Management of the Adverse Effects of Immune Checkpoint Inhibitors
Abstract
:1. Introduction
2. Immunological Checkpoint Inhibitors Available in the Pharmaceutical Market and Main Associated Adverse Effects
3. Relevant Issues for the Monitoring and Treatment of Immune-Related Adverse Reactions
4. Management of Adverse Effects Associated with Immunological Checkpoint Inhibitors

5. Treatment of Steroid-Refractory Immune-Related Adverse Effects
6. Role of the Hospital Pharmacist in Educating the Patient and Family or Caregivers
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Dine, J.; Gordon, R.; Shames, Y.; Kasler, M.K.; Barton-Burke, M. Immune checkpoint inhibitors: An innovation in immunotherapy for the treatment and management of patients with cancer. Asia-Pacific J. Oncol. Nurs. 2017, 4, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Haanen, J.B.A.G.; Carbonnel, F.; Robert, C.; Kerr, K.M.; Peters, S.; Larkin, J.; Jordan, K.; on behalf of the ESMO Guidelines Committee. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv119–iv142. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.A.; Schneider, B.J.; Brahmer, J.; Andrews, S.; Armand, P.; Bhatia, S.; Budde, L.E.; Costa, L.; Davies, M.; Dunnington, D.; et al. Management of immunotherapy-related toxicities. J. Natl. Compr. Cancer Netw. 2019, 17, 255–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Immune Checkpoint Inhibitors Unleashed to Fight Cancer. Available online: https://www.the-rheumatologist.org/article/immune-checkpoint-inhibitors-unleashed-fight-cancer/ (accessed on 8 September 2020).
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham, C.O.; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.E.; LeBoeuf, N.R.; et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 2017, 5, 95. [Google Scholar] [CrossRef] [Green Version]
- Ogino, S.; Nowak, J.; Hamada, T.; Milner, D.A., Jr.; Nishihara, R. Insights into pathogenic interactions among environment, host, and tumor at the crossroads of molecular pathology and epidemiology. Annu. Rev. Pathol. Mech. Dis. 2018, 14, 83–103. [Google Scholar] [CrossRef]
- Hamada, T.; Nowak, J.A.; Milner, D.A.; Song, M.; Ogino, S. Integration of microbiology, molecular pathology, and epidemiology: A new paradigm to explore the pathogenesis of microbiome-driven neoplasms. J. Pathol. 2019, 247, 615–628. [Google Scholar] [CrossRef] [Green Version]
- Kanz, B.A.; Pollack, M.H.; Johnpulle, R.A.N.; Puzanov, I.; Horn, L.; Morgans, A.K.; Sosman, J.A.; Rapisuwon, S.; Conry, R.; Eroglu, Z.; et al. Safety and efficacy of anti-PD-1 in patients with baseline cardiac, renal, or hepatic dysfunction. J. Immunother. Cancer 2016, 4, 60. [Google Scholar] [CrossRef] [Green Version]
- Westfield, C. Managing immune checkpoint inhibitor side effects: Key recommendations. In Oncology Practice Management, Engage Healthcare Communications; DBuffery, D.B., Lorton, L.J., Guglielmon, C., Eds.; Engage Healthcare Communications, LLC: Cranbury, NJ, USA, 2018; Volume 8. [Google Scholar]
- The National Cancer Institute. Can. Med. Assoc. J. 1947, 56, 558.
- Castinetti, F.; Albarel, F.; Archambeaud, F.; Bertherat, J.; Bouillet, B.; Buffier, P.; Briet, C.; Cariou, B.; Caron, P.; Chabre, O.; et al. French Endocrine Society Guidance on endocrine side effects of immunotherapy. Endocr.-Related Cancer 2018, 26, G1–G18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacDougall, A.K. The Pharmacist’s Role in Educating the Health-Care Team about Adverse Effects of Immune Checkpoint Inhibitors; The ASCO Post: Newyork, NY, USA, 2018. [Google Scholar]
- Weber, J.S.; Yang, J.C.; Atkins, M.B.; Disis, M.L. Toxicities of immunotherapy for the practitioner. J. Clin. Oncol. 2015, 33, 2092–2099. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.; Sykiotis, G.P.; Maillard, M.; Fraga, M.; Ribi, C.; Kuntzer, T.; Michielin, O.; Peters, S.; Coukos, G.; Spertini, F.; et al. New therapeutic perspectives to manage refractory immune checkpoint-related toxicities. Lancet Oncol. 2019, 20, e54–e64. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Lee, S.K.; Oo, T.H.; Rojas-Hernandez, C.M. Management of immune-mediated cytopenias in the era of cancer immunotherapy. J. Immunother. 2018, 41, 32–34. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.H.; Zobniw, C.M.; Trinh, V.A.; Ma, J.; Bassett, R.L.; Abdel-Wahab, N.; Anderson, J.; Davis, J.E.; Joseph, J.; Uemura, M.; et al. Infliximab associated with faster symptom resolution compared with corticosteroids alone for the management of immune-related enterocolitis. J. Immunother. Cancer 2018, 6, 103. [Google Scholar] [CrossRef]
- Stroud, C.R.; Hegde, A.; Cherry, C.; Naqash, A.R.; Sharma, N.; Addepalli, S.; Cherukuri, S.; Parent, T.; Hardin, J.; Walker, P. Tocilizumab for the management of immune mediated adverse events secondary to PD-1 blockade. J. Oncol. Pharm. Pr. 2017, 25, 551–557. [Google Scholar] [CrossRef]
- Fujita, K.; Kim, Y.H.; Kanai, O.; Yoshida, H.; Mio, T.; Hirai, T. Emerging concerns of infectious diseases in lung cancer patients receiving immune checkpoint inhibitor therapy. Respir. Med. 2019, 146, 66–70. [Google Scholar] [CrossRef]
- Brand, F.Z.A.; Suter, N.; Adam, J.-P.; Faulques, B.; Maietta, A.; Soulieres, D.; Blais, N. Severe immune mucositis and esophagitis in metastatic squamous carcinoma of the larynx associated with pembrolizumab. J. Immunother. Cancer 2018, 6, 22. [Google Scholar] [CrossRef] [Green Version]
- Indini, A.; Di Guardo, L.; Cimminiello, C.; Prisciandaro, M.; Randon, G.; De Braud, F.; Del Vecchio, M. Immune-related adverse events correlate with improved survival in patients undergoing anti-PD1 immunotherapy for metastatic melanoma. J. Cancer Res. Clin. Oncol. 2018, 145, 511–521. [Google Scholar] [CrossRef]
- Ariane, B.; Maliha, P.G.; Hudson, M.; Small, D.; Barbacki, A. A case of severe Pembrolizumab-induced neutropenia. Anti-Cancer Drugs 2018, 29, 817–819. [Google Scholar] [CrossRef]
- Horisberger, A.; La Rosa, S.; Zurcher, J.-P.; Zimmermann, S.; Spertini, F.; Coukos, G.; Obeid, M. A severe case of refractory esophageal stenosis induced by nivolumab and responding to tocilizumab therapy. J. Immunother. Cancer 2018, 6, 156. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, H.; Fujieda, K.; Miyashita, A.; Fukushima, S.; Ikeda, T.; Kubo, Y.; Senju, S.; Ihn, H.; Nishimura, Y.; Oshiumi, H. Combined blockade of IL6 and PD-1/PD-L1 signaling abrogates mutual regulation of their immunosuppressive effects in the tumor microenvironment. Cancer Res. 2018, 78, 5011–5022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lomax, A.J.; Lim, J.; Cheng, R.; Sweeting, A.; Lowe, P.; McGill, N.; Shackel, N.; Chua, E.; McNeil, C. Immune toxicity with checkpoint inhibition for metastatic melanoma: Case series and clinical management. J. Ski. Cancer 2018, 2018, 9602540. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, K.; Thomas, M.; Dougan, M. Diagnosis and Management of Hepatitis in Patients on Checkpoint Blockade. Oncologist 2018, 23, 991–997. [Google Scholar] [CrossRef] [Green Version]
- Matsubara, T.; Nishida, T.; Mukai, K.; Yamamoto, M.; Fukui, K.; Adachi, S.; Inada, M.; Higaki, Y.; Tomita, R.; Shimakoshi, H.; et al. Nivolumab induces sustained liver injury in a patient with malignant melanoma. Intern. Med. 2018, 57, 1789–1792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganatra, S.; Neilan, T.G. Immune Checkpoint Inhibitor-Associated Myocarditis. Oncology 2018, 23, 879–886. [Google Scholar] [CrossRef] [Green Version]
- Suresh, K.; Voong, K.R.; Shankar, B.; Forde, P.M.; Ettinger, D.S.; Marrone, K.A.; Kelly, R.J.; Hann, C.L.; Levy, B.; Feliciano, J.L.; et al. Pneumonitis in non-small cell lung cancer patients receiving immune checkpoint immunotherapy: Incidence and risk factors. J. Thorac. Oncol. 2018, 13, 1930–1939. [Google Scholar] [CrossRef] [Green Version]
- Huffman, B.M.; Kottschade, L.A.; Kamath, P.S.; Markovic, S.N. Hepatotoxicity after immune checkpoint inhibitor therapy in melanoma. Am. J. Clin. Oncol. 2018, 41, 760–765. [Google Scholar] [CrossRef]
- Iyoda, T.; Kurita, N.; Takada, A.; Watanabe, H.; Ando, M. Resolution of infliximab-refractory nivolumab-induced acute severe enterocolitis after cyclosporine treatment in a patient with non-small cell lung cancer. Am. J. Case Rep. 2018, 19, 360–364. [Google Scholar] [CrossRef]
- Nassri, A.; Muenyi, V.; Alkhasawneh, A.; Ribeiro, B.D.S.; Scolapio, J.S.; Malespin, M.; de Melo, S.W., Jr. Ipilimumab and Nivolumab induced steroid-refractory colitis treated with infliximab: A case report. World J. Gastrointest. Pharmacol. Ther. 2019, 10, 29–34. [Google Scholar] [CrossRef]
- Kashima, J.; Okuma, Y.; Shimizuguchi, R.; Chiba, K. Bile duct obstruction in a patient treated with nivolumab as second-line chemotherapy for advanced non-small-cell lung cancer: A case report. Cancer Immunol. Immunother. 2017, 67, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Schadendorf, D.; Wolchok, J.D.; Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.-J.; Cowey, C.L.; Lao, C.D.; Chesney, J.; et al. Efficacy and safety outcomes in patients with advanced melanoma who discontinued treatment with nivolumab and ipilimumab because of adverse events: A pooled analysis of randomized phase II and III trials. J. Clin. Oncol. 2017, 35, 3807–3814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horvat, T.Z.; Adel, N.G.; Panageas, K.S.; Wolchok, J.D.; Chapman, P.B.; Dang, T.-O.; Momtaz, P.; Postow, M.A.; Callahan, M.K.; Carvajal, R.D.; et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with Ipilimumab at Memorial Sloan Kettering Cancer Center. J. Clin. Oncol. 2015, 33, 3193–3198. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| ICPI Monoclonal Antibody | Trade Name | Mechanism of Action |
|---|---|---|
| Ipilimumab | Yervoy | CTLA-4 inhibitor |
| Pembrolizumab | Keytruda | PD-1 inhibitor |
| Nivolumab | Opdivo | PD-1 inhibitor |
| Atezolizumab | Tecentriq | PD-L1 inhibitor |
| Avelumab | Bavencio | PD-L1 inhibitor |
| Durvalumab | Imfinzi | PD-L1 inhibitor |
| Cemiplimab | Libtayo | PD-1 inhibitor |
| Ipilimu. | Pembrolizu. | Nivolu. | Atezolizu. | Avelu. | Durvalu. | Cemipli. | |
|---|---|---|---|---|---|---|---|
| Breast cancer | ✓ | ||||||
| Cervical cancer | ✓ | ||||||
| Colorectal cancer | ✓ | ✓ | ✓ | ||||
| Cutaneous squamous cell carcinoma | ✓ | ✓ | |||||
| Endometrial carcinoma | ✓ | ||||||
| Esophageal cancer | ✓ | ✓ | |||||
| Gastric cancer | ✓ | ||||||
| Head and neck cancer | ✓ | ✓ | |||||
| Hepatocellular carcinoma | ✓ | ✓ | ✓ | ✓ | |||
| Hodgkin lymphoma | ✓ | ✓ | |||||
| Large B-cell lymphoma | ✓ | ||||||
| Melanoma | ✓ | ✓ | ✓ | ✓ | |||
| Merkel cell carcinoma | ✓ | ✓ | |||||
| Microsatellite instability-high tumors | ✓ | ||||||
| Non-small cell lung cancer | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Renal cell carcinoma | ✓ | ✓ | ✓ | ✓ | |||
| Small cell lung cancer | ✓ | ✓ | ✓ | ✓ | |||
| Tumor mutation burden-high tumors | ✓ | ||||||
| Urothelial carcinoma | ✓ | ✓ | ✓ | ✓ | ✓ |
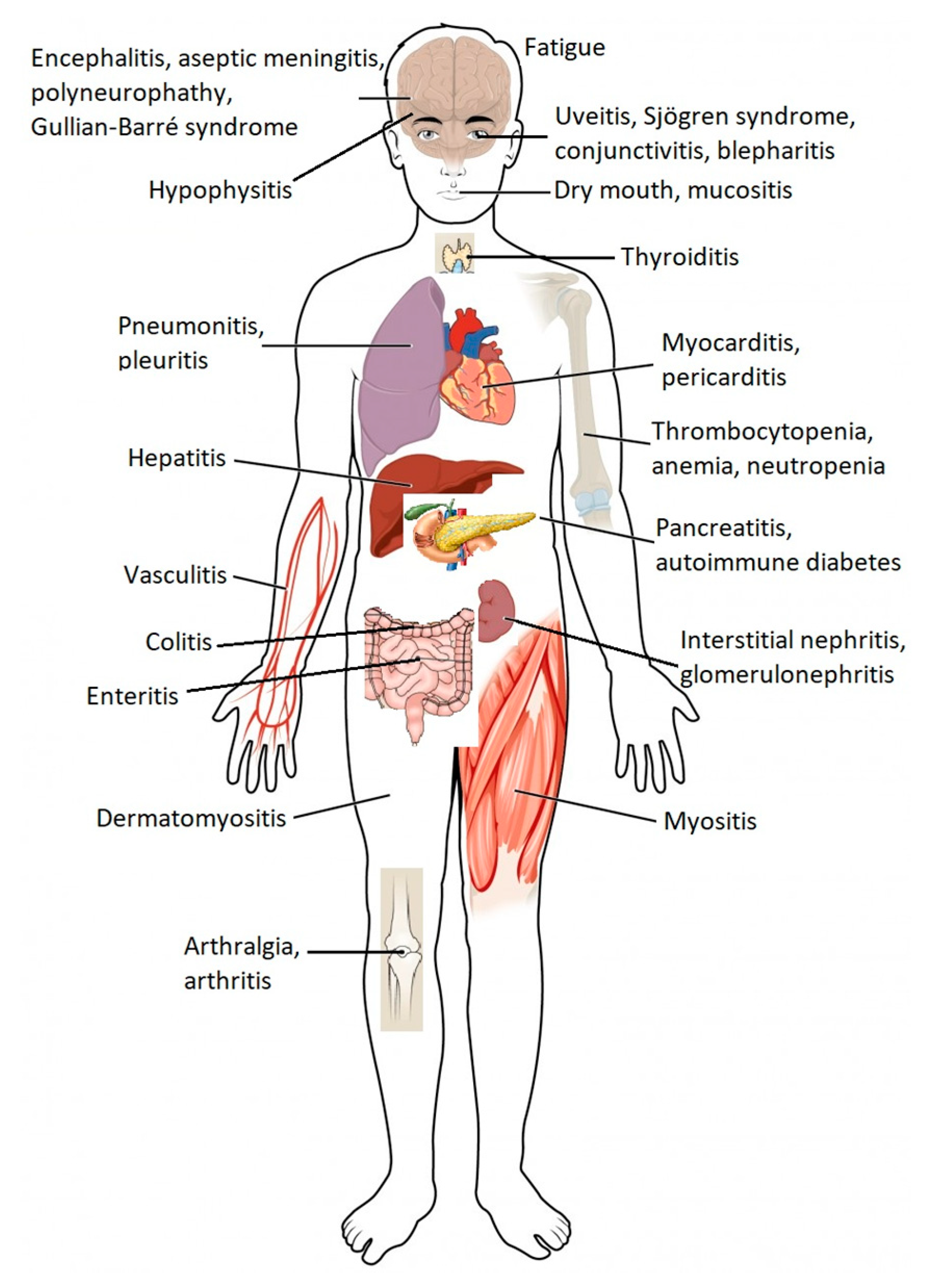
| Affected Organ or System of the Human Body | Immune Related Adverse Effects |
|---|---|
| Skin | Bullous dermatosis Skin rash/Inflammatory dermatitis Severe skin reactions |
| Gastrointestinal | Colitis Hepatitis |
| Lung | Pneumonitis |
| Endocrine | Diabetes Hyperthyroidism (primary) Hypophysitis Primary adrenal insufficiency |
| Musculoskeletal | Inflammatory arthritis Myositis Polymyalgia rheumatica |
| Renal | Nephritis |
| Nervous System | Myasthenia gravis Guillain–Barré syndrome Peripheral neuropathy Autonomic neuropathy Aseptic meningitis Encephalitis Transverse myelitis |
| Hematological | Autoimmune hemolytic anemia Acquired thrombotic thrombocytopenic purpura Uremic hemolytic syndrome Aplastic anemia Lymphopenia Immune thrombocytopenia Acquired hemophilia |
| Cardiovascular | Myocarditis Pericarditis ArrhythmiasHeart failure associated with ventricular failure Vasculitis Venous thromboembolism |
| Ocular | Uveitis/Iritis Episcleritis Blepharitis |
| Common Adverse Reactions | Research of Alternative/Non-Inflammatory Etiologies | Degree of Toxicity | Recommended Management of Immune-Related Adverse Events (irAEs) |
|---|---|---|---|
| Gastrointestinal Diarrhea/Colitis | Exclude infectious etiology (Clostridium difficile) | Grade 1 (Mild) | Symptomatic treatment Consider budesonide 9 mg/day Continue immunotherapy |
| Grade 2 (Moderate) | Delay immunotherapy Methylprednisolone IV 0.5–1 mg/kg/day (or oral equivalent) Consider gastroenterology and colonoscopy consultation When improving to ≤ grade 1, reduce the dose for at least 4 weeks | ||
| Grade 3–4 (Severe) | Stop immunotherapy Methylprednisolone IV 1–2 mg/kg/day When improving to ≤ grade 1, reduce the dose for at least 4 weeks If no improvement in symptoms within 48–72 h, consider 2nd line immunosuppression (infliximab) | ||
| Hepatitis | Evaluate for: - Alcohol intake - Concomitant drugs with hepatotoxic potential - Exclude biliary disease/biliary obstruction | Grade 1 (Mild) | Continue immunotherapy Repeat LFTs within 1 week |
| Grade 2 (Moderate) | Delay immunotherapy Repeat LFTs every 3–5 days Methylprednisolone IV 0.5–1 mg/kg/day (or oral equivalent) When improving to mild or baseline, reduce the dose of steroids for at least 4 weeks | ||
| Grade 3–4 (Severe) | Stop immunotherapy Increase the frequency of LFTs to 1–2 days Methylprednisolone IV 1–2 mg/kg/day Gastroenterology consultation If no improvement in symptoms within 48-72 h, consider 2nd line immunosuppression (infliximab) | ||
| Pneumonitis | Evaluate for: - Pulmonary embolism - Cardiac causes - Infectious etiology - COPD - Seasonal allergies/cough post-nasal drip | Grade 1 (Mild) | Delay immunotherapy Monitor symptoms Repeat chest X-ray in 2–4 weeks |
| Grade 2 (Moderate) | Delay immunotherapy Monitor symptoms closely, consider hospitalization Re-image every 1–3 days Pneumology and infectious disease consultations, consider bronchoscopy Methylprednisolone IV 1–2 mg/kg/day (or oral equivalent) When symptoms improve, reduce the dose of steroids for at least 4 weeks | ||
| Grade 3–4 (Severe) | Stop immunotherapy Methylprednisolone IV 2–4 mg/kg/day, discontinue steroids for a period of at least 6 weeks If no improvement in symptoms within 48–72 h, consider 2nd line immunosuppression (infliximab, mycophenolate mofetil, IVIG) | ||
| Dermatological adverse reactions | Exclude non-inflammatory causes (allergic reaction to other drugs, photosensitivity, etc.) | Grade 1 (Mild) | Continue immunotherapy Supportive therapy emollients, low-potency topical steroids, antihistamines |
| Grade 2 (Moderate) | Continue immunotherapy Topical steroids of moderate-high potency If persistent, despite optimized topical treatment, consider methylprednisolone 0.5–1 mg/kg/day (or oral equivalent) If it improves slightly or resolves, reduce the dose of steroids for at least 4 weeks Consider dermatological evaluation and skin biopsy | ||
| Grade 3–4 (Severe) | Delay immunotherapy Methylprednisolone IV 1–2 mg/kg/day (or oral equivalent) If it improves to mild or resolves, reduce the dose of steroids for at least 4 weeksConsider skin biopsy | ||
| Endocrinopathies | Exclude non-inflammatory etiology of symptoms | Grade 1 (Mild) | Continue immunotherapy If TSH is abnormal, add free T4 and T3 Consider morning cortisol and ACTH |
| Grade 2 (Moderate) | TSH, free T4, morning cortisol and ACTHConsider pituitary MRIMethylprednisolone IV 1–2 mg/kg/day (or oral equivalent)If it improves, reduce the dose of steroids for at least 4 weeksHormone replacement therapy if indicatedEndocrinology consultation | ||
| Grade 3–4 (Severe) | Delay or discontinue immunotherapy If adrenal crisis is suspected, exclude infection/sepsis, BP support Stress doses of mineralocorticosteroid |
| Immunosuppressive Drug/Commentary | Immune-Related Adverse Events (irAEs) Treated |
|---|---|
| Anti-IL6: tocilizumab. It is not expected that anti-tumoral response will cease, since concomitant blockade of PD-1/PD-L1 and IL-6 have a synergic anti-tumoral effect [17,24,25]. | Severe or refractory arthritis, large vessel vasculitis, uveitis, myocarditis, myastenia gravis, pneumonitis, hepatitis, hypophisitis, colitis, pancreatitis, mediated coagulopathy. |
| Sulfasalazine [26]. | Refractory rheumatologic irAEs. |
| Mycophenolate mofetil [17,27,28,29,30]. | Hepatitis, pneumonitis, myocarditis. |
| Calcineurin inhibitors: tacrolimus (dose based on blood results) or cyclosporine [17,27,29,31,32]. | Tacrolimus: hepatitis, myocarditis. Cyclosporine: enterocolitis, hepatitis. |
| Anti-TNFα blockade: infliximab, adalimumab, golimumab, etanercept, certolizumab [18,19,29,30,33,34]. | May be a good option especially in cases of steroid refractory colitis, myocarditis, bile duct obstruction and pneumonitis, arthritis, nephritis, and uveitis. |
| IV immunoglobulins [16]. | Guillain–Barré syndrome, subacute and chronic inflammatory demyelinating polineuropathy, thromnocytopenia, enteric neuropathy, ocular myositis, encephalitis, facial nerve palsy, myasthenia gravis, transverse myelitis. |
| Plasmapheresis [16]. | Myastenia gravis, acute inflammatory demyelinating poliradiculoneuropathy, encephalitis. |
| Azathioprine: before administration, a thiopurine S-methyltransferase (TPMT) genotype or enzyme function test should be carried, because patients with lower TPMT activity have an increased risk of manifesting life-threatening bone marrow suppression [16,27]. | Hepatitis. |
| Anti-CD20 depletion: rituximab, ofatumumab, obinutuzumab, ocrelizumab [16]. | Systemic lupus erythematosus, antineutrophil cytoplasmic antibody-associated vasculitis, severe Sjögren’s syndrome, cutaneous vasculitis, nephritis, autoimmune autonomic ganglionopathy, sensory ganglionopathy, myasthenia gravis, transverse myelitis, enteric neuropathy, encephalitis, aseptic meningitis, hepatitis. |
| Anti-IL-17-blockade: ixekizumab, brodalumab, secukinumab [16]. | Colitis, severe psoriasis refractory to anti-TNFα therapy, rheumatoid arthritis, anti-IL6 refractory irAEs. |
| Anti-IL-23 and anti-IL-12 blockade: ustekinumab [16]. | Psoriasis and psoriatic arthritis. |
| Janus kinase inhibitor: tofacitinib [16]. | Rheumatoid arthritis and ulcerative colitis. |
| Anti-B-cell strategy: belimumab [16]. | Lupus erythematosus. |
| Cyclophosphamide [16]. | Symptomatic sarcoidosis, pneumonitis, Guillian–Barré syndrome, Stevens–Johnson syndrome with central and neurologic symptoms, autoimmune autonomic ganglionopathy, sensory ganglionopathy, polyneuropathy, central neuritis. |
| Immunotherapy Alert and Education Card for Patients |
|---|
| What is immunotherapy? Cancer immunotherapy is a type of drug therapy that stimulates the body’s natural defenses to fight cancer. |
| How will immunotherapy be administered? Immunotherapy is administered intravenously (IV). It can be administered via peripheral or central venous (IV) access. |
| What adverse effects may arise during treatment? The onset of adverse effects may vary from patient to patient and with the prescribed therapy. Adverse effects can occur from 1–3 weeks after the start of treatment to months after the end of therapy. It is recommended to discuss any new adverse effects immediately with the oncologist, nurse, or pharmacist. The general adverse effects to be observed include: (1) Dermatological problems: rash, itching, dry skin, rash, changes in color; (2) Gastrointestinal problems: flatulence, abdominal bloating, nausea or vomiting, changes in the usual pattern of bowel movements, black, sticky, tar-like stools, or blood or mucus stools; (3) Liver problems: nausea or vomiting, loss of appetite, pain in the right side of the stomach, yellowing of the skin or whites of the eyes, dark urine, bleeding or bruising more often than normal; (4) Endocrine problems (especially thyroid, pituitary, and adrenal): fatigue, rapid heartbeat, weight loss or gain, increased sweating, hair loss, feeling cold, constipation, lower voice, muscle pain, dizziness or fainting, headaches that are persistent or headache that is unusual; (5) Lung problems: shortness of breath, chest pain, cough. |
| Who can I contact if I develop adverse effects? Name and contact of the oncologist: Contact of the hospital where the treatment is being carried out: Emergency phone: Nursing team contact: Hospital pharmacy contact: |
| How will adverse effects be treated? Adverse effects are treated based on the nature and severity of the symptoms. However, in general, most adverse effects may require the administration of a corticosteroid for treatment. The dosage and duration of treatment may vary depending on the intensity and severity of the symptoms and will be discussed with the attending physician. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morgado, M.; Plácido, A.; Morgado, S.; Roque, F. Management of the Adverse Effects of Immune Checkpoint Inhibitors. Vaccines 2020, 8, 575. https://doi.org/10.3390/vaccines8040575
Morgado M, Plácido A, Morgado S, Roque F. Management of the Adverse Effects of Immune Checkpoint Inhibitors. Vaccines. 2020; 8(4):575. https://doi.org/10.3390/vaccines8040575
Chicago/Turabian StyleMorgado, Manuel, Ana Plácido, Sandra Morgado, and Fátima Roque. 2020. "Management of the Adverse Effects of Immune Checkpoint Inhibitors" Vaccines 8, no. 4: 575. https://doi.org/10.3390/vaccines8040575
APA StyleMorgado, M., Plácido, A., Morgado, S., & Roque, F. (2020). Management of the Adverse Effects of Immune Checkpoint Inhibitors. Vaccines, 8(4), 575. https://doi.org/10.3390/vaccines8040575





