Budget Impact Analysis of Fixed Dose Versus Weight-Based Dosing Regimen of Nivolumab and Pembrolizumab in the Treatment of Non-Small Cell Lung Cancer
Abstract
:1. Introduction
1.1. Economic Impact
1.2. New Dosage Strategy
2. Materials and Methods
2.1. Methodological Overview
2.2. Database
- -
- the number of patients treated with each drug according to the treatment line,
- -
- the number of cures received per year per patient,
- -
- the average number of cures received per year by all patients treated.
2.3. Time Horizon
2.4. Target Population
- ▪
- in 1st line, patients treated with pembrolizumab as monotherapy indicated for metastatic NSCLC in adults whose tumors express PD-L1 with a ≥ 50% tumor proportion score (TPS), with no EGFR or ALK positive tumor mutations.
- ▪
- in 2nd line, patients included were:
- ◦
- patients treated with nivolumab, as monotherapy for the treatment of locally advanced or metastatic NSCLC, without scoring condition,
- ◦
- patients treated with pembrolizumab as monotherapy for the treatment of locally advanced or metastatic NSCLC in adults whose tumors express PD-L1 with a TPS greater than or equal to 1%.
- ▪
- In the first line, due to an altered general condition, an advanced age of the patients or the presence of comorbidities, the HAS, in its opinion, considers that around 80% of patients will receive treatment. In addition, in the first line, the number of patients likely to benefit from pembrolizumab is low due to tumor overexpression of PD-L1 ≥ 50%. Among the patients included in the pivotal Keynote 024 study [28], approximately 25% had a PD-L1 status ≥ 50%. More conservatively, in this study, we assumed that 20% of patients have a PD-L1 status ≥ 50%. Those 20% patients are then potentially affected by a prescription for pembrolizumab.
- ▪
- In the 2nd line, in its opinion, the HAS estimates that among the patients treated in the first line, only 40% of patients will be eligible for a new treatment. However, with regard to treatment with ICI, which is theoretically better tolerated than treatment with chemotherapy, it is assumed that 50% of patients will be eligible for second-line treatment. In addition, we assume that patients who received first-line treatment with ICI will not be eligible for a new immunotherapy treatment even if there are no data on the prescription of ICI in the 2nd line. Finally, among the patients still to be treated in the 2nd line, the number of patients likely to benefit from nivolumab and/or pembrolizumab is estimated at 100% due to the fact that nivolumab prescription does not depend on PD-L1 status.
2.5. Evolution of the Analysis Population between 2018 and 2019
2.6. Cost of Nivolumab and Pembrolizumab
2.7. Patients’ Weight
2.8. Duration of Treatment
2.9. Scenarios
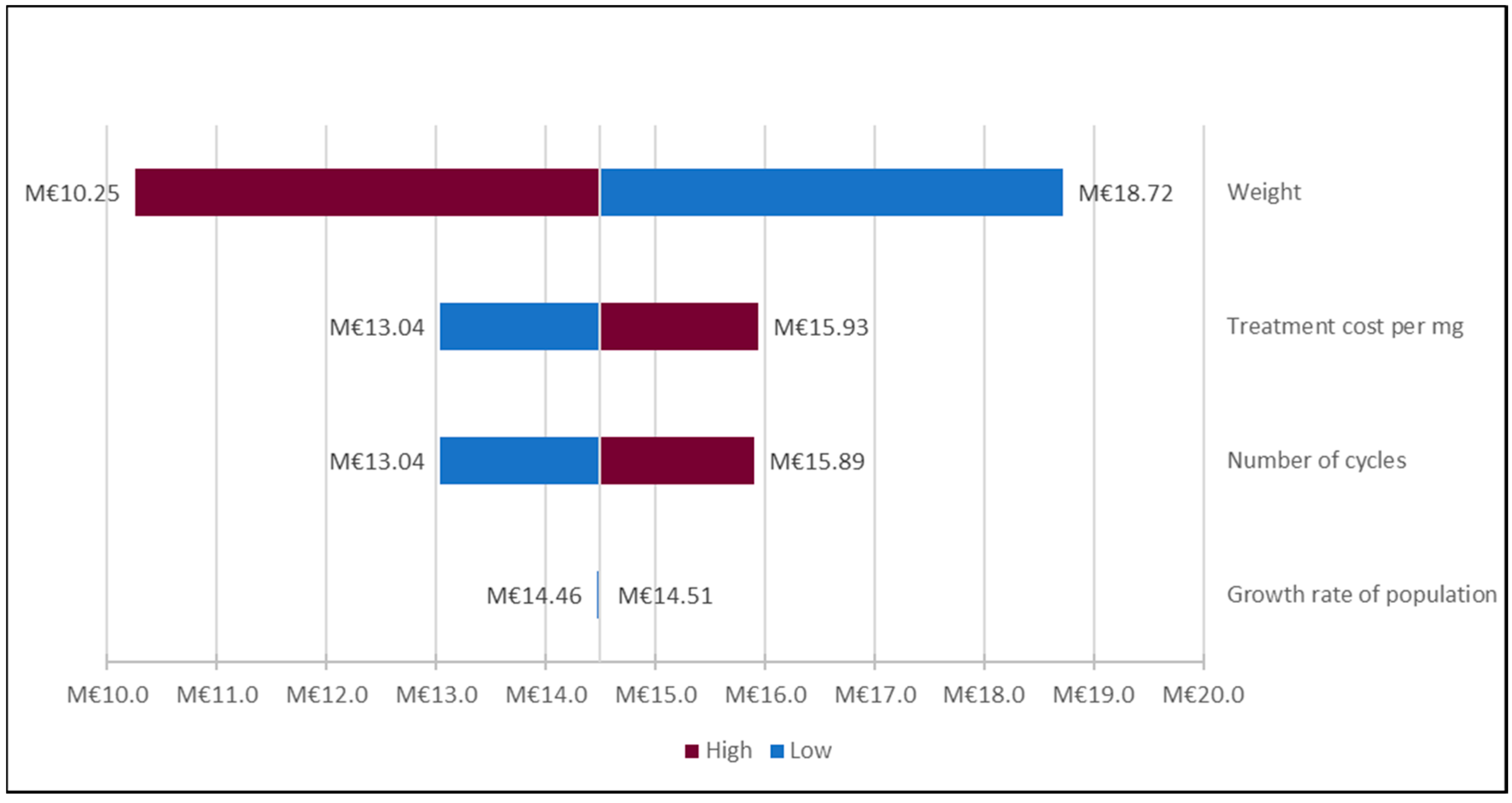
2.10. Sensitivity Analyses
3. Results
4. Discussion
Comparison with the Literature
- ▪
- For the largest centers, regroup patients receiving nivolumab visits over a few targeted days each week in order to be able to use the rest of each vial for the next patient.
- ▪
- In centers with a smaller volume of patients, the use of dose standardization (dose-banding) up to a maximum dose is proposed. In the PGTM model, the standard doses are rounded to the nearest 20 mg, unless this exceeds 5% of the difference from the dose he or she would have received based on his or her weight (3 mg/kg), in which case, they increase to the higher dose (rounded up to the next 20 mg), up to a maximum of 240 mg.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fact Sheets by Cancer. Available online: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx (accessed on 30 October 2020).
- Le Cancer du Poumon—Les Cancers les Plus Fréquents. Available online: http://www.e-cancer.fr/Professionnels-de-sante/Les-chiffres-du-cancer-en-France/Epidemiologie-des-cancers/Les-cancers-les-plus-frequents/Cancer-du-poumon (accessed on 30 October 2020).
- Haute Autorité de Santé—Cancer Broncho-Pulmonaire: Le Parcours de Soins Doit Préserver en Priorité Une Qualité de Vie. Available online: https://www.has-sante.fr/portail/jcms/c_1651595/fr/cancer-broncho-pulmonaire-le-parcours-de-soins-doit-preserver-en-priorite-une-qualite-de-vie (accessed on 30 October 2020).
- Schiller, J.H.; Harrington, D.; Belani, C.P.; Langer, C.; Sandler, A.; Krook, J.; Zhu, J.; Johnson, D.H. Comparison of Four Chemotherapy Regimens for Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2002, 346, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Barlesi, F.; Mazieres, J.; Merlio, J.-P.; Debieuvre, D.; Mosser, J.; Lena, H.; Ouafik, L.; Besse, B.; Rouquette, I.; Westeel, V.; et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: Results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet 2016, 387, 1415–1426. [Google Scholar] [CrossRef]
- Remon, J.; Passiglia, F.; Ahn, M.-J.; Barlesi, F.; Forde, P.M.; Garon, E.B.; Gettinger, S.; Goldberg, S.B.; Herbst, R.S.; Horn, L.; et al. Immune Checkpoint Inhibitors in Thoracic Malignancies: Review of the Existing Evidence by an IASLC Expert Panel and Recommendations. J. Thorac. Oncol. 2020, 15, 914–947. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.; Horn, L.; Hossein, B.; Ramalingam, S.; Pluzanski, A.; Burgio, M.; Garassino, M.; Chow, L.; Gettinger, S.; Crino, L.; et al. O.02 Long-term Survival Outcomes with Nivolumab (NIVO) in Pts with Previously Treated Advanced Non-Small Cell Lung Cancer (NSCLC): Impact of Early Disease Control and Response. J. Thorac. Oncol. 2019, 14, S1152–S1153. [Google Scholar] [CrossRef]
- Herbst, R.S.; Garon, E.B.; Kim, D.-W.; Cho, B.C.; Perez-Gracia, J.L.; Han, J.-Y.; Arvis, C.D.; Majem, M.; Forster, M.D.; Monnet, I.; et al. Long-Term Outcomes and Retreatment among Patients With Previously Treated, Programmed Death-Ligand 1‒Positive, Advanced Non‒Small-Cell Lung Cancer in the KEYNOTE-010 Study. J. Clin. Oncol. 2020, 38, 1580–1590. [Google Scholar] [CrossRef]
- Aguilar, E.; Ricciuti, B.; Gainor, J.; Kehl, K.; Kravets, S.; Dahlberg, S.; Nishino, M.; Sholl, L.; Adeni, A.; Subegdjo, S.; et al. Outcomes to first-line pembrolizumab in patients with non-small-cell lung cancer and very high PD-L1 expression. Ann. Oncol. 2019, 30, 1653–1659. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gümüş, M.; Mazières, J.; Hermes, B.; Çay Şenler, F.; Csőszi, T.; Fülöp, A.; et al. Pembrolizumab plus Chemotherapy for Squamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef]
- Institut National du Cancer, Le Prix des Médicaments Anticancéreux, Jun. Available online: https://www.e-cancer.fr/Expertises-et-publications/Catalogue-des-publications/Le-prix-des-medicaments-anticancereux (accessed on 30 October 2020).
- FDA, Approved Drugs—Modification of the Dosage Regimen for Nivolumab. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/modification-dosage-regimen-nivolumab (accessed on 30 October 2020).
- Zhao, X.; Suryawanshi, S.; Hruska, M.; Feng, Y.; Wang, X.; Shen, J.; Vezina, H.E.; McHenry, M.B.; Waxman, I.M.; Achanta, A.; et al. Assessment of nivolumab benefit–risk profile of a 240-mg flat dose relative to a 3-mg/kg dosing regimen in patients with advanced tumors. Ann. Oncol. 2017, 28, 2002–2008. [Google Scholar] [CrossRef]
- Freshwater, T.; Kondic, A.; Ahamadi, M.; Li, C.H.; De Greef, R.; De Alwis, D.; Stone, J.A. Evaluation of dosing strategy for pembrolizumab for oncology indications. J. Immunother. Cancer 2017, 5, 43. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Ribas, A.; Wolchok, J.D.; Hodi, F.S.; Hamid, O.; Kefford, R.; Weber, J.S.; Joshua, A.M.; Hwu, W.-J.; Gangadhar, T.C.; et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: A randomised dose-comparison cohort of a phase 1 trial. Lancet 2014, 384, 1109–1117. [Google Scholar] [CrossRef]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; Von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2017, 389, 255–265. [Google Scholar] [CrossRef]
- Fehrenbacher, L.; Spira, A.; Ballinger, M.; Kowanetz, M.; Vansteenkiste, J.; Mazieres, J.; Park, K.; Smith, D.; Artal-Cortes, A.; Lewanski, C.; et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): A multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016, 387, 1837–1846. [Google Scholar] [CrossRef]
- HAS. Guide Méthodologique—Choix méthodologiques pour l’évaluation économique à la HAS, October. Available online: https://www.hassante.fr/portail/upload/docs/application/pdf/2011-11/guide_methodo_vf.pdf (accessed on 30 October 2020).
- Haute Autorité de Santé—Analyse d’Impact Budgétaire: La HAS Enrichit l’Évaluation Médico-Économique des Produits de Santé. Available online: https://www.has-sante.fr/portail/jcms/c_2747893/en/analyse-d-impact-budgetaire-la-has-enrichit-l-evaluation-medico-economique-des-produits-de-sante (accessed on 30 October 2020).
- Population Légale de l’Île-de-FRANCE—Insee Flash Ile-de-France. Available online: https://www.insee.fr/fr/statistiques/4270719 (accessed on 15 November 2020).
- Dossier Complet—Département de la Seine-Saint-Denis (93) | Insee. Available online: https://www.insee.fr/fr/statistiques/2011101?geo=DEP-93 (accessed on 17 November 2020).
- Keyrus, Les Agences Régionales de Santé se Dotent d’un Outil de Pilotage Mutualisé à la Pointe de Diamant. Available online: http://keyrus-prod.s3.amazonaws.com/uploads/media/SuccessStories_ARS-2012.pdf (accessed on 30 October 2020).
- ScanSanté—Accès Aux Données Facilité | ATIH. Available online: https://www.atih.sante.fr/actualites/scansante-acces-aux-donnees-facilite (accessed on 15 November 2020).
- HAS. Tumeur Maligne, Affection Maligne du Tissu Lymphatique ou Hématopoïétique Mélanome Cutané, January. Available online: https://www.has-sante.fr/upload/docs/application/pdf/2012-03/ald_30_guide_melanome_web.pdf (accessed on 15 November 2020).
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [Green Version]
- Assurance Maladie. OPDIVO, Base des Médicaments et Informations Tarifaires (BDM_IT). Available online: http://www.codage.ext.cnamts.fr/codif/bdm_it//fiche/index_lis_ucd.php?p_code_cip=&p_nom_commercial=OPDIVO&p_nb=2&p_site=AMELI&p_homol_retro=&p_homol_taa=taa (accessed on 20 February 2020).
- Assurance Maladie. KEYTRUDA, Base des Médicaments et Informations Tarifaires (BDM_IT). Available online: http://www.codage.ext.cnamts.fr/codif/bdm_it//fiche/index_lis_ucd.php?p_code_cip=&p_nom_commercial=KEYTRUDA&p_nb=2&p_site=AMELI&p_homol_retro=&p_homol_taa=taa (accessed on 20 February 2020).
- Herbst, R.S.; Baas, P.; Kim, D.-W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.-Y.; Molina, J.; Kim, J.-H.; Arvis, C.D.; Ahn, M.-J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Morelle, M.; Plantier, M.; Dervaux, B.; Pagès, A.; Deniès, F.; Havet, N.; Perrier, L. Méthodes d’analyse et de traitement des données de coût : Approches par « micro-costing » et « gross-costing ». Revue d’Épidémiologie et de Santé Publique 2018, 66, S101–S118. [Google Scholar] [CrossRef]
- Patnaik, A.; Kang, S.P.; Rasco, D.; Papadopoulos, K.P.; Elassaiss-Schaap, J.; Beeram, M.; Drengler, R.; Chen, C.; Smith, L.; Espino, G.; et al. Phase I Study of Pembrolizumab (MK-3475; Anti-PD-1 Monoclonal Antibody) in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2015, 21, 4286–4293. [Google Scholar] [CrossRef] [Green Version]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the Treatment of Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef]
- Garon, E.; Reck, M.; Rodriguez-Abreu, D.; Robinson, A.; Hui, R.; Tibor, C.; Fulop, A.; Gottfried, M.; Peled, N.; Tafreshi, A. Use of a 200-mg fixed dose of pembrolizumab for the treatment of advanced non–small cell lung cancer (NSCLC). In Proceedings of the the 17th World Conference on Lung Cancer, Vienna, Austria, 4–7 December 2016; International Association for the Study of Lung Cancer (IASLC). Available online: https://library.iaslc.org/search-speaker?search_speaker=42008 (accessed on 30 October 2020).
- Hamid, O.; Puzanov, I.; Dummer, R.; Schachter, J.; Daud, A.; Schadendorf, D.; Blank, C.U.; Cranmer, L.D.; Robert, C.; Pavlick, A.C.; et al. Final analysis of a randomised trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory advanced melanoma. Eur. J. Cancer 2017, 86, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Ribas, A.; Hamid, O.; Daud, A.I.; Wolchok, J.D.; Joshua, A.M.; Hwu, W.-J.; Weber, J.S.; Gangadhar, T.C.; Joseph, R.W.; et al. Three-year overall survival for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. J. Clin. Oncol. 2016, 34, 9503. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Drake, C.G.; Wollner, I.; Powderly, J.D.; Picus, J.; Sharfman, W.H.; Stankevich, E.; Pons, A.; Salay, T.M.; McMiller, T.L.; et al. Phase I Study of Single-Agent Anti-Programmed Death-1 (MDX-1106) in Refractory Solid Tumors: Safety, Clinical Activity, Pharmacodynamics, and Immunologic Correlates. J. Clin. Oncol. 2010, 28, 3167–3175. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, X.; Bajaj, G.; Agrawal, S.; Bello, A.; Lestini, B.; Finckenstein, F.G.; Park, J.-S.; Roy, A. Nivolumab Exposure–Response Analyses of Efficacy and Safety in Previously Treated Squamous or Nonsquamous Non–Small Cell Lung Cancer. Clin. Cancer Res. 2017, 23, 5394–5405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- L’obésité en France: Les Écarts Entre Catégories Sociales S’accroissent—Insee Première. Available online: https://www.insee.fr/fr/statistiques (accessed on 30 October 2020).
- A McDowell, M.; Fryar, C.D.; Ogden, C.L. Anthropometric reference data for children and adults: United States, 1988-1994. Vital- Health Stat. Data Natl. Health Surv. 2009, 11, 1–68. [Google Scholar]
- Norum, J.; Antonsen, M.A.; Tollåli, T.; Al-Shibli, K.; Andersen, G.; Svanqvist, K.H.; Helbekkmo, N. Pembrolizumab as second-line therapy in non-small cell lung cancer in northern Norway: Budget impact and expected gain—A model-based analysis. ESMO Open 2017, 2, e000222. [Google Scholar] [CrossRef] [Green Version]
- INESS. Opdivo—Cancer du Poumon Non à Petites Cellules, August. Available online: https://www.inesss.qc.ca/fileadmin/doc/INESSS/Inscription_medicaments/Avis_au_ministre/Aout_2016/Opdivo_CPNPC_2016_08.pdf (accessed on 30 October 2020).
- (PGTM) Programme de Gestion Thérapeutique des Médicaments. NIVOLUMAB—Quelle Stratégie Posologique Devrait-on Privilégier: Dose en Fonction du Poids, Dose Fixe ou Dose en Fonction du Poids Avec Une Dose Maximale? Rapport d’Évaluation, September. Available online: http://www.pgtm.org/documentation/FSW/Nivolumab_Strat%C3%A9gie%20posologique.pdf (accessed on 30 October 2020).
- (PGTM) Programme de Gestion Thérapeutique des Médicaments. PEMBROLIZUMAB—Quelle Stratégie Posologique Devrait-on Privilégier: Dose en Fonction du Poids, Dose Fixe ou Dose en Fonction du Poids Avec Une Dose Maximale? Rapport d’Évaluation, September. Available online: http://www.pgtm.org/documentation/FSW/Pembrolizumab_Strat%C3%A9gie%20posologique.pdf (accessed on 30 October 2020).
- INESS. Keytruda—Cancer du Poumon Non à Petites Cellules—Avis d’Ajout d’Une Indication Reconnue à la Liste Établissements, August. Available online: https://www.inesss.qc.ca/fileadmin/doc/INESSS/Inscription_medicaments/Avis_au_ministre/Novembre_2017/Keytruda_1re_int_CPNPC_2017_08.pdf (accessed on 30 October 2020).
- Goldstein, D.A.; Gordon, N.; Davidescu, M.; Leshno, M.; Steuer, C.E.; Patel, N.; Stemmer, S.M.; Zer, A. A Phamacoeconomic Analysis of Personalized Dosing vs Fixed Dosing of Pembrolizumab in Firstline PD-L1-Positive Non–Small Cell Lung Cancer. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef]
- Bayle, A.; Besse, B.; Annereau, M.; Bonastre, J. Switch to anti-programmed cell death protein 1 (anti-PD-1) fixed-dose regimen: What is the economic impact? Eur. J. Cancer 2019, 113, 28–31. [Google Scholar] [CrossRef]




| Year | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 |
|---|---|---|---|---|---|---|---|
| Number of patients | 6981 | 7036 | 7207 | 7351 | 7574 | 7449 | 7642 |
| Growth rates | +0.8% | +2.4% | +2.0% | +3.0% | −1.7% | 2.6% |
| Pembrolizumab | Nivolumab | |
|---|---|---|
| 2018 (PMSI data) | ||
| Avicenne Hospital | 20 | 84 |
| Ile-de-France | 261 | 1876 |
| 2019 (projection after application of population change rate) | ||
| Avicenne Hospital | 20 * | 85 * |
| Ile-de-France | 265 * | 1904 * |
| Posology A | Weight (kg) B | Total (mg) C = A × B | Price (All Taxes Included)/mg D | Cost per Administration C × D | Number of Cycles E | Treatment Cost per Patient (C × D) × E |
|---|---|---|---|---|---|---|
| Pembrolizumab | ||||||
| 2 mg/kg | 68 | 136 | €26.84 * | €3650 | 10.5 | €38,325 |
| 200 mg | 68 | 200 | €26.84 * | €5368 | 10.5 | €56,364 |
| Posology A | Weight (kg) B | Total (mg) C = A × B | Price (All Taxes Included)/mg D | Cost per Administration C × D | Number of Cycles E | Treatment Cost per Patient (C × D) × E |
|---|---|---|---|---|---|---|
| Nivolumab | ||||||
| 3 mg/kg | 68 | 204 | €10.58 * | €2158 | 8.5 | €18,343 |
| 240 mg | 68 | 240 | €10.58 * | €2539 | 8.5 | €21,582 |
| Pembrolizumab | ||||||
| 2 mg/kg | 68 | 136 | €26.84 * | €3650 | 4.9 | €17,885 |
| 200 mg | 68 | 200 | €26.84 * | €5368 | 4.9 | €26,303 |
| ICI | Nivolumab | Pembrolizumab | |
|---|---|---|---|
| 1st Line | 2nd Line | ||
| Cost of Weight-based dose | €18,343 | €38,325 | €17,885 |
| Cost of fixed dose | €21,582 | €56,364 | €26,303 |
| Additional treatment cost per year and per patient | €3239 | €18,039 | €8418 |
| Nivolumab | 2018 | 2019 | |
|---|---|---|---|
| Low Hypothesis | High Hypothesis | ||
| Avicenne Hospital | €272,076 | €277,032 | €152,215 |
| Ile-de-France | €6,076,364 | €5,948,192 | €3,268,238 |
| Pembrolizumab (1st line + 2nd line) | 2018 | 2019 | |
| Low hypothesis | High hypothesis | ||
| Avicenne Hospital | €278,039 | €279,937 | €604,329 |
| Ile-de-France | €3,628,414 | €4,252,835 | €11,217,904 |
| Nivolumab + Pembrolizumab | 2018 | 2019 | |
|---|---|---|---|
| Low Hypothesis | High Hypothesis | ||
| Avicenne Hospital | €550,115 | €556,969 | €756,544 |
| Ile-de-France | €9,704,778 | €10,201,027 | €14,486,141 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monirul, S.; Rigal, M.; Chouahnia, K.; Le Jouan, M.; Apparuit, M.; Paix, A.; Jacolot, A.; Zelek, L.; Duchemann, B. Budget Impact Analysis of Fixed Dose Versus Weight-Based Dosing Regimen of Nivolumab and Pembrolizumab in the Treatment of Non-Small Cell Lung Cancer. Vaccines 2020, 8, 730. https://doi.org/10.3390/vaccines8040730
Monirul S, Rigal M, Chouahnia K, Le Jouan M, Apparuit M, Paix A, Jacolot A, Zelek L, Duchemann B. Budget Impact Analysis of Fixed Dose Versus Weight-Based Dosing Regimen of Nivolumab and Pembrolizumab in the Treatment of Non-Small Cell Lung Cancer. Vaccines. 2020; 8(4):730. https://doi.org/10.3390/vaccines8040730
Chicago/Turabian StyleMonirul, Sanjana, Marthe Rigal, Kader Chouahnia, Mélisande Le Jouan, Maxime Apparuit, Adrien Paix, Anne Jacolot, Laurent Zelek, and Boris Duchemann. 2020. "Budget Impact Analysis of Fixed Dose Versus Weight-Based Dosing Regimen of Nivolumab and Pembrolizumab in the Treatment of Non-Small Cell Lung Cancer" Vaccines 8, no. 4: 730. https://doi.org/10.3390/vaccines8040730
APA StyleMonirul, S., Rigal, M., Chouahnia, K., Le Jouan, M., Apparuit, M., Paix, A., Jacolot, A., Zelek, L., & Duchemann, B. (2020). Budget Impact Analysis of Fixed Dose Versus Weight-Based Dosing Regimen of Nivolumab and Pembrolizumab in the Treatment of Non-Small Cell Lung Cancer. Vaccines, 8(4), 730. https://doi.org/10.3390/vaccines8040730






