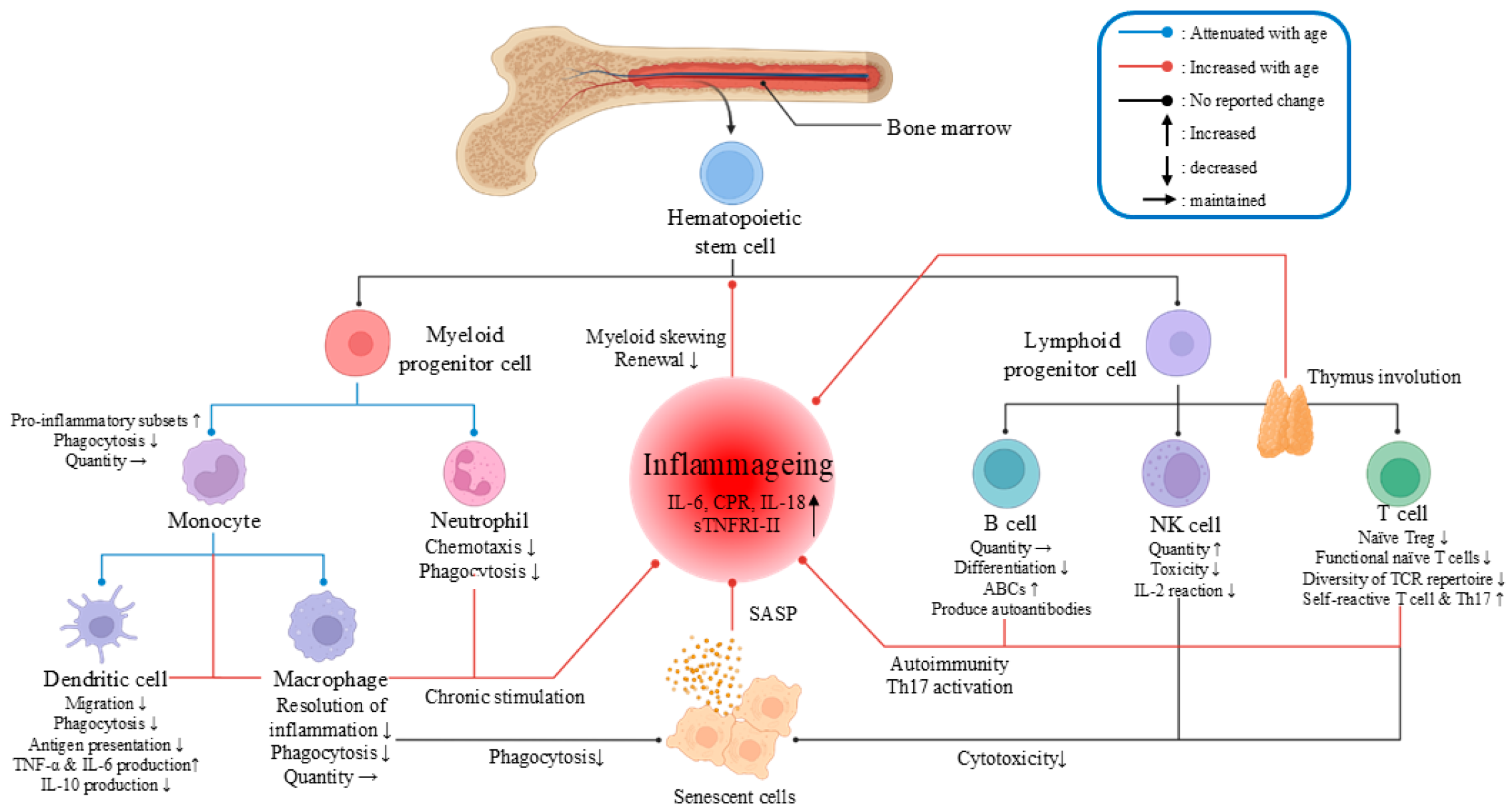
4.1. Inflammaging and Immunosenescence Increase Morbidity and Mortality
4.1.1. Viral Infections May Exacerbate the Pre-Existing Inflammatory State in Elderly Individuals
4.1.2. Immunosenescence Increases Susceptibility to and Mortality Rates from Viral Infections
4.1.3. Aging Weakens the Innate Immune Response, Increasing Viral Susceptibility
4.1.4. Aging Weakens the Adaptive Immune Response, Increasing Viral Susceptibility
4.2. Aging Leads to Increased Viral Load in Older Patients
4.2.1. Aging Contributes to Obesity, Which Increases the Viral Load
4.2.2. Aging Increases the Gene Expression of Virus-Specific Receptors
4.3. Aging Increases Severity Due to Frailty and Chronic Diseases
5. Potential Clinical Manifestations and Complications of SARS-CoV-2 and Influenza Coinfection in Elderly Individuals
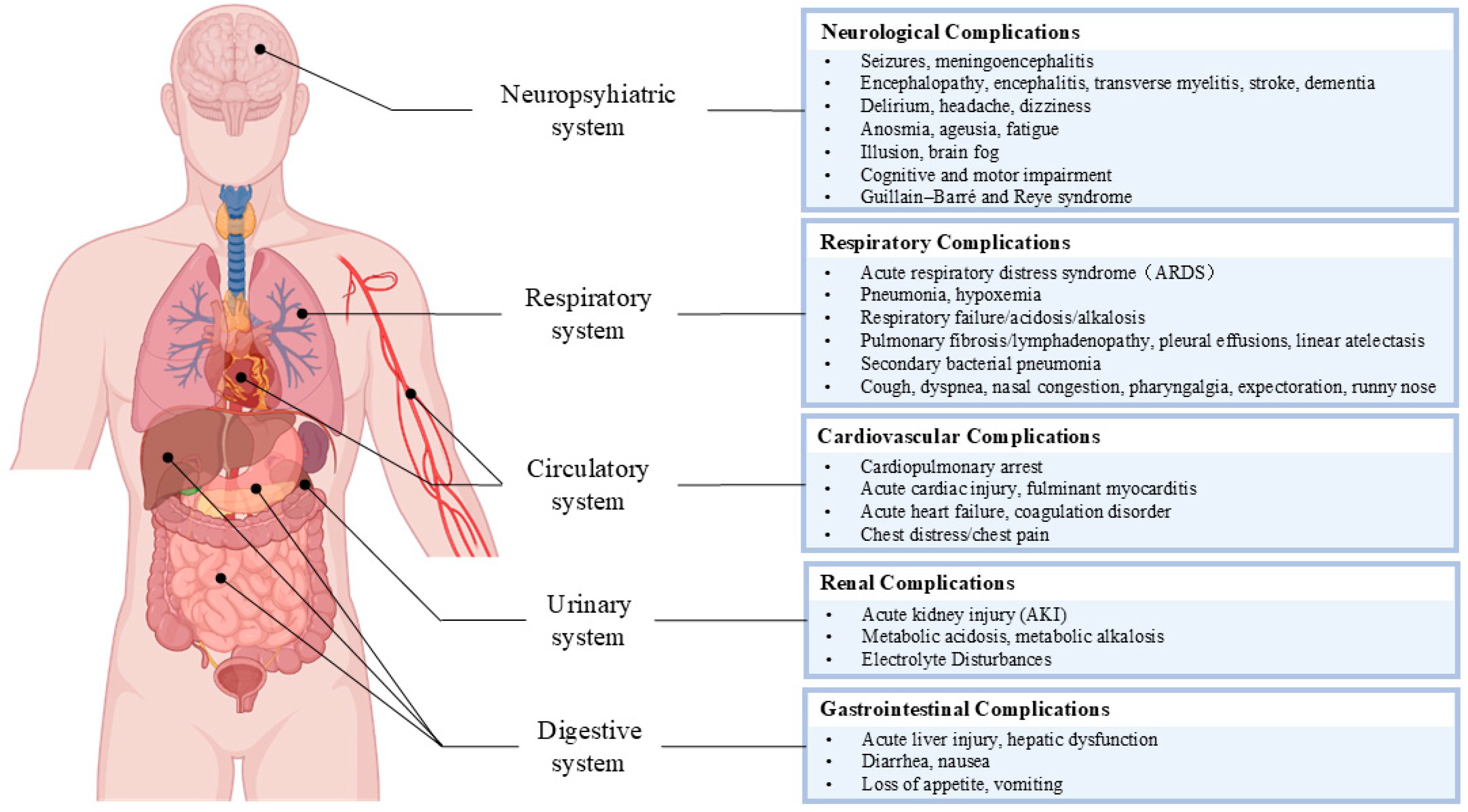
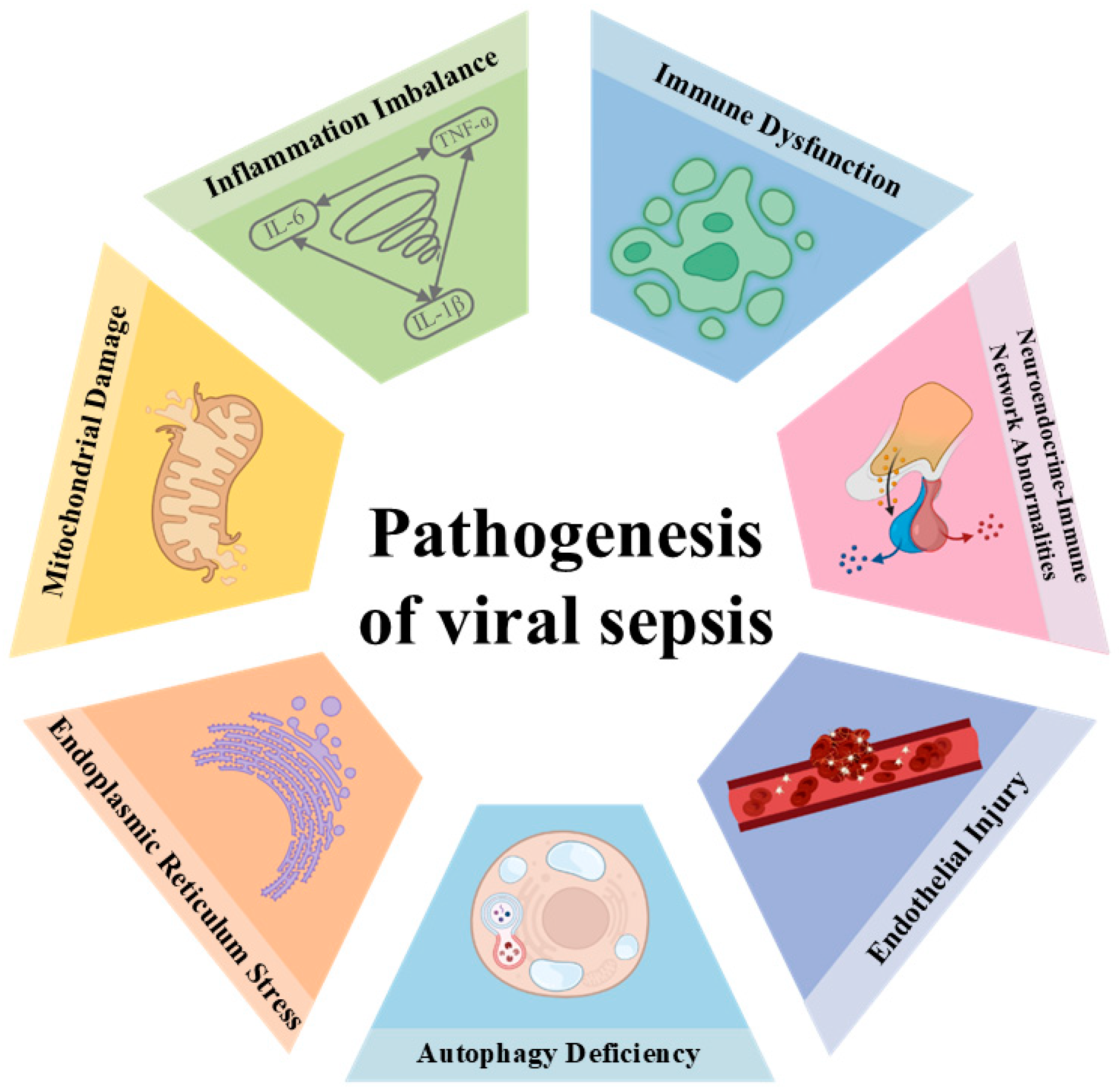
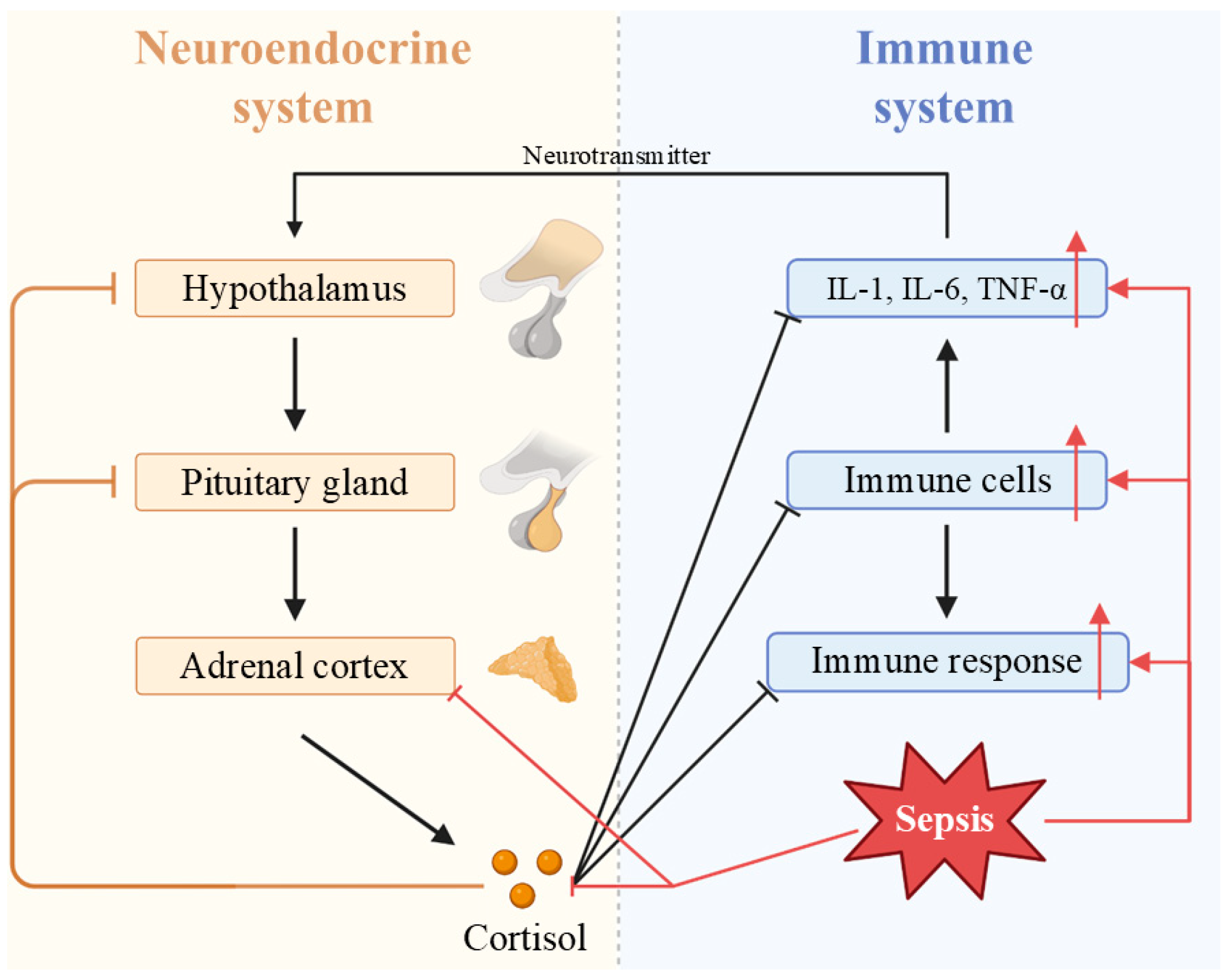
5.1. Viral Sepsis Involves Multiorgan Dysfunction
5.2. Clinical Manifestations of Viral Coinfection in the Neuropsychiatric System
5.3. Clinical Manifestations of Viral Coinfection in the Respiratory System
5.4. Clinical Manifestations of Viral Coinfection in the Cardiovascular System
5.5. Clinical Manifestations of Viral Coinfection in the Urinary System
5.6. Clinical Manifestations of Viral Coinfection in the Digestive System
6. Prevention, Diagnosis, and Treatment Recommendations for SARS-CoV-2 and Influenza Coinfections
6.1. Prevention
6.2. Diagnosis
6.3. Treatment
6.4. Conclusions
7. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| CFR | case fatality ratio |
| IAV | influenza A virus |
| IBV | influenza B virus |
| HA | hemagglutinin |
| NA | neuraminidase |
| SASP | senescence-associated secretory phenotype |
| HSCs | hematopoietic stem cells |
| ICU | intensive care unit |
| ETC | electron transport chain |
| HPA | hypothalamic–pituitary–adrenal |
| HSCs | hematopoietic stem cells |
| ICU | intensive care unit |
| ETC | electron transport chain |
| HPA | hypothalamic–pituitary–adrenal |
| TF | tissue factor |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| GGT | gamma glutamyl transferase |
| LDH | lactate dehydrogenase |
| COVID-19 | Coronavirus Disease 2019 |
| VOCs | variants of concern |
| ACE2 | angiotensin-converting enzyme 2 |
| RBD | receptor-binding domain |
| SARS | severe acute respiratory syndrome |
| RAS | renin–angiotensin System |
| ARDS | acute respiratory distress syndrome |
| AKI | acute kidney injury |
| Ang II | angiotensin II |
| vWF | von Willebrand factor |
| WHO | World Health Organization |
| NPIs | nonpharmaceutical interventions |
| PCR | polymerase chain reaction |
| Ct | cycle threshold |
| IL-1 | interleukin-1 |
| IL-6 | interleukin-6 |
| TNF-α | tumor necrosis factor-alpha |
| ER | endoplasmic reticulum |
| PERK | protein kinase R-like endoplasmic reticulum kinase |
| eIF2α | eukaryotic initiation factor 2 alpha |
| ATF-4 | activating transcription factor 4 |
| ATF-6 | activating transcription factor 6 |
| CHOP | C/EBP homologous protein |
| S1P | serineproteasesite-1 |
| S2P | serine 534 proteasesite-2 |
| IRE1 | inositol-requiringenzyme1 |
| JNK | c-Jun N-terminal kinase |
| BBB | blood–brain barrier |
| CK | creatine kinase |
| CK-MB | creatine kinase isoenzyme MB |
| cTnI | cardiac troponin I |
| BNP | brain natriuretic peptide |
| PT | prothrombin time |
| APTT | activated partial thromboplastin time |
| ACS | acute coronary syndrome |
| MMP-13 | matrix metalloproteinase-13 |
| DCs | dendritic cells |
| APCs | antigen-presenting cells |
| MHC-II | MHC class II molecules |
| NK | natural killer |
| ABCs | age-associated B cells |
| TCR | T cell receptor |
| Treg | regulatory T cell |
| Teff | effector T cell |
| Th17 | T helper17 |
| BMI | body mass index |
| CARS | anti-inflammatory response syndrome |
| UPR | Unfolded Protein Response |
| CNS | central nervous system |
| VLP | virus-like particle |
| M2e | matrix 2 ectodomain of the influenza virus |
References
- Hua, K.; Pan, Y.; Fang, J.; Wu, H.; Hua, Y. Integrating social, climate and environmental changes to confront accelerating global aging. BMC Public Health 2024, 24, 2838. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.F.; Xie, C.; Schenkel, J.A.; Wu, C.; Long, Q.; Cui, H.; Aman, Y.; Frank, J.; Liao, J.; Zou, H.; et al. A research agenda for ageing in China in the 21st century (2nd edition): Focusing on basic and translational research, long-term care, policy and social networks. Ageing Res. Rev. 2020, 64, 101174. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Klein, S.L.; Garibaldi, B.T.; Li, H.; Wu, C.; Osevala, N.M.; Li, T.; Margolick, J.B.; Pawelec, G.; Leng, S.X. Aging in COVID-19: Vulnerability, immunity and intervention. Ageing Res. Rev. 2021, 65, 101205. [Google Scholar] [CrossRef] [PubMed]
- Uyeki, T.M.; Hui, D.S.; Zambon, M.; Wentworth, D.E.; Monto, A.S. Influenza. Lancet 2022, 400, 693–706. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; Wang, X.; Yu, H. Influenza-associated disease burden in mainland China: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 2886. [Google Scholar] [CrossRef]
- Fahim, M.; Roshdy, W.H.; Deghedy, O.; Kamel, R.; Naguib, A.; Showky, S.; Elguindy, N.; Abdel Fattah, M.; Afifi, S.; Mohsen, A.; et al. Epidemiology, Disease Severity and Outcome of Severe Acute Respiratory Syndrome Coronavirus 2 and Influenza Viruses Coinfection Seen at Egypt Integrated Acute Respiratory Infections Surveillance, 2020–2022. Can. J. Infect. Dis. Med. Microbiol. = J. Can. Des Mal. Infect. Microbiol. Medicale 2022, 2022, 7497500. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, F.; Wu, K.; Wang, J.; Li, F.; Huang, S.; Lu, J.; Huang, J.; Liu, H.; Zhou, R.; et al. Clinical and virological impact of single and dual infections with influenza A (H1N1) and SARS-CoV-2 in adult inpatients. PLoS Negl. Trop. Dis. 2021, 15, e0009997. [Google Scholar] [CrossRef]
- Bai, L.; Zhao, Y.; Dong, J.; Liang, S.; Guo, M.; Liu, X.; Wang, X.; Huang, Z.; Sun, X.; Zhang, Z.; et al. Coinfection with influenza A virus enhances SARS-CoV-2 infectivity. Cell Res. 2021, 31, 395–403. [Google Scholar] [CrossRef]
- Xie, Y.; Choi, T.; Al-Aly, Z. Long-term outcomes following hospital admission for COVID-19 versus seasonal influenza: A cohort study. Lancet. Infect. Dis. 2024, 24, 239–255. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, F.; Roy, A.; Yang, J.; Luo, W.; Shen, X.; Irwin, D.M.; Chen, R.A.; Shen, Y. Evolutionary perspectives and adaptation dynamics of human seasonal influenza viruses from 2009 to 2019: An insight from codon usage. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2021, 96, 105067. [Google Scholar] [CrossRef]
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef]
- Aufi, I.M.; Khudhair, A.M.; Ghaeb Al-Saadi, L.; Almoneem Ahmed, M.A.; Mahdi Shukur, F.M. Epidemiology and Molecular Characterization of Seasonal Influenza Viruses in Iraq. Arch. Razi Inst. 2021, 76, 871–877. [Google Scholar] [CrossRef]
- Uyeki, T.M. Influenza. Ann. Intern. Med. 2021, 174, Itc161–Itc176. [Google Scholar] [CrossRef]
- Virk, R.K.; Jayakumar, J.; Mendenhall, I.H.; Moorthy, M.; Lam, P.; Linster, M.; Lim, J.; Lin, C.; Oon, L.L.E.; Lee, H.K.; et al. Divergent evolutionary trajectories of influenza B viruses underlie their contemporaneous epidemic activity. Proc. Natl. Acad. Sci. USA 2020, 117, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tang, F.; Cao, Z.; Zeng, J.; Qiu, Z.; Zhang, C.; Long, H.; Cheng, P.; Sun, Q.; Han, W.; et al. Global pattern and determinant for interaction of seasonal influenza viruses. J. Infect. Public Health 2024, 17, 1086–1094. [Google Scholar] [CrossRef]
- Huang, W.; Li, X.; Tan, M.; Cheng, Y.; Chen, T.; Wei, H.; Zeng, X.; Xie, Y.; Liu, J.; Xiao, N.; et al. Epidemiological and Virological Surveillance of Seasonal Influenza Viruses—China, 2020–2021. China CDC Wkly. 2021, 3, 918–922. [Google Scholar] [CrossRef]
- Lipsitch, M.; Viboud, C. Influenza seasonality: Lifting the fog. Proc. Natl. Acad. Sci. USA 2009, 106, 3645–3646. [Google Scholar] [CrossRef]
- Viboud, C.; Alonso, W.J.; Simonsen, L. Influenza in tropical regions. PLoS Med. 2006, 3, e89. [Google Scholar] [CrossRef]
- Azziz Baumgartner, E.; Dao, C.N.; Nasreen, S.; Bhuiyan, M.U.; Mah, E.M.S.; Al Mamun, A.; Sharker, M.A.; Zaman, R.U.; Cheng, P.Y.; Klimov, A.I.; et al. Seasonality, timing, and climate drivers of influenza activity worldwide. J. Infect. Dis. 2012, 206, 838–846. [Google Scholar] [CrossRef]
- Zhu, A.Q.; Li, Z.J.; Zhang, H.J. Spatial timing of circulating seasonal influenza A and B viruses in China from 2014 to 2018. Sci. Rep. 2023, 13, 7149. [Google Scholar] [CrossRef]
- Caini, S.; Spreeuwenberg, P.; Kusznierz, G.F.; Rudi, J.M.; Owen, R.; Pennington, K.; Wangchuk, S.; Gyeltshen, S.; Ferreira de Almeida, W.A.; Pessanha Henriques, C.M.; et al. Distribution of influenza virus types by age using case-based global surveillance data from twenty-nine countries, 1999–2014. BMC Infect. Dis. 2018, 18, 269. [Google Scholar] [CrossRef]
- He, X.; He, C.; Hong, W.; Zhang, K.; Wei, X. The challenges of COVID-19 Delta variant: Prevention and vaccine development. MedComm 2021, 2, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.L.; Morris, C.P.; Betz, J.F.; Zhang, Y.; Bollinger, R.; Wang, N.; Thiemann, D.R.; Fall, A.; Eldesouki, R.E.; Norton, J.M.; et al. Impact of Severe Acute Respiratory Syndrome Coronavirus 2 Variants on Inpatient Clinical Outcome. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2023, 76, 1539–1549. [Google Scholar] [CrossRef] [PubMed]
- Alhamlan, F.S.; Al-Qahtani, A.A. SARS-CoV-2 Variants: Genetic Insights, Epidemiological Tracking, and Implications for Vaccine Strategies. Int. J. Mol. Sci. 2025, 26, 1263. [Google Scholar] [CrossRef]
- Rodrigues, E.S.; Slavov, S.N.; de La Roque, D.G.L.; Santos, E.V.; Borges, J.S.; Evaristo, M.; da Costa, P.N.M.; de Matos Maçonetto, J.; Marques, A.A.; Baccarin, A.D.; et al. Epidemiology of the SARS-CoV-2 Omicron Variant Emergence in the Southeast Brazilian Population. Microorganisms 2024, 12, 449. [Google Scholar] [CrossRef] [PubMed]
- Ode, H.; Nakata, Y.; Nagashima, M.; Hayashi, M.; Yamazaki, T.; Asakura, H.; Suzuki, J.; Kubota, M.; Matsuoka, K.; Matsuda, M.; et al. Molecular epidemiological features of SARS-CoV-2 in Japan, 2020–2021. Virus Evol. 2022, 8, veac034. [Google Scholar] [CrossRef]
- Wiemken, T.L.; Khan, F.; Puzniak, L.; Yang, W.; Simmering, J.; Polgreen, P.; Nguyen, J.L.; Jodar, L.; McLaughlin, J.M. Seasonal trends in COVID-19 cases, hospitalizations, and mortality in the United States and Europe. Sci. Rep. 2023, 13, 3886. [Google Scholar] [CrossRef]
- Inaida, S.; Paul, R.E.; Matsuno, S. Viral transmissibility of SARS-CoV-2 accelerates in the winter, similarly to influenza epidemics. Am. J. Infect. Control 2022, 50, 1070–1076. [Google Scholar] [CrossRef]
- Callaway, E. COVID’s future: Mini-waves rather than seasonal surges. Nature 2023, 617, 229–230. [Google Scholar] [CrossRef]
- Ye, Y. China’s rolling COVID waves could hit every six months—Infecting millions. Nature 2023, 618, 442–443. [Google Scholar] [CrossRef]
- Mandal, M.; Mandal, S. Spatiotemporal genome diversity of SARS-CoV-2 in wastewater: A two-year global epidemiological study. Environ. Monit. Assess. 2023, 196, 44. [Google Scholar] [CrossRef] [PubMed]
- Kampenusa, I.; Niedre-Otomere, B.; Trofimova, J.; Pole, I.; Pakarna, G.; Savicka, O.; Nikisins, S. Circulation and Codetections of Influenza Virus, SARS-CoV-2, Respiratory Syncytial Virus, Rhinovirus, Adenovirus, Bocavirus, and Other Respiratory Viruses During 2022-2023 Season in Latvia. Viruses 2024, 16, 1650. [Google Scholar] [CrossRef]
- Smit, A.J.; Fitchett, J.M.; Engelbrecht, F.A.; Scholes, R.J.; Dzhivhuho, G.; Sweijd, N.A. Winter Is Coming: A Southern Hemisphere Perspective of the Environmental Drivers of SARS-CoV-2 and the Potential Seasonality of COVID-19. Int. J. Environ. Res. Public Health 2020, 17, 5634. [Google Scholar] [CrossRef] [PubMed]
- Mai, K.L.; Pan, W.Q.; Lin, Z.S.; Wang, Y.; Yang, Z.F. Pathogenesis of influenza and SARS-CoV-2 co-infection at the extremes of age: Decipher the ominous tales of immune vulnerability. Adv. Biotechnol. 2025, 3, 5. [Google Scholar] [CrossRef]
- Yue, H.; Zhang, M.; Xing, L.; Wang, K.; Rao, X.; Liu, H.; Tian, J.; Zhou, P.; Deng, Y.; Shang, J. The epidemiology and clinical characteristics of co-infection of SARS-CoV-2 and influenza viruses in patients during COVID-19 outbreak. J. Med. Virol. 2020, 92, 2870–2873. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Ma, J.; Wang, H.; Wang, X.; Hu, Z.; Li, H.; Zhang, H.; Liu, X. Co-infection of influenza A virus and SARS-CoV-2: A retrospective cohort study. J. Med. Virol. 2021, 93, 2947–2954. [Google Scholar] [CrossRef]
- Hashemi, S.A.; Safamanesh, S.; Ghasemzadeh-Moghaddam, H.; Ghafouri, M.; Azimian, A. High prevalence of SARS-CoV-2 and influenza A virus (H1N1) coinfection in dead patients in Northeastern Iran. J. Med. Virol. 2021, 93, 1008–1012. [Google Scholar] [CrossRef]
- Yan, X.; Li, K.; Lei, Z.; Luo, J.; Wang, Q.; Wei, S. Prevalence and associated outcomes of coinfection between SARS-CoV-2 and influenza: A systematic review and meta-analysis. Int. J. Infect. Dis. 2023, 136, 29–36. [Google Scholar] [CrossRef]
- Cong, B.; Deng, S.; Wang, X.; Li, Y. The role of respiratory co-infection with influenza or respiratory syncytial virus in the clinical severity of COVID-19 patients: A systematic review and meta-analysis. J. Glob. Health 2022, 12, 05040. [Google Scholar] [CrossRef]
- Varshney, K.; Pillay, P.; Mustafa, A.D.; Shen, D.; Adalbert, J.R.; Mahmood, M.Q. A systematic review of the clinical characteristics of influenza-COVID-19 co-infection. Clin. Exp. Med. 2023, 23, 3265–3275. [Google Scholar] [CrossRef]
- Jeong, S.; Lee, N.; Park, Y.; Kim, J.; Jeon, K.; Park, M.J.; Song, W. Prevalence and Clinical Impact of Coinfection in Patients with Coronavirus Disease 2019 in Korea. Viruses 2022, 14, 446. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.H.; Ragusa, M.; Tortosa, F.; Torres, A.; Gresh, L.; Méndez-Rico, J.A.; Alvarez-Moreno, C.A.; Lisboa, T.C.; Valderrama-Beltrán, S.L.; Aldighieri, S.; et al. Viral reactivations and co-infections in COVID-19 patients: A systematic review. BMC Infect. Dis. 2023, 23, 259. [Google Scholar] [CrossRef]
- Maltezou, H.C.; Papanikolopoulou, A.; Vassiliu, S.; Theodoridou, K.; Nikolopoulou, G.; Sipsas, N.V. COVID-19 and Respiratory Virus Co-Infections: A Systematic Review of the Literature. Viruses 2023, 15, 865. [Google Scholar] [CrossRef]
- Pan, Q.; Tang, Z.; Yu, Y.; Zang, G.; Chen, X. Co-circulation and co-infection of COVID-19 and influenza in China: Challenges and implications. Front. Public Health 2023, 11, 1295877. [Google Scholar] [CrossRef]
- Achdout, H.; Vitner, E.B.; Politi, B.; Melamed, S.; Yahalom-Ronen, Y.; Tamir, H.; Erez, N.; Avraham, R.; Weiss, S.; Cherry, L.; et al. Increased lethality in influenza and SARS-CoV-2 coinfection is prevented by influenza immunity but not SARS-CoV-2 immunity. Nat. Commun. 2021, 12, 5819. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Skarlupka, A.L.; Jang, H.; Blas-Machado, U.; Holladay, N.; Hogan, R.J.; Ross, T.M. SARS-CoV-2 and Influenza A Virus Coinfections in Ferrets. J. Virol. 2022, 96, e0179121. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Nguyen, T.Q.; Casel, M.A.B.; Rollon, R.; Kim, S.M.; Kim, Y.I.; Yu, K.M.; Jang, S.G.; Yang, J.; Poo, H.; et al. Coinfection with SARS-CoV-2 and Influenza A Virus Increases Disease Severity and Impairs Neutralizing Antibody and CD4(+) T Cell Responses. J. Virol. 2022, 96, e0187321. [Google Scholar] [CrossRef]
- Lingani, M.; Cissé, A.; Tialla, D.; Ilboudo, A.K.; Savadogo, M.; Sawadogo, C.; Gampini, S.; Tarnagda, G.; Tao, M.; Diagbouga, S.; et al. Coinfections with SARS-CoV-2 variants and influenza virus during the 2019 Coronavirus disease pandemic in Burkina Faso: A surveillance study. Health Sci. Rep. 2023, 6, e1041. [Google Scholar] [CrossRef]
- Rezaee, D.; Bakhtiari, S.; Jalilian, F.A.; Doosti-Irani, A.; Asadi, F.T.; Ansari, N. Coinfection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and influenza virus during the COVID-19 pandemic. Arch. Virol. 2023, 168, 53. [Google Scholar] [CrossRef]
- Tang, C.Y.; Boftsi, M.; Staudt, L.; McElroy, J.A.; Li, T.; Duong, S.; Ohler, A.; Ritter, D.; Hammer, R.; Hang, J.; et al. SARS-CoV-2 and influenza co-infection: A cross-sectional study in central Missouri during the 2021-2022 influenza season. Virology 2022, 576, 105–110. [Google Scholar] [CrossRef]
- Ntagereka, P.B.; Basengere, R.A.; Baharanyi, T.C.; Kashosi, T.M.; Buhendwa, J.C.; Bisimwa, P.B.; Kusinza, A.B.; Mugumaarhahama, Y.; Shukuru, D.W.; Patrick, S.B.; et al. Molecular Evidence of Coinfection with Acute Respiratory Viruses and High Prevalence of SARS-CoV-2 among Patients Presenting Flu-Like Illness in Bukavu City, Democratic Republic of Congo. Can. J. Infect. Dis. Med. Microbiol. = J. Can. Des Mal. Infect. Microbiol. Medicale 2022, 2022, 1553266. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Zhang, X.Q.; Yin, W.Q.; Li, Z.Y.; Li, X.N.; Gao, M.; Shi, Y.L.; Guo, H.W.; Chen, Z.M. The relationship among socioeconomic status, social support and frailty: Is there a gender difference? Aging Clin. Exp. Res. 2025, 37, 111. [Google Scholar] [CrossRef]
- Pawelec, G. Age and immunity: What is “immunosenescence”? Exp. Gerontol. 2018, 105, 4–9. [Google Scholar] [CrossRef]
- Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 2013, 14, R115. [Google Scholar] [CrossRef] [PubMed]
- Dou, Z.; Ghosh, K.; Vizioli, M.G.; Zhu, J.; Sen, P.; Wangensteen, K.J.; Simithy, J.; Lan, Y.; Lin, Y.; Zhou, Z.; et al. Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature 2017, 550, 402–406. [Google Scholar] [CrossRef]
- Teissier, T.; Boulanger, E.; Cox, L.S. Interconnections between Inflammageing and Immunosenescence during Ageing. Cells 2022, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Malaquin, N.; Martinez, A.; Rodier, F. Keeping the senescence secretome under control: Molecular reins on the senescence-associated secretory phenotype. Exp. Gerontol. 2016, 82, 39–49. [Google Scholar] [CrossRef]
- Coppé, J.P.; Desprez, P.Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef]
- Freund, A.; Orjalo, A.V.; Desprez, P.Y.; Campisi, J. Inflammatory networks during cellular senescence: Causes and consequences. Trends Mol. Med. 2010, 16, 238–246. [Google Scholar] [CrossRef]
- Corona, G.; Pizzocaro, A.; Vena, W.; Rastrelli, G.; Semeraro, F.; Isidori, A.M.; Pivonello, R.; Salonia, A.; Sforza, A.; Maggi, M. Diabetes is most important cause for mortality in COVID-19 hospitalized patients: Systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2021, 22, 275–296. [Google Scholar] [CrossRef]
- Poly, T.N.; Islam, M.M.; Yang, H.C.; Lin, M.C.; Jian, W.S.; Hsu, M.H.; Jack Li, Y.C. Obesity and Mortality Among Patients Diagnosed With COVID-19: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 620044. [Google Scholar] [CrossRef]
- Jiang, G.; Zou, Y.; Zhao, D.; Yu, J. Optimising vaccine immunogenicity in ageing populations: Key strategies. Lancet. Infect. Dis. 2025, 25, e23–e33. [Google Scholar] [CrossRef] [PubMed]
- Wijsman, C.A.; Maier, A.B.; de Craen, A.J.; van den Biggelaar, A.H.; Westendorp, R.G. An unopposed proinflammatory response is beneficial for survival in the oldest old. Results of the Leiden 85-plus Study. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2011, 66, 393–399. [Google Scholar] [CrossRef]
- Reber, A.J.; Chirkova, T.; Kim, J.H.; Cao, W.; Biber, R.; Shay, D.K.; Sambhara, S. Immunosenescence and Challenges of Vaccination against Influenza in the Aging Population. Aging Dis. 2012, 3, 68–90. [Google Scholar]
- Zimmermann, P.; Curtis, N. Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections. Arch. Dis. Child. 2021, 106, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Gollapudi, S.; Gupta, S.; Agrawal, A. Dendritic cells from the elderly display an intrinsic defect in the production of IL-10 in response to lithium chloride. Exp. Gerontol. 2013, 48, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Agrawal, S.; Cao, J.N.; Su, H.; Osann, K.; Gupta, S. Altered innate immune functioning of dendritic cells in elderly humans: A role of phosphoinositide 3-kinase-signaling pathway. J. Immunol. 2007, 178, 6912–6922. [Google Scholar] [CrossRef]
- Agrawal, A.; Tay, J.; Ton, S.; Agrawal, S.; Gupta, S. Increased reactivity of dendritic cells from aged subjects to self-antigen, the human DNA. J. Immunol. 2009, 182, 1138–1145. [Google Scholar] [CrossRef]
- Wenisch, C.; Patruta, S.; Daxböck, F.; Krause, R.; Hörl, W. Effect of age on human neutrophil function. J. Leukoc. Biol. 2000, 67, 40–45. [Google Scholar] [CrossRef]
- Sapey, E.; Greenwood, H.; Walton, G.; Mann, E.; Love, A.; Aaronson, N.; Insall, R.H.; Stockley, R.A.; Lord, J.M. Phosphoinositide 3-kinase inhibition restores neutrophil accuracy in the elderly: Toward targeted treatments for immunosenescence. Blood 2014, 123, 239–248. [Google Scholar] [CrossRef]
- Campos, C.; Pera, A.; Sanchez-Correa, B.; Alonso, C.; Lopez-Fernandez, I.; Morgado, S.; Tarazona, R.; Solana, R. Effect of age and CMV on NK cell subpopulations. Exp. Gerontol. 2014, 54, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Albright, J.M.; Dunn, R.C.; Shults, J.A.; Boe, D.M.; Afshar, M.; Kovacs, E.J. Advanced Age Alters Monocyte and Macrophage Responses. Antioxid. Redox Signal. 2016, 25, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Hearps, A.C.; Martin, G.E.; Angelovich, T.A.; Cheng, W.J.; Maisa, A.; Landay, A.L.; Jaworowski, A.; Crowe, S.M. Aging is associated with chronic innate immune activation and dysregulation of monocyte phenotype and function. Aging Cell 2012, 11, 867–875. [Google Scholar] [CrossRef]
- Linehan, E.; Dombrowski, Y.; Snoddy, R.; Fallon, P.G.; Kissenpfennig, A.; Fitzgerald, D.C. Aging impairs peritoneal but not bone marrow-derived macrophage phagocytosis. Aging Cell 2014, 13, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Sette, A.; Crotty, S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880. [Google Scholar] [CrossRef]
- Frasca, D.; Diaz, A.; Romero, M.; Blomberg, B.B. The generation of memory B cells is maintained, but the antibody response is not, in the elderly after repeated influenza immunizations. Vaccine 2016, 34, 2834–2840. [Google Scholar] [CrossRef]
- Cancro, M.P.; Hao, Y.; Scholz, J.L.; Riley, R.L.; Frasca, D.; Dunn-Walters, D.K.; Blomberg, B.B. B cells and aging: Molecules and mechanisms. Trends Immunol. 2009, 30, 313–318. [Google Scholar] [CrossRef]
- Labrie, J.E., 3rd; Sah, A.P.; Allman, D.M.; Cancro, M.P.; Gerstein, R.M. Bone marrow microenvironmental changes underlie reduced RAG-mediated recombination and B cell generation in aged mice. J. Exp. Med. 2004, 200, 411–423. [Google Scholar] [CrossRef]
- Ma, S.; Wang, C.; Mao, X.; Hao, Y. B Cell Dysfunction Associated With Aging and Autoimmune Diseases. Front. Immunol. 2019, 10, 318. [Google Scholar] [CrossRef]
- Fletcher, A.L.; Seach, N.; Reiseger, J.J.; Lowen, T.E.; Hammett, M.V.; Scott, H.S.; Boyd, R.L. Reduced thymic Aire expression and abnormal NF-kappa B2 signaling in a model of systemic autoimmunity. J. Immunol. 2009, 182, 2690–2699. [Google Scholar] [CrossRef]
- Sudo, K.; Ema, H.; Morita, Y.; Nakauchi, H. Age-associated characteristics of murine hematopoietic stem cells. J. Exp. Med. 2000, 192, 1273–1280. [Google Scholar] [CrossRef]
- Goronzy, J.J.; Lee, W.W.; Weyand, C.M. Aging and T-cell diversity. Exp. Gerontol. 2007, 42, 400–406. [Google Scholar] [CrossRef] [PubMed]
- van der Geest, K.S.; Abdulahad, W.H.; Tete, S.M.; Lorencetti, P.G.; Horst, G.; Bos, N.A.; Kroesen, B.J.; Brouwer, E.; Boots, A.M. Aging disturbs the balance between effector and regulatory CD4+ T cells. Exp. Gerontol. 2014, 60, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Coder, B.; Su, D.M. Thymic involution beyond T-cell insufficiency. Oncotarget 2015, 6, 21777–21778. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Zitvogel, L. CD4(+) T Cells at the Center of Inflammaging. Cell Metab. 2020, 32, 4–5. [Google Scholar] [CrossRef]
- Goronzy, J.J.; Fang, F.; Cavanagh, M.M.; Qi, Q.; Weyand, C.M. Naive T cell maintenance and function in human aging. J. Immunol. 2015, 194, 4073–4080. [Google Scholar] [CrossRef]
- Ovadya, Y.; Landsberger, T.; Leins, H.; Vadai, E.; Gal, H.; Biran, A.; Yosef, R.; Sagiv, A.; Agrawal, A.; Shapira, A.; et al. Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat. Commun. 2018, 9, 5435. [Google Scholar] [CrossRef]
- Voehringer, D.; Koschella, M.; Pircher, H. Lack of proliferative capacity of human effector and memory T cells expressing killer cell lectinlike receptor G1 (KLRG1). Blood 2002, 100, 3698–3702. [Google Scholar] [CrossRef]
- Rodier, F.; Campisi, J. Four faces of cellular senescence. J. Cell Biol. 2011, 192, 547–556. [Google Scholar] [CrossRef]
- Yang, G.; Cao, J.; Qin, J.; Mei, X.; Deng, S.; Xia, Y.; Zhao, J.; Wang, J.; Luan, T.; Chen, D.; et al. Initial COVID-19 severity influenced by SARS-CoV-2-specific T cells imprints T-cell memory and inversely affects reinfection. Signal Transduct. Target. Ther. 2024, 9, 141. [Google Scholar] [CrossRef]
- Josset, L.; Engelmann, F.; Haberthur, K.; Kelly, S.; Park, B.; Kawoaka, Y.; García-Sastre, A.; Katze, M.G.; Messaoudi, I. Increased viral loads and exacerbated innate host responses in aged macaques infected with the 2009 pandemic H1N1 influenza A virus. J. Virol. 2012, 86, 11115–11127. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Shen, Z.; Gu, W.; Lyu, Z.; Qi, X.; Mu, Y.; Ning, Y. Prevalence of obesity and associated complications in China: A cross-sectional, real-world study in 15.8 million adults. Diabetes Obes. Metab. 2023, 25, 3390–3399. [Google Scholar] [CrossRef]
- Zhao, X.; Gang, X.; He, G.; Li, Z.; Lv, Y.; Han, Q.; Wang, G. Obesity Increases the Severity and Mortality of Influenza and COVID-19: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2020, 11, 595109. [Google Scholar] [CrossRef]
- Cai, Q.; Chen, F.; Wang, T.; Luo, F.; Liu, X.; Wu, Q.; He, Q.; Wang, Z.; Liu, Y.; Liu, L.; et al. Obesity and COVID-19 Severity in a Designated Hospital in Shenzhen, China. Diabetes Care 2020, 43, 1392–1398. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, E.A.; Meliopoulos, V.A.; van de Velde, N.C.; van de Velde, L.A.; Mann, B.; Gao, G.; Rosch, J.; Tuomanen, E.; McCullers, J.; Vogel, P.; et al. A Perfect Storm: Increased Colonization and Failure of Vaccination Leads to Severe Secondary Bacterial Infection in Influenza Virus-Infected Obese Mice. mBio 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Segaloff, H.E.; Evans, R.; Arshad, S.; Zervos, M.J.; Archer, C.; Kaye, K.S.; Martin, E.T. The impact of obesity and timely antiviral administration on severe influenza outcomes among hospitalized adults. J. Med. Virol. 2018, 90, 212–218. [Google Scholar] [CrossRef]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Andersen, C.J.; Murphy, K.E.; Fernandez, M.L. Impact of Obesity and Metabolic Syndrome on Immunity. Adv. Nutr. 2016, 7, 66–75. [Google Scholar] [CrossRef]
- Honce, R.; Schultz-Cherry, S. Impact of Obesity on Influenza A Virus Pathogenesis, Immune Response, and Evolution. Front. Immunol. 2019, 10, 1071. [Google Scholar] [CrossRef]
- Jungreis, I.; Sealfon, R.; Kellis, M. SARS-CoV-2 gene content and COVID-19 mutation impact by comparing 44 Sarbecovirus genomes. Nat. Commun. 2021, 12, 2642. [Google Scholar] [CrossRef]
- Yang, Z.; Macdonald-Dunlop, E.; Chen, J.; Zhai, R.; Li, T.; Richmond, A.; Klarić, L.; Pirastu, N.; Ning, Z.; Zheng, C.; et al. Genetic Landscape of the ACE2 Coronavirus Receptor. Circulation 2022, 145, 1398–1411. [Google Scholar] [CrossRef] [PubMed]
- Bunyavanich, S.; Do, A.; Vicencio, A. Nasal Gene Expression of Angiotensin-Converting Enzyme 2 in Children and Adults. JAMA 2020, 323, 2427–2429. [Google Scholar] [CrossRef]
- Oboza, P.; Ogarek, N.; Olszanecka-Glinianowicz, M.; Kocelak, P. COVID-19 and obesity: The confrontation of two pandemics. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 695–709. [Google Scholar] [CrossRef] [PubMed]
- Li, G.M.; Li, Y.G.; Yamate, M.; Li, S.M.; Ikuta, K. Lipid rafts play an important role in the early stage of severe acute respiratory syndrome-coronavirus life cycle. Microbes Infect. 2007, 9, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Ciarambino, T.; Crispino, P.; Minervini, G.; Giordano, M. COVID-19 and Frailty. Vaccines 2023, 11, 606. [Google Scholar] [CrossRef]
- Hu, T.; Liu, C.H.; Lei, M.; Zeng, Q.; Li, L.; Tang, H.; Zhang, N. Metabolic regulation of the immune system in health and diseases: Mechanisms and interventions. Signal Transduct. Target. Ther. 2024, 9, 268. [Google Scholar] [CrossRef]
- Ahmad Malik, J.; Ahmed, S.; Shinde, M.; Almermesh, M.H.S.; Alghamdi, S.; Hussain, A.; Anwar, S. The Impact of COVID-19 On Comorbidities: A Review Of Recent Updates For Combating It. Saudi J. Biol. Sci. 2022, 29, 3586–3599. [Google Scholar] [CrossRef]
- Stowe, J.; Tessier, E.; Zhao, H.; Guy, R.; Muller-Pebody, B.; Zambon, M.; Andrews, N.; Ramsay, M.; Lopez Bernal, J. Interactions between SARS-CoV-2 and influenza, and the impact of coinfection on disease severity: A test-negative design. Int. J. Epidemiol. 2021, 50, 1124–1133. [Google Scholar] [CrossRef]
- Ding, Q.; Lu, P.; Fan, Y.; Xia, Y.; Liu, M. The clinical characteristics of pneumonia patients coinfected with 2019 novel coronavirus and influenza virus in Wuhan, China. J. Med. Virol. 2020, 92, 1549–1555. [Google Scholar] [CrossRef]
- Ma, S.; Lai, X.; Chen, Z.; Tu, S.; Qin, K. Clinical characteristics of critically ill patients co-infected with SARS-CoV-2 and the influenza virus in Wuhan, China. Int. J. Infect. Dis. 2020, 96, 683–687. [Google Scholar] [CrossRef]
- Valikhani, M.; Feyzmanesh, A.; Daliri, S. A case of Co-infection COVID-19 and influenza with psychotic symptoms. Heliyon 2023, 9, e15501. [Google Scholar] [CrossRef]
- Farias, L.; Silva, F.; Maia, K.M.; Cavalcante, K.F.; Damasceno, L.S. A fatal case of COVID-19-associated meningoencephalitis in a patient coinfected with influenza A. Rev. Do Inst. Med. Trop. Sao Paulo 2023, 65, e22. [Google Scholar] [CrossRef] [PubMed]
- Taquet, M.; Geddes, J.R.; Husain, M.; Luciano, S.; Harrison, P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: A retrospective cohort study using electronic health records. Lancet Psychiatry 2021, 8, 416–427. [Google Scholar] [CrossRef]
- Rubin, R. As Their Numbers Grow, COVID-19 “Long Haulers” Stump Experts. JAMA 2020, 324, 1381–1383. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.; Patel, A.; Chan, K.H.; Veeraballi, S.; Slim, J. A Case Series of SARS-CoV-2 and Influenza Co-infection. Cureus 2021, 13, e17597. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Miyazaki, S.; Yamashita, R.; Ikeda, T. Coinfection with SARS-CoV-2 and influenza A virus. BMJ Case Rep. 2020, 13. [Google Scholar] [CrossRef]
- Helms, J.; Kremer, S.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Kummerlen, C.; Collange, O.; Boulay, C.; Fafi-Kremer, S.; Ohana, M.; et al. Neurologic Features in Severe SARS-CoV-2 Infection. N. Engl. J. Med. 2020, 382, 2268–2270. [Google Scholar] [CrossRef]
- Toovey, S.; Jick, S.S.; Meier, C.R. Parkinson’s disease or Parkinson symptoms following seasonal influenza. Influenza Other Respir Viruses 2011, 5, 328–333. [Google Scholar] [CrossRef]
- Grisanti, S.G.; Franciotta, D.; Garnero, M.; Zuppa, A.; Massa, F.; Mobilia, E.M.; Pesce, G.; Schenone, A.; Benedetti, L. A case series of parainfectious Guillain-Barré syndrome linked to influenza A (H1N1) virus infection. J. Neuroimmunol. 2021, 357, 577605. [Google Scholar] [CrossRef]
- Meidaninikjeh, S.; Sabouni, N.; Taheri, M.; Borjkhani, M.; Bengar, S.; Majidi Zolbanin, N.; Khalili, A.; Jafari, R. SARS-CoV-2 and Guillain-Barré Syndrome: Lessons from Viral Infections. Viral Immunol. 2022, 35, 404–417. [Google Scholar] [CrossRef]
- Antoon, J.W.; Hall, M.; Howard, L.M.; Herndon, A.; Freundlich, K.L.; Grijalva, C.G.; Williams, D.J. COVID-19 and Acute Neurologic Complications in Children. Pediatrics 2022, 150, e2022058167. [Google Scholar] [CrossRef] [PubMed]
- Noor, A.; Gradidge, E. A Case of Reye Syndrome Caused by Influenza A Virus. Ochsner. J. 2018, 18, 425–427. [Google Scholar] [CrossRef]
- Wu, X.; Cai, Y.; Huang, X.; Yu, X.; Zhao, L.; Wang, F.; Li, Q.; Gu, S.; Xu, T.; Li, Y.; et al. Co-infection with SARS-CoV-2 and Influenza A Virus in Patient with Pneumonia, China. Emerg. Infect. Dis. 2020, 26, 1324–1326. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, A.; Riaz, A.; Makan, A.; Crawford, E.; Dev, D.; Srinivasan, K.; Ahmad, N.; Moudgil, H. Lessons of the month: Co-infection with SARS-CoV-2 and influenza B virus in a patient with community-acquired pneumonia. Clin. Med. 2020, 20, e262–e263. [Google Scholar] [CrossRef] [PubMed]
- Nechipurenko, Y.D.; Semyonov, D.A.; Lavrinenko, I.A.; Lagutkin, D.A.; Generalov, E.A.; Zaitceva, A.Y.; Matveeva, O.V.; Yegorov, Y.E. The Role of Acidosis in the Pathogenesis of Severe Forms of COVID-19. Biology 2021, 10, 852. [Google Scholar] [CrossRef]
- Seers, T.; Davenport, R. Phosphate metabolism and respiratory alkalosis: A forgotten lesson in COVID-19. Age Ageing 2020, 49, 927. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, N.; Potdar, V.; Vijay, N.; Mukhopadhyay, L.; Borkakoty, B.; Manjusree, S.; Choudhary, M.L.; Chowdhury, D.; Verma, R.; Bhardwaj, S.D.; et al. SARS-CoV-2 and Influenza Virus Co-Infection Cases Identified through ILI/SARI Sentinel Surveillance: A Pan-India Report. Viruses 2022, 14, 627. [Google Scholar] [CrossRef]
- Sang, C.J., 3rd; Heindl, B.; Von Mering, G.; Brott, B.; Kopf, R.S.; Benson, P.V.; Rajapreyar, I. Stress-Induced Cardiomyopathy Precipitated by COVID-19 and Influenza A Coinfection. JACC Case Rep. 2020, 2, 1356–1358. [Google Scholar] [CrossRef]
- Tong, X.; Xu, X.; Lv, G.; Wang, H.; Cheng, A.; Wang, D.; Fan, G.; Zhang, Y.; Li, Y. Clinical characteristics and outcome of influenza virus infection among adults hospitalized with severe COVID-19: A retrospective cohort study from Wuhan, China. BMC Infect. Dis. 2021, 21, 341. [Google Scholar] [CrossRef]
- Ozaras, R.; Cirpin, R.; Duran, A.; Duman, H.; Arslan, O.; Bakcan, Y.; Kaya, M.; Mutlu, H.; Isayeva, L.; Kebanlı, F.; et al. Influenza and COVID-19 coinfection: Report of six cases and review of the literature. J. Med. Virol. 2020, 92, 2657–2665. [Google Scholar] [CrossRef]
- Lew, S.; Manes, P.; Smith, B. Coinfection with SARS-CoV-2 and Influenza A Virus in a 32-Year-Old Man. Am. J. Case Rep. 2020, 21, e926092. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P. Metabolic Acidosis as a Possible Cause of CKD: What Should Clinicians Do? Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2014, 64, 481–483. [Google Scholar] [CrossRef]
- Alfano, G.; Fontana, F.; Mori, G.; Giaroni, F.; Ferrari, A.; Giovanella, S.; Ligabue, G.; Ascione, E.; Cazzato, S.; Ballestri, M.; et al. Acid base disorders in patients with COVID-19. Int. Urol. Nephrol. 2022, 54, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Mabillard, H.; Sayer, J.A. Electrolyte Disturbances in SARS-CoV-2 Infection. F1000Research 2020, 9, 587. [Google Scholar] [CrossRef]
- Antony, S.J.; Almaghlouth, N.K.; Heydemann, E.L. Are coinfections with COVID-19 and influenza low or underreported? An observational study examining current published literature including three new unpublished cases. J. Med. Virol. 2020, 92, 2489–2497. [Google Scholar] [CrossRef]
- Hashemi, S.A.; Safamanesh, S.; Ghafouri, M.; Taghavi, M.R.; Mohajer Zadeh Heydari, M.S.; Namdar Ahmadabad, H.; Ghasemzadeh-Moghaddam, H.; Azimian, A. Co-infection with COVID-19 and influenza A virus in two died patients with acute respiratory syndrome, Bojnurd, Iran. J. Med. Virol. 2020, 92, 2319–2321. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.L.; McGinley, J.P.; Drysdale, S.B.; Pollard, A.J. Epidemiology and Immune Pathogenesis of Viral Sepsis. Front. Immunol. 2018, 9, 2147. [Google Scholar] [CrossRef]
- Gupta, N.; Richter, R.; Robert, S.; Kong, M. Viral Sepsis in Children. Front. Pediatr. 2018, 6, 252. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Shenoy, S. Coronavirus (Covid-19) sepsis: Revisiting mitochondrial dysfunction in pathogenesis, aging, inflammation, and mortality. Inflamm. Res. 2020, 69, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Cillóniz, C.; Dominedò, C.; Magdaleno, D.; Ferrer, M.; Gabarrús, A.; Torres, A. Pure Viral Sepsis Secondary to Community-Acquired Pneumonia in Adults: Risk and Prognostic Factors. J. Infect. Dis. 2019, 220, 1166–1171. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Cai, S.; Su, J. The Pathogenesis of Sepsis and Potential Therapeutic Targets. Int. J. Mol. Sci. 2019, 20, 5376. [Google Scholar] [CrossRef]
- Iwashyna, T.J.; Cooke, C.R.; Wunsch, H.; Kahn, J.M. Population burden of long-term survivorship after severe sepsis in older Americans. J. Am. Geriatr. Soc. 2012, 60, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.; Herance, R.; Rovira, S.; Hernández-Mijares, A.; Victor, V.M. Mitochondrial dysfunction and antioxidant therapy in sepsis. Infect. Disord. Drug Targets 2012, 12, 161–178. [Google Scholar] [CrossRef]
- Quoilin, C.; Mouithys-Mickalad, A.; Lécart, S.; Fontaine-Aupart, M.P.; Hoebeke, M. Evidence of oxidative stress and mitochondrial respiratory chain dysfunction in an in vitro model of sepsis-induced kidney injury. Biochim. Biophys. Acta 2014, 1837, 1790–1800. [Google Scholar] [CrossRef]
- Ren, C.; Yao, R.Q.; Zhang, H.; Feng, Y.W.; Yao, Y.M. Sepsis-associated encephalopathy: A vicious cycle of immunosuppression. J. Neuroinflammat. 2020, 17, 14. [Google Scholar] [CrossRef]
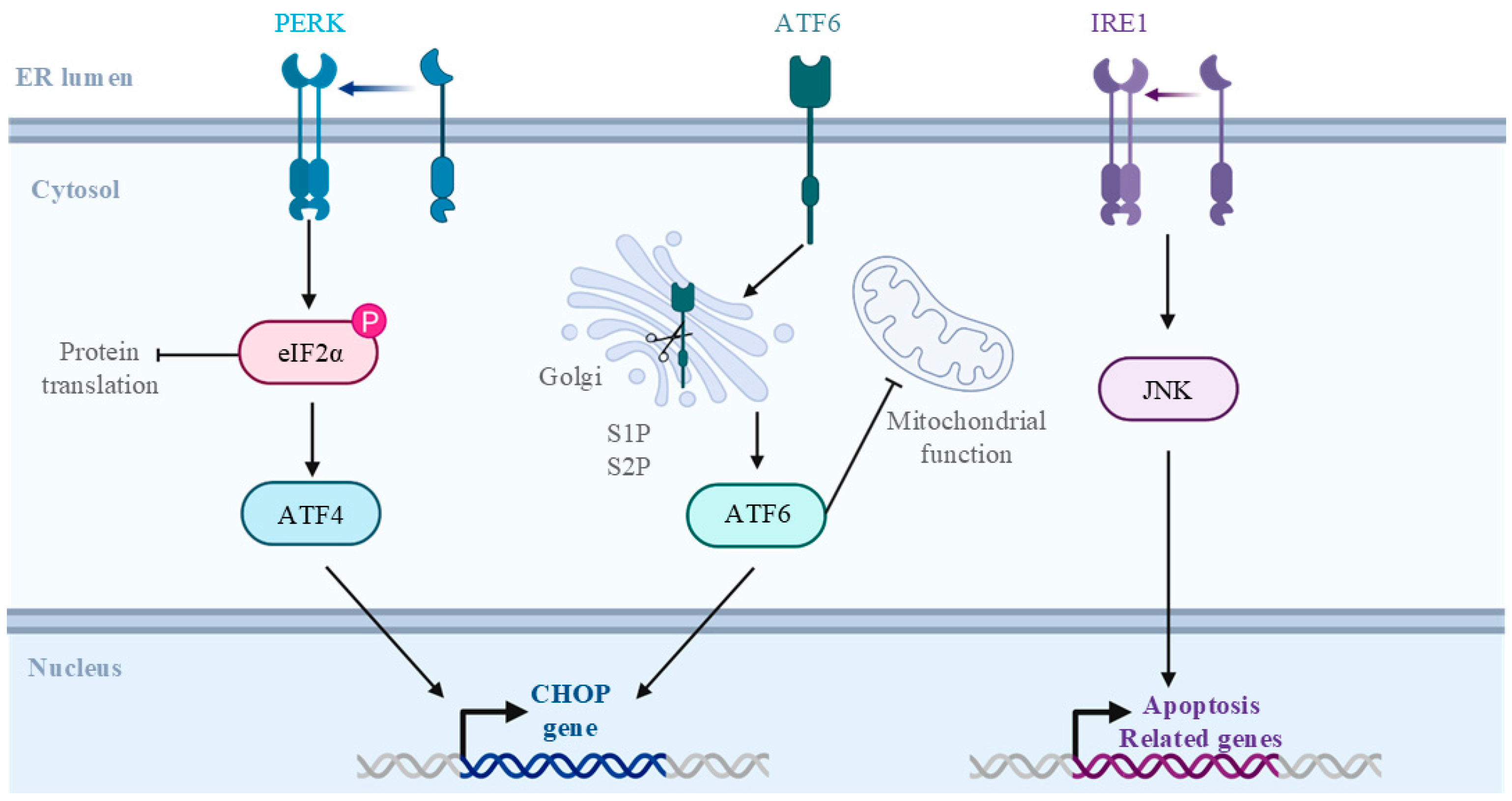
- Khan, M.M.; Yang, W.L.; Wang, P. Endoplasmic reticulum Stress in Sepsis. Shock 2015, 44, 294–304. [Google Scholar] [CrossRef]
- Bermejo-Martin, J.F.; Martín-Fernandez, M.; López-Mestanza, C.; Duque, P.; Almansa, R. Shared Features of Endothelial Dysfunction between Sepsis and Its Preceding Risk Factors (Aging and Chronic Disease). J. Clin. Med. 2018, 7, 400. [Google Scholar] [CrossRef]
- Ince, C.; Mayeux, P.R.; Nguyen, T.; Gomez, H.; Kellum, J.A.; Ospina-Tascón, G.A.; Hernandez, G.; Murray, P.; De Backer, D. The Endothelium in Sepsis. Shock 2016, 45, 259–270. [Google Scholar] [CrossRef]
- Aman, Y.; Schmauck-Medina, T.; Hansen, M.; Morimoto, R.I.; Simon, A.K.; Bjedov, I.; Palikaras, K.; Simonsen, A.; Johansen, T.; Tavernarakis, N.; et al. Autophagy in healthy aging and disease. Nat. Aging 2021, 1, 634–650. [Google Scholar] [CrossRef] [PubMed]
- Oami, T.; Watanabe, E.; Hatano, M.; Teratake, Y.; Fujimura, L.; Sakamoto, A.; Ito, C.; Toshimori, K.; Swanson, P.E.; Oda, S. Blocking Liver Autophagy Accelerates Apoptosis and Mitochondrial Injury in Hepatocytes and Reduces Time to Mortality in a Murine Sepsis Model. Shock 2018, 50, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhou, J.; Ye, L.C.; Li, J.; Wu, Z.; Li, Y.; Li, C. Autophagy maintains the integrity of endothelial barrier in LPS-induced lung injury. J. Cell. Physiol. 2018, 233, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, K.; Iwasaki, Y.; Daimon, M. Hypothalamic Regulation of Corticotropin-Releasing Factor under Stress and Stress Resilience. Int. J. Mol. Sci. 2021, 22, 12242. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Y.; Tang, J.; Jiang, J.; Chen, Z. New insights into the roles of CHOP-induced apoptosis in ER stress. Acta Biochim. Biophys. Sin. 2014, 46, 629–640. [Google Scholar] [CrossRef]
- Walter, P.; Ron, D. The unfolded protein response: From stress pathway to homeostatic regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef]
- Burkewitz, K.; Feng, G.; Dutta, S.; Kelley, C.A.; Steinbaugh, M.; Cram, E.J.; Mair, W.B. Atf-6 Regulates Lifespan through ER-Mitochondrial Calcium Homeostasis. Cell Rep. 2020, 32, 108125. [Google Scholar] [CrossRef]
- García de la Cadena, S.; Massieu, L. Caspases and their role in inflammation and ischemic neuronal death. Focus on caspase-12. Apoptosis Int. J. Program. Cell Death 2016, 21, 763–777. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
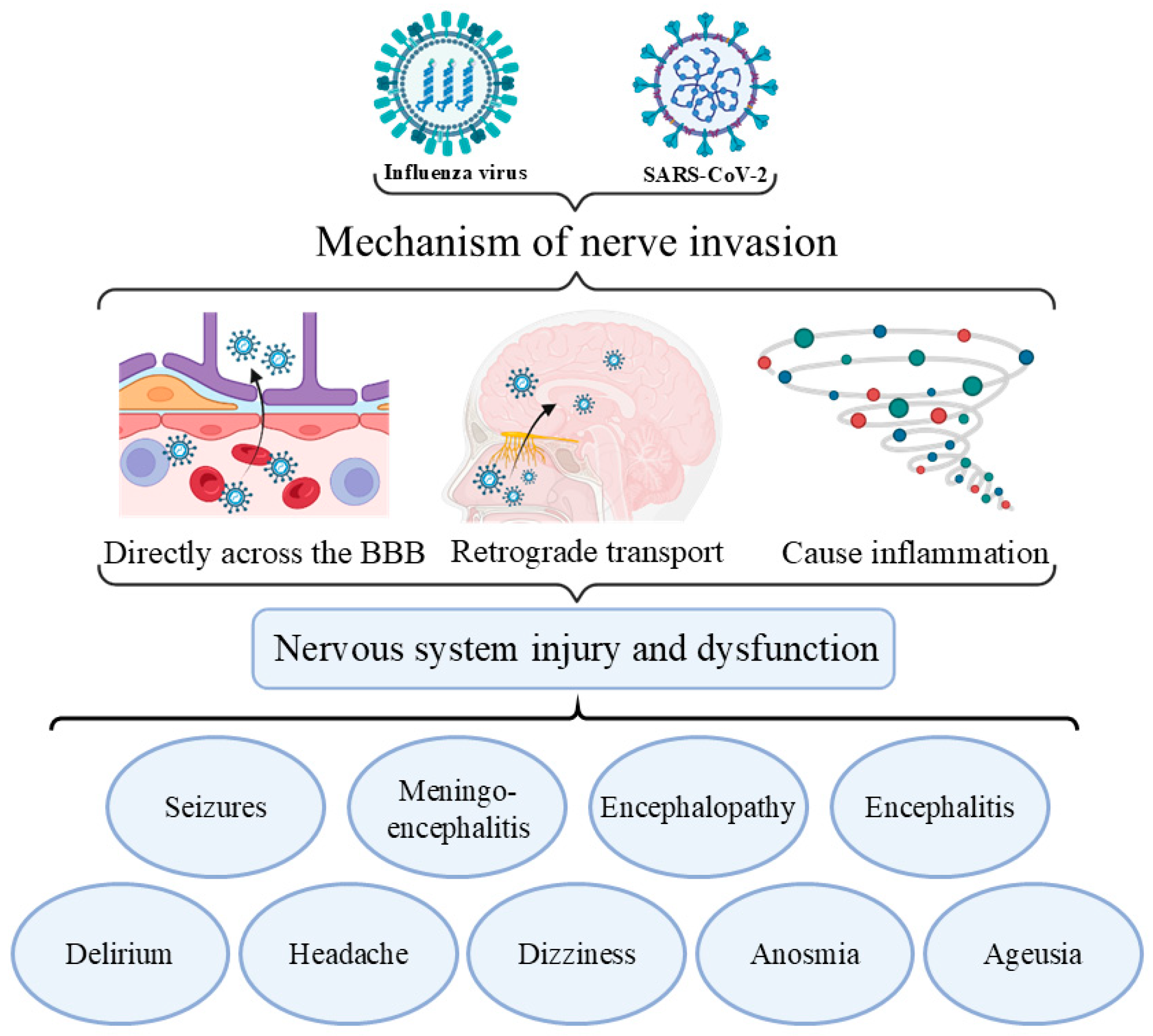
- Meinhardt, J.; Radke, J.; Dittmayer, C.; Franz, J.; Thomas, C.; Mothes, R.; Laue, M.; Schneider, J.; Brünink, S.; Greuel, S.; et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2021, 24, 168–175. [Google Scholar] [CrossRef]
- Iadecola, C.; Anrather, J.; Kamel, H. Effects of COVID-19 on the Nervous System. Cell 2020, 183, 16–27.e11. [Google Scholar] [CrossRef]
- Kimura-Ohba, S.; Kitamura, M.; Tsukamoto, Y.; Kogaki, S.; Sakai, S.; Fushimi, H.; Matsuoka, K.; Takeuchi, M.; Itoh, K.; Ueda, K.; et al. Viral entry and translation in brain endothelia provoke influenza-associated encephalopathy. Acta Neuropathol. 2024, 147, 77. [Google Scholar] [CrossRef] [PubMed]
- van Riel, D.; Verdijk, R.; Kuiken, T. The olfactory nerve: A shortcut for influenza and other viral diseases into the central nervous system. J. Pathol. 2015, 235, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Park, C.H.; Sunden, Y.; Kimura, T.; Ochiai, K.; Kida, H.; Umemura, T. The vagus nerve is one route of transneural invasion for intranasally inoculated influenza a virus in mice. Vet. Pathol. 2004, 41, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Rothan, H.A.; Natekar, J.P.; Stone, S.; Pathak, H.; Strate, P.G.; Arora, K.; Brinton, M.A.; Kumar, M. Neuroinvasion and Encephalitis Following Intranasal Inoculation of SARS-CoV-2 in K18-hACE2 Mice. Viruses 2021, 13, 132. [Google Scholar] [CrossRef]
- Chen, Z.; Li, G. Immune response and blood-brain barrier dysfunction during viral neuroinvasion. Innate Immun. 2021, 27, 109–117. [Google Scholar] [CrossRef]
- Siahaan, Y.M.T.; Puspitasari, V.; Pangestu, A. COVID-19-Associated Encephalopathy: Systematic Review of Case Reports. J. Clin. Neurol. 2022, 18, 194–206. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Cavallazzi, R.; Ramirez, J.A. Influenza and Viral Pneumonia. Infect. Dis. Clin. N. Am. 2024, 38, 183–212. [Google Scholar] [CrossRef]
- Cao, X. COVID-19: Immunopathology and its implications for therapy. Nat. Rev. Immunol. 2020, 20, 269–270. [Google Scholar] [CrossRef]
- Santos, R.A.S.; Sampaio, W.O.; Alzamora, A.C.; Motta-Santos, D.; Alenina, N.; Bader, M.; Campagnole-Santos, M.J. The ACE2/Angiotensin-(1-7)/MAS Axis of the Renin-Angiotensin System: Focus on Angiotensin-(1-7). Physiol. Rev. 2018, 98, 505–553. [Google Scholar] [CrossRef] [PubMed]
- Amor, S.; Fernández Blanco, L.; Baker, D. Innate immunity during SARS-CoV-2: Evasion strategies and activation trigger hypoxia and vascular damage. Clin. Exp. Immunol. 2020, 202, 193–209. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wu, D.; Chen, H.; Yan, W.; Yang, D.; Chen, G.; Ma, K.; Xu, D.; Yu, H.; Wang, H.; et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ (Clin. Res. Ed.) 2020, 368, m1091. [Google Scholar] [CrossRef]
- Chen, H.; Li, X.; Marmar, T.; Xu, Q.; Tu, J.; Li, T.; Han, J.; Xu, D.; Shen, T. Cardiac Troponin I association with critical illness and death risk in 726 seriously ill COVID-19 patients: A retrospective cohort study. Int. J. Med. Sci. 2021, 18, 1474–1483. [Google Scholar] [CrossRef]
- Duan, J.; Wu, Y.; Liu, C.; Yang, C.; Yang, L. Deleterious effects of viral pneumonia on cardiovascular system. Eur. Heart J. 2020, 41, 1833–1838. [Google Scholar] [CrossRef]
- Nishiga, M.; Wang, D.W.; Han, Y.; Lewis, D.B.; Wu, J.C. COVID-19 and cardiovascular disease: From basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 2020, 17, 543–558. [Google Scholar] [CrossRef]
- Corrales-Medina, V.F.; Madjid, M.; Musher, D.M. Role of acute infection in triggering acute coronary syndromes. Lancet Infect. Dis. 2010, 10, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Muscente, F.; De Caterina, R. Causal relationship between influenza infection and risk of acute myocardial infarction: Pathophysiological hypothesis and clinical implications. Eur. Heart J. Suppl. 2020, 22, E68–E72. [Google Scholar] [CrossRef]
- Chiang, M.H.; Wu, H.H.; Shih, C.J.; Chen, Y.T.; Kuo, S.C.; Chen, T.L. Association between influenza vaccination and reduced risks of major adverse cardiovascular events in elderly patients. Am. Heart J. 2017, 193, 1–7. [Google Scholar] [CrossRef]
- Lee, H.S.; Noh, J.Y.; Shin, O.S.; Song, J.Y.; Cheong, H.J.; Kim, W.J. Matrix Metalloproteinase-13 in Atherosclerotic Plaque Is Increased by Influenza A Virus Infection. J. Infect. Dis. 2020, 221, 256–266. [Google Scholar] [CrossRef]
- Wool, G.D.; Miller, J.L. The Impact of COVID-19 Disease on Platelets and Coagulation. Pathobiology 2021, 88, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Konala, V.M.; Adapa, S.; Naramala, S.; Chenna, A.; Lamichhane, S.; Garlapati, P.R.; Balla, M.; Gayam, V. A Case Series of Patients Coinfected with Influenza and COVID-19. J. Investig. Med. High Impact Case Rep. 2020, 8, 2324709620934674. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, Y.; Gao, R.; Lu, R.; Han, K.; Wu, G.; Tan, W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA 2020, 323, 1843–1844. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Ahmadian, E.; Hosseiniyan Khatibi, S.M.; Razi Soofiyani, S.; Abediazar, S.; Shoja, M.M.; Ardalan, M.; Zununi Vahed, S. Covid-19 and kidney injury: Pathophysiology and molecular mechanisms. Rev. Med. Virol. 2021, 31, e2176. [Google Scholar] [CrossRef]
- Alhoufie, S.T.; Alsharif, N.H.; Alfarouk, K.O.; Ibrahim, N.A.; Kheyami, A.M.; Aljifri, A.A. COVID-19 with underdiagnosed influenza B and parainfluenza-2 co-infections in Saudi Arabia: Two case reports. J. Infect. Public Health 2021, 14, 1567–1570. [Google Scholar] [CrossRef] [PubMed]
- Shafran, N.; Issachar, A.; Shochat, T.; Shafran, I.H.; Bursztyn, M.; Shlomai, A. Abnormal liver tests in patients with SARS-CoV-2 or influenza—Prognostic similarities and temporal disparities. JHEP Rep. Innov. Hepatol. 2021, 3, 100258. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.W.; Tian, J.H.; Pei, Y.Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef]
- Yao, X.H.; Li, T.Y.; He, Z.C.; Ping, Y.F.; Liu, H.W.; Yu, S.C.; Mou, H.M.; Wang, L.H.; Zhang, H.R.; Fu, W.J.; et al. A pathological report of three COVID-19 cases by minimal invasive autopsies. Zhonghua Bing Li Xue Za Zhi 2020, 49, 411–417. [Google Scholar] [CrossRef]
- Lei, F.; Liu, Y.M.; Zhou, F.; Qin, J.J.; Zhang, P.; Zhu, L.; Zhang, X.J.; Cai, J.; Lin, L.; Ouyang, S.; et al. Longitudinal Association Between Markers of Liver Injury and Mortality in COVID-19 in China. Hepatology 2020, 72, 389–398. [Google Scholar] [CrossRef]
- Abels, S.; Nadal, D.; Stroehle, A.; Bossart, W. Reliable detection of respiratory syncytial virus infection in children for adequate hospital infection control management. J. Clin. Microbiol. 2001, 39, 3135–3139. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Fan, W.; Tingting, D.; Zuoming, S.; Qiang, Z. 4-phenylbutyric acid attenuates endoplasmic reticulum stress-mediated apoptosis and protects the hepatocytes from intermittent hypoxia-induced injury. Sleep Breath 2019, 23, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Tsui, J.L.; Gutierrez, B.; Busch Moreno, S.; du Plessis, L.; Deng, X.; Cai, J.; Bajaj, S.; Suchard, M.A.; Pybus, O.G.; et al. COVID-19 pandemic interventions reshaped the global dispersal of seasonal influenza viruses. Science 2024, 386, eadq3003. [Google Scholar] [CrossRef]
- Ayouni, I.; Maatoug, J.; Dhouib, W.; Zammit, N.; Fredj, S.B.; Ghammam, R.; Ghannem, H. Effective public health measures to mitigate the spread of COVID-19: A systematic review. BMC Public Health 2021, 21, 1015. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Wang, Y.; Lin, Z.; He, W.; Sun, J.; Li, Q.; Zhang, M.; Chang, Z.; Guo, Y.; Zeng, W.; et al. Influenza and COVID-19 co-infection and vaccine effectiveness against severe cases: A mathematical modeling study. Front. Cell. Infect. Microbiol. 2024, 14, 1347710. [Google Scholar] [CrossRef]
- Behrouzi, B.; Araujo Campoverde, M.V.; Liang, K.; Talbot, H.K.; Bogoch, I.I.; McGeer, A.; Fröbert, O.; Loeb, M.; Vardeny, O.; Solomon, S.D.; et al. Influenza Vaccination to Reduce Cardiovascular Morbidity and Mortality in Patients with COVID-19: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 1777–1794. [Google Scholar] [CrossRef]
- Wilcox, C.R.; Islam, N.; Dambha-Miller, H. Association between influenza vaccination and hospitalisation or all-cause mortality in people with COVID-19: A retrospective cohort study. BMJ Open Respir. Res. 2021, 8, e000857. [Google Scholar] [CrossRef]
- Katsiroumpa, A.; Sourtzi, P.; Kaitelidou, D.; Siskou, O.; Konstantakopoulou, O.; Galanis, P. Predictors of Seasonal Influenza Vaccination Willingness among High-Risk Populations Three Years after the Onset of the COVID-19 Pandemic. Vaccines 2023, 11, 331. [Google Scholar] [CrossRef]
- Wu, N.; Joyal-Desmarais, K.; Ribeiro, P.A.B.; Vieira, A.M.; Stojanovic, J.; Sanuade, C.; Yip, D.; Bacon, S.L. Long-term effectiveness of COVID-19 vaccines against infections, hospitalisations, and mortality in adults: Findings from a rapid living systematic evidence synthesis and meta-analysis up to December, 2022. Lancet Respir. Med. 2023, 11, 439–452. [Google Scholar] [CrossRef]
- Tokars, J.I.; Patel, M.M.; Foppa, I.M.; Reed, C.; Fry, A.M.; Ferdinands, J.M. Waning of Measured Influenza Vaccine Effectiveness Over Time: The Potential Contribution of Leaky Vaccine Effect. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020, 71, e633–e641. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, Q.; Li, M.; Mai, Q.; Ma, L.; Zhang, H.; Zhong, H.; Mai, K.; Cheng, N.; Feng, P.; et al. A decavalent composite mRNA vaccine against both influenza and COVID-19. mBio 2024, 15, e0066824. [Google Scholar] [CrossRef] [PubMed]
- Lennon, R.P.; Block, R., Jr.; Schneider, E.C.; Zephrin, L.; Shah, A. Underserved population acceptance of combination influenza-COVID-19 booster vaccines. Vaccine 2022, 40, 562–567. [Google Scholar] [CrossRef]
- Ye, Q.; Wu, M.; Zhou, C.; Lu, X.; Huang, B.; Zhang, N.; Zhao, H.; Chi, H.; Zhang, X.; Ling, D.; et al. Rational development of a combined mRNA vaccine against COVID-19 and influenza. NPJ Vaccines 2022, 7, 84. [Google Scholar] [CrossRef]
- Huang, Y.; Shi, H.; Forgacs, D.; Ross, T.M. Flu-COVID combo recombinant protein vaccines elicited protective immune responses against both influenza and SARS-CoV-2 viruses infection. Vaccine 2024, 42, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ye, Z.; Li, C.; Zhou, J.; Xue, W.; Xiang, L.; Chen, Y.; Chen, S.; Ye, R.; Dong, J.; et al. A subunit-based influenza/SARS-CoV-2 Omicron combined vaccine induced potent protective immunity in BALB/c mice. J. Med. Virol. 2024, 96, e29479. [Google Scholar] [CrossRef]
- Shi, R.; Zeng, J.; Xu, L.; Wang, F.; Duan, X.; Wang, Y.; Wu, Z.; Yu, D.; Huang, Q.; Yao, Y.G.; et al. A combination vaccine against SARS-CoV-2 and H1N1 influenza based on receptor binding domain trimerized by six-helix bundle fusion core. EBioMedicine 2022, 85, 104297. [Google Scholar] [CrossRef]
- Li, Y.; Liu, P.; Hao, T.; Liu, S.; Wang, X.; Xie, Y.; Xu, K.; Lei, W.; Zhang, C.; Han, P.; et al. Rational design of an influenza-COVID-19 chimeric protective vaccine with HA-stalk and S-RBD. Emerg. Microbes Infect. 2023, 12, 2231573. [Google Scholar] [CrossRef]
- Bommireddy, R.; Stone, S.; Bhatnagar, N.; Kumari, P.; Munoz, L.E.; Oh, J.; Kim, K.H.; Berry, J.T.L.; Jacobsen, K.M.; Jaafar, L.; et al. Influenza Virus-like Particle-Based Hybrid Vaccine Containing RBD Induces Immunity against Influenza and SARS-CoV-2 Viruses. Vaccines 2022, 10, 944. [Google Scholar] [CrossRef] [PubMed]
- Chaparian, R.R.; Harding, A.T.; Hamele, C.E.; Riebe, K.; Karlsson, A.; Sempowski, G.D.; Heaton, N.S.; Heaton, B.E. A Virion-Based Combination Vaccine Protects against Influenza and SARS-CoV-2 Disease in Mice. J. Virol. 2022, 96, e0068922. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; He, J.; Chen, J.; Qi, R.; Yuan, L.; Shao, T.; Zhao, H.; Chen, C.; Chen, Y.; et al. Intranasal influenza-vectored COVID-19 vaccine restrains the SARS-CoV-2 inflammatory response in hamsters. Nat. Commun. 2023, 14, 4117. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Z.; Shi, W.; Zhu, D.; Hu, S.; Dinh, P.C.; Cheng, K. A SARS-CoV-2 and influenza double hit vaccine based on RBD-conjugated inactivated influenza A virus. Sci. Adv. 2023, 9, eabo4100. [Google Scholar] [CrossRef] [PubMed]
- Loes, A.N.; Gentles, L.E.; Greaney, A.J.; Crawford, K.H.D.; Bloom, J.D. Attenuated Influenza Virions Expressing the SARS-CoV-2 Receptor-Binding Domain Induce Neutralizing Antibodies in Mice. Viruses 2020, 12, 987. [Google Scholar] [CrossRef] [PubMed]
- Ao, Z.; Ouyang, M.J.; Olukitibi, T.A.; Warner, B.; Vendramelli, R.; Truong, T.; Meilleur, C.; Zhang, M.; Kung, S.; Fowke, K.R.; et al. A Recombinant VSV-Based Bivalent Vaccine Effectively Protects against Both SARS-CoV-2 and Influenza A Virus Infection. J. Virol. 2022, 96, e0133722. [Google Scholar] [CrossRef]
- Cao, K.; Wang, X.; Peng, H.; Ding, L.; Wang, X.; Hu, Y.; Dong, L.; Yang, T.; Hong, X.; Xing, M.; et al. A Single Vaccine Protects against SARS-CoV-2 and Influenza Virus in Mice. J. Virol. 2022, 96, e0157821. [Google Scholar] [CrossRef]
- Xiang, X.; Wang, Z.H.; Ye, L.L.; He, X.L.; Wei, X.S.; Ma, Y.L.; Li, H.; Chen, L.; Wang, X.R.; Zhou, Q. Co-infection of SARS-COV-2 and Influenza A Virus: A Case Series and Fast Review. Curr. Med. Sci. 2021, 41, 51–57. [Google Scholar] [CrossRef]
- Heshmat-Ghahdarijani, K.; Vaseghi, G.; Nasirian, M.; Javanmard, S.H. Co-infection between the severe acute respiratory syndrome coronavirus 2 and the influenza Type B in Isfahan, Iran. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2021, 26, 51. [Google Scholar] [CrossRef]
- Guan, Z.; Chen, C.; Li, Y.; Yan, D.; Zhang, X.; Jiang, D.; Yang, S.; Li, L. Impact of Coinfection with SARS-CoV-2 and Influenza on Disease Severity: A Systematic Review and Meta-Analysis. Front. Public Health 2021, 9, 773130. [Google Scholar] [CrossRef]
- Huang, B.R.; Lin, Y.L.; Wan, C.K.; Wu, J.T.; Hsu, C.Y.; Chiu, M.H.; Huang, C.H. Co-infection of influenza B virus and SARS-CoV-2: A case report from Taiwan. J. Microbiol. Immunol. Infect. = Wei Mian Yu Gan Ran Za Zhi 2021, 54, 336–338. [Google Scholar] [CrossRef] [PubMed]
- Karami, H.; Derakhshani, A.; Ghasemigol, M.; Fereidouni, M.; Miri-Moghaddam, E.; Baradaran, B.; Tabrizi, N.J.; Najafi, S.; Solimando, A.G.; Marsh, L.M.; et al. Weighted Gene Co-Expression Network Analysis Combined with Machine Learning Validation to Identify Key Modules and Hub Genes Associated with SARS-CoV-2 Infection. J. Clin. Med. 2021, 10, 3567. [Google Scholar] [CrossRef]
- Liu, D.; Leung, K.Y.; Lam, H.Y.; Zhang, R.; Fan, Y.; Xie, X.; Chan, K.H.; Hung, I.F. Interaction and antiviral treatment of coinfection between SARS-CoV-2 and influenza in vitro. Virus Res. 2024, 345, 199371. [Google Scholar] [CrossRef]
- Ray, W.A.; Murray, K.T.; Hall, K.; Arbogast, P.G.; Stein, C.M. Azithromycin and the risk of cardiovascular death. N. Engl. J. Med. 2012, 366, 1881–1890. [Google Scholar] [CrossRef] [PubMed]

| Virus | SARS-CoV-2 | Influenza Virus | Reference |
|---|---|---|---|
| Nucleic acid | Single-stranded Positive-sense RNA virus | Single-stranded Negative-sense RNAvirus | |
| Infection target | S protein and ACE2 | HA protein and Sia | |
| Coinfection mechanism | IAV infection induces elevated ACE2 expression | [8] | |
| Coinfection increases the viral load of SARS-CoV-2 and influenza | [8,45] | ||
| Coinfection extends the duration of the initial viral infection | [46] | ||
| Coinfection leads to heightened immune cell infiltration | [46] | ||
| Coinfection leads to severe lymphopenia in peripheral blood | [47] | ||
| Neuropsychiatric system | Seizures and meningoencephalitis | [111,112] |
| Encephalopathy, encephalitis, transverse myelitis, stroke, and dementia | [4,113,114] | |
| Delirium, headache, and dizziness | [109,111,115] | |
| Anosmia, ageusia, and fatigue | [109,116] | |
| Illusion and brain fog | [4,113,114] | |
| Cognitive and motor impairment | [117,118] | |
| Guillain–Barré and Reye syndrome | [119,120,121,122] | |
| Respiratory system | Acute respiratory distress syndrome (ARDS) | [40,110] |
| Pneumonia and hypoxemia | [40,123] | |
| Respiratory failure/acidosis/alkalosis | [107,124,125,126] | |
| Pulmonary fibrosis/lymphadenopathy, pleural effusions, and linear atelectasis | [36,40] | |
| Secondary bacterial pneumonia | [7] | |
| Cough, dyspnea, nasal congestion, pharyngalgia, expectoration, and runny nose | [36,40,109,127] | |
| Cardiovascular system | Cardiopulmonary arrest | [40,112,128] |
| Acute cardiac injury and fulminant myocarditis | [40,110,128] | |
| Acute heart failure and coagulation disorder | [7,129] | |
| Chest distress/chest pain | [110,130] | |
| Urinary system | Acute kidney injury (AKI) and anuric | [7,110,131] |
| Metabolic acidosis and metabolic alkalosis | [132,133] | |
| Electrolyte disturbances | [133,134] | |
| Digestive system | Acute liver injury and hepatic dysfunction | [40,110,135] |
| Diarrhea and nausea | [136] | |
| Loss of appetite and vomiting | [115] | |
| Others | Cytokine storm | [110] |
| Viral sepsis and sepsis | [137,138] | |
| Dehydration and exacerbation of chronic disease | [4] |
| Platform | Name | Vaccine Effectiveness | Reference |
|---|---|---|---|
| mRNA vaccines | AR-CoV/IAV | HAI Ab (>1: 1024) SARS-CoV-2 NAb (>1: 849) After 2 doses | [203] |
| FLUCOV-10 | Provided complete protection to immunized mice | [201] | |
| Recombinant protein vaccines | FLU-COVID | As efficient as influenza or SARS-CoV-2 mono vaccines | [204] |
| - | High level IgG, HAI Ab High titers NAb against SARS-CoV-2 Omicron BA.5 | [205] | |
| - | HAI Ab (>1: 1024) Long-lasting and high-titer Nabs against SARS-CoV-2 | [206] | |
| - | Broad NAbs against VOCs Cross-protection against IAVs | [207] | |
| Virus-like-particle vaccine | VLP-RBD-GM-CSF-IL-12 | Long-lasting and high-titer Nabs against H1N1 Abs against ACE2 binding to RBD | [208] |
| Influenza-based vaccines | TM-RBD-HA | Elicited Nabs and provided protection with IAV and SARS-CoV-2 in mice. | [209] |
| Pneucolin (dNS1-RBD) | Exhibited great safety and efficacy in the elderly | [210] | |
| Flu-RBD | High titers of HA-specific and RBD-specific IgG Abs | [211] | |
| ΔNA(RBD)-Flu | A single dose can generate equivalent Nab titers to those produced by two doses of mRNA vaccines. | [212] | |
| VSV-based vaccines | - | Effectively protected hamsters or mice against SARS-CoV-2 Delta, H1N1, and H3N2 | [213] |
| Adenoviral vectors vaccines | Adc68-CoV/Flu | Effectively induced SARS-CoV-2-targeting Abs and anti-influenza Abs in mice | [214] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Li, S.; Ye, W.; Wang, H.; Su, J.; Gao, L.; Shi, R.; Mou, X.; Leng, S.X.; Xiao, C.; et al. Viral Infections in Elderly Individuals: A Comprehensive Overview of SARS-CoV-2 and Influenza Susceptibility, Pathogenesis, and Clinical Treatment Strategies. Vaccines 2025, 13, 431. https://doi.org/10.3390/vaccines13040431
Huang Y, Li S, Ye W, Wang H, Su J, Gao L, Shi R, Mou X, Leng SX, Xiao C, et al. Viral Infections in Elderly Individuals: A Comprehensive Overview of SARS-CoV-2 and Influenza Susceptibility, Pathogenesis, and Clinical Treatment Strategies. Vaccines. 2025; 13(4):431. https://doi.org/10.3390/vaccines13040431
Chicago/Turabian StyleHuang, Yanhao, Shumin Li, Wenjie Ye, Haoyun Wang, Jun Su, Lijuan Gao, Ruohu Shi, Xinyi Mou, Sean Xiao Leng, Chanchan Xiao, and et al. 2025. "Viral Infections in Elderly Individuals: A Comprehensive Overview of SARS-CoV-2 and Influenza Susceptibility, Pathogenesis, and Clinical Treatment Strategies" Vaccines 13, no. 4: 431. https://doi.org/10.3390/vaccines13040431
APA StyleHuang, Y., Li, S., Ye, W., Wang, H., Su, J., Gao, L., Shi, R., Mou, X., Leng, S. X., Xiao, C., & Chen, G. (2025). Viral Infections in Elderly Individuals: A Comprehensive Overview of SARS-CoV-2 and Influenza Susceptibility, Pathogenesis, and Clinical Treatment Strategies. Vaccines, 13(4), 431. https://doi.org/10.3390/vaccines13040431






