Improved CO2/CH4 Separation in Carbon Molecular Sieve Membranes via Copolymerization of Long-Chain Flexible Structures
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
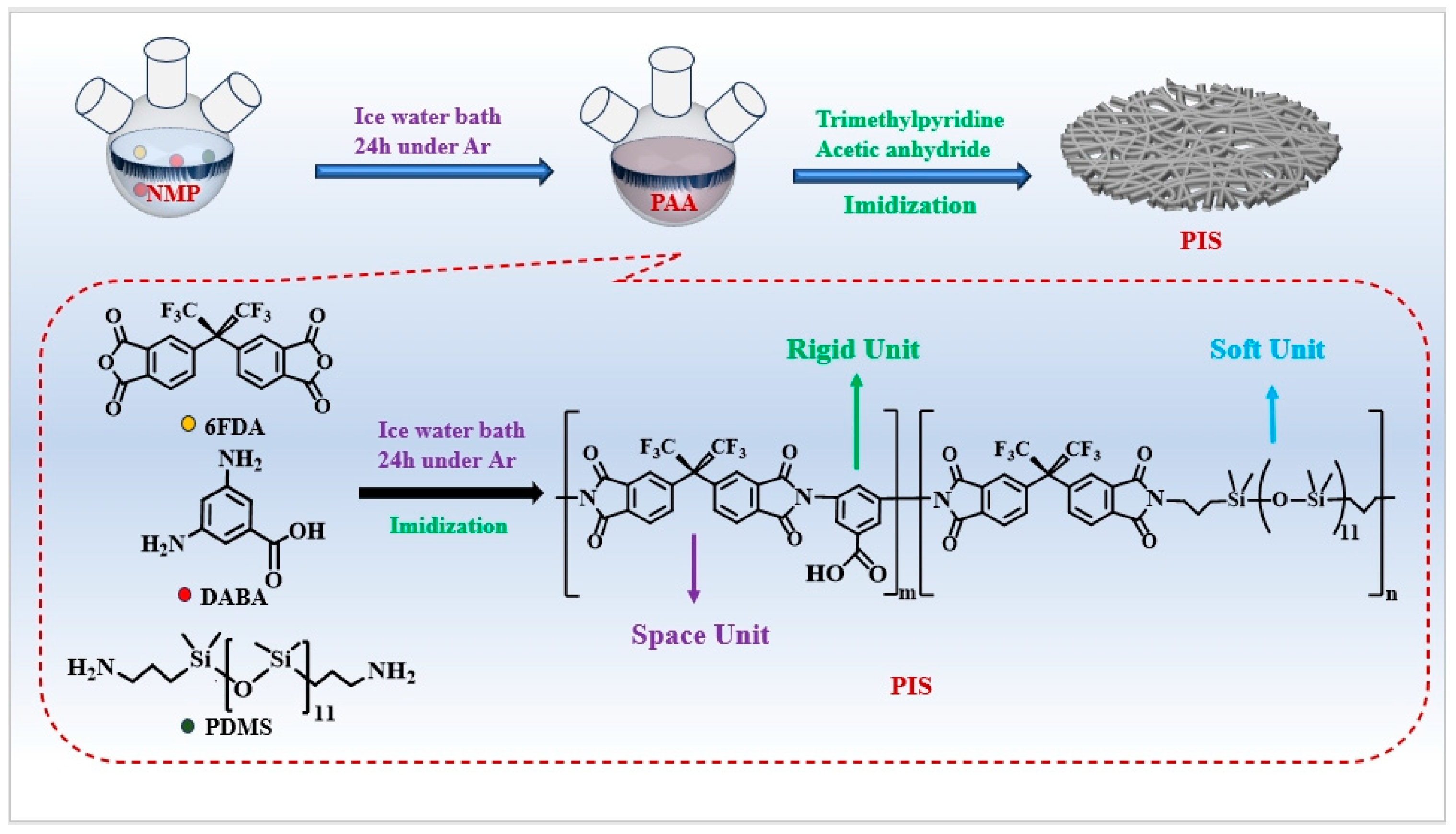
2.2. Synthesis of Polysiloxane-Based Polyimides (PIS)
2.3. Preparation of CMS Membranes
2.4. Permeation Measurement
2.5. Characterizations
3. Results
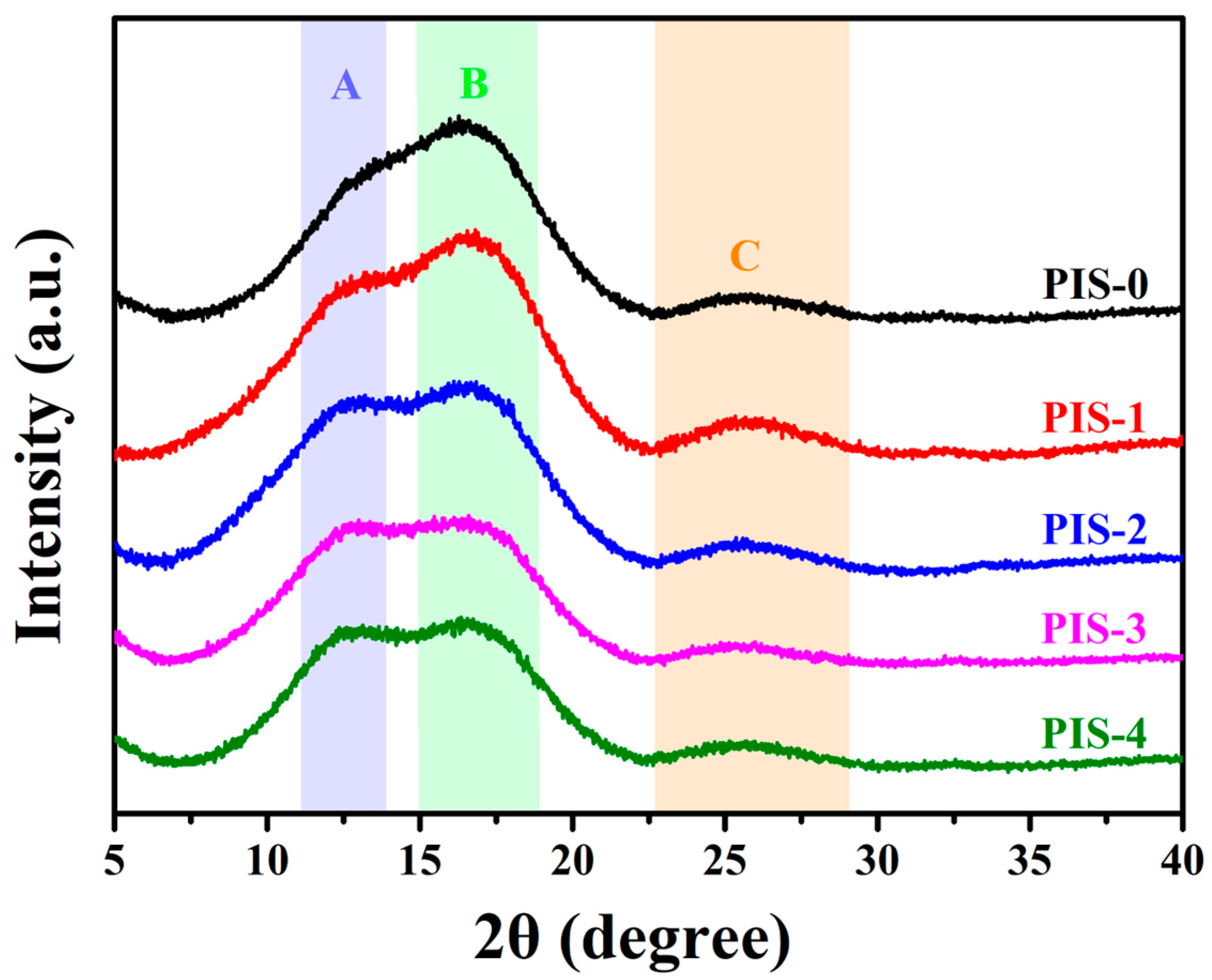
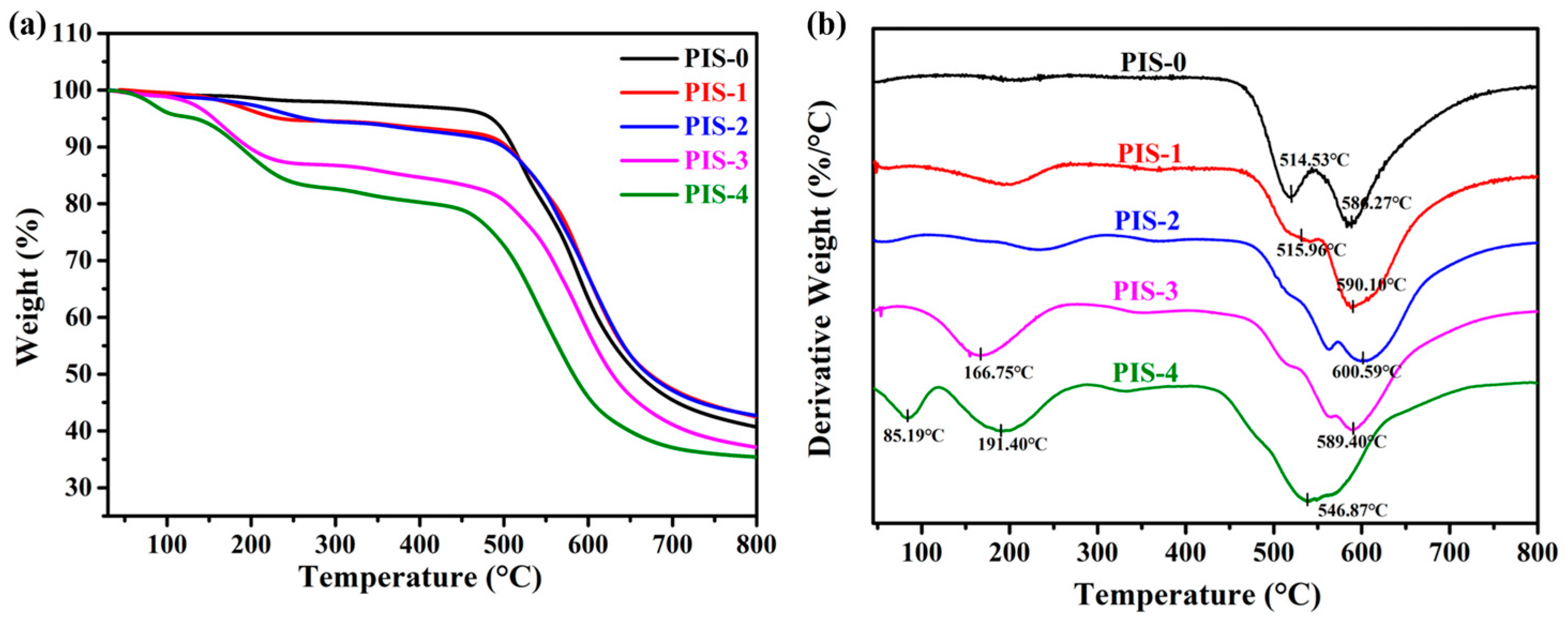
3.1. Characterization of PISs
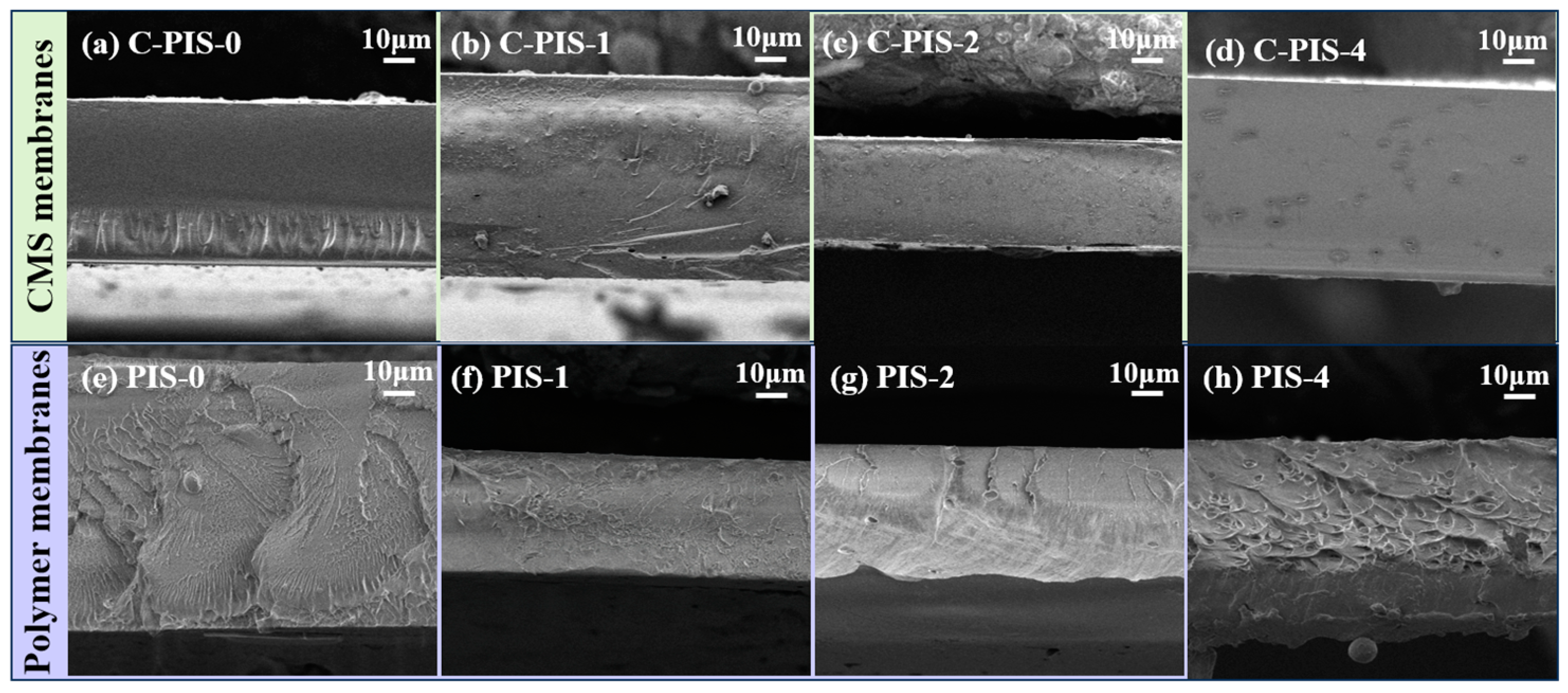
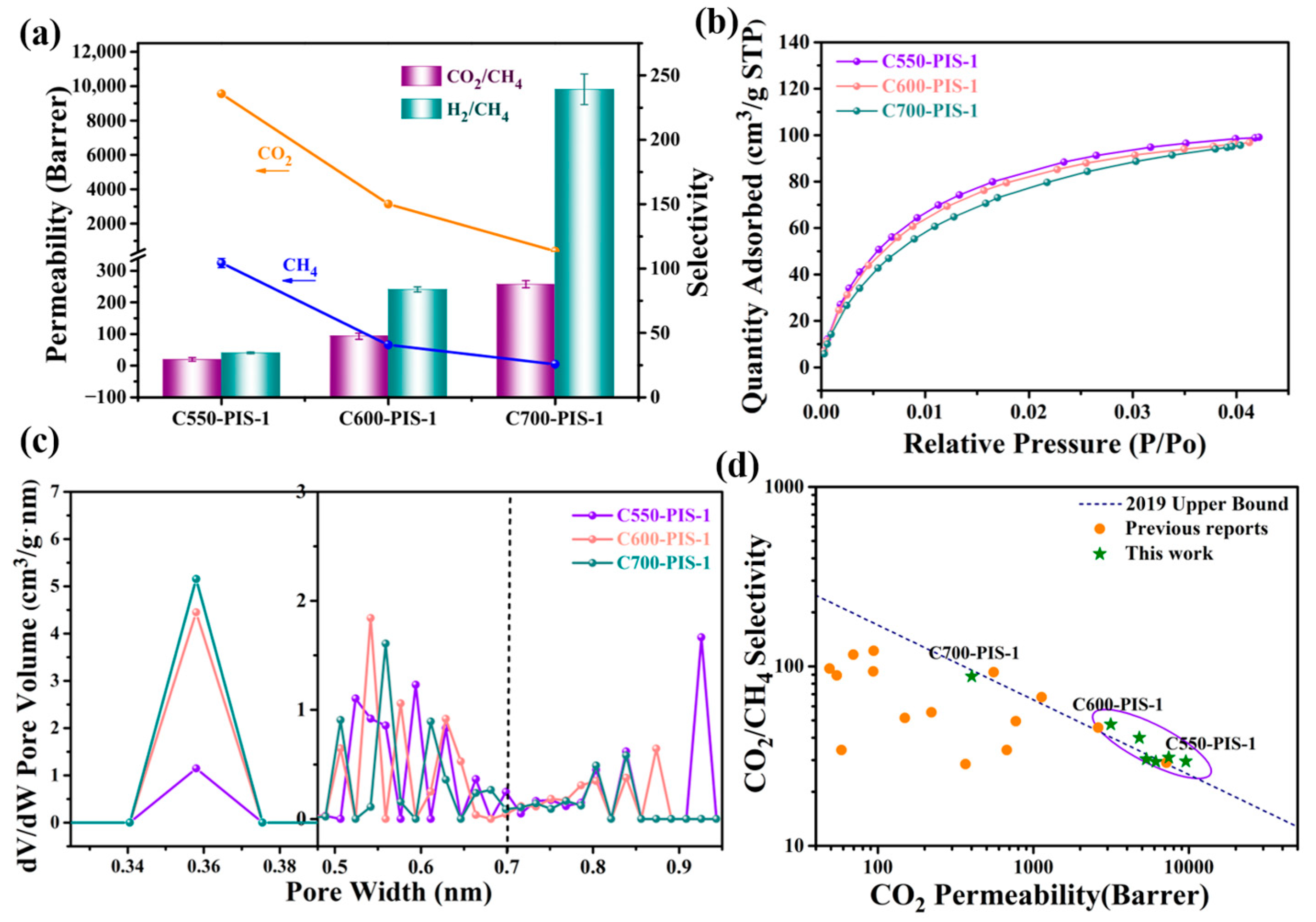
3.2. Characterization of CMS Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- IEA. Gas Market Report, Q3; IEA: Paris, France, 2024. [Google Scholar]
- Iarikov, D.D.; Oyama, S.T. Chapter 5—Review of CO2/CH4 Separation Membranes. In Membrane Science and Technology; Oyama, S.T., Stagg-Williams, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 91–115. [Google Scholar]
- Rodero, M.d.R.; Muñoz, R.; González-Sánchez, A.; Ruiz, H.A.; Quijano, G. Membrane materials for biogas purification and upgrading: Fundamentals, recent advances and challenges. J. Environ. Chem. Eng. 2024, 12, 114106. [Google Scholar] [CrossRef]
- Lei, L.; Lindbråthen, A.; Zhang, X.; Favvas, E.P.; Sandru, M.; Hillestad, M.; He, X. Preparation of carbon molecular sieve membranes with remarkable CO2/CH4 selectivity for high-pressure natural gas sweetening. J. Membr. Sci. 2020, 614, 118529. [Google Scholar] [CrossRef]
- Du, N.; Park, H.; Robertson, G.; Dal-Cin, M.M.; Visser, T.; Scoles, L.; Guiver, M. Polymer nanosieve membranes for CO2-capture applications. Nat. Mater. 2011, 10, 372–375. [Google Scholar] [CrossRef]
- Sanders, D.F.; Smith, Z.P.; Guo, R.; Robeson, L.M.; McGrath, J.E.; Paul, D.R.; Freeman, B.D. Energy-efficient polymeric gas separation membranes for a sustainable future: A review. Polymer 2013, 54, 4729–4761. [Google Scholar] [CrossRef]
- Ghanem, B.S.; Swaidan, R.; Ma, X.H.; Litwiller, E.; Pinnau, I. Energy-Efficient Hydrogen Separation by AB-Type Ladder-Polymer Molecular Sieves. Adv. Mater. 2014, 26, 6696–6700. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Leisen, J.E.; Liu, Z.Y.; Quan, W.Y.; Koros, W.J. Key Features of Polyimide-Derived Carbon Molecular Sieves. Angew. Chem. Int. Ed. Engl. 2021, 60, 22322–22331. [Google Scholar] [CrossRef]
- Yang, X.; Li, Z.Y.; Zhang, C.Z.; Wang, H.Y.; Zhang, E.; Xing, Y.; Xiao, P.; Yang, R.T.; Liu, Y.S.; Webley, P.A. Practical separation performance evaluation of coal mine methane upgrading with carbon molecular sieves. Chem. Eng. J. 2019, 367, 295–303. [Google Scholar] [CrossRef]
- Lei, L.; Bai, L.; Lindbråthen, A.; Pan, F.J.; Zhang, X.P.; He, X.Z. Carbon membranes for CO2 removal: Status and perspectives from materials to processes. Chem. Eng. J. 2020, 401, 126084. [Google Scholar] [CrossRef]
- Xin, J.; Zhou, X.; Huo, G.; Zhang, Z.; Zhang, Y.; Kang, S.; Dai, Z.; Li, N. Development of high performance carbon molecular sieve membranes via tuning the side groups on PI precursors. J. Membr. Sci. 2023, 688, 122124. [Google Scholar] [CrossRef]
- Karousos, D.S.; Lei, L.F.; Lindbråthen, A.; Sapalidis, A.A.; Kouvelos, E.P.; He, X.Z.; Favvas, E.P. Cellulose-based carbon hollow fiber membranes for high-pressure mixed gas separations of CO2/CH4 and CO2/N2. Sep. Purif. Technol. 2020, 253, 117473. [Google Scholar] [CrossRef]
- Lei, L.; Pan, F.; Lindbrathen, A.; Zhang, X.; Hillestad, M.; Nie, Y.; Bai, L.; He, X.; Guiver, M.D. Carbon hollow fiber membranes for a molecular sieve with precise-cutoff ultramicropores for superior hydrogen separation. Nat. Commun. 2021, 12, 268. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Li, H.; Yu, Y.; Liu, Y. Improving gas separation performances of poly(furfuryl alcohol)-based carbon molecular sieve membrane by tuning furan ring opening and membrane structures. J. Membr. Sci. 2023, 688, 122117. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, H.; Hu, Y.; Li, X.; Chen, X.; Kim, S.; Wang, Y.; Simon, G.P.; Wang, H. Carbon Nanotube Networks as Nanoscaffolds for Fabricating Ultrathin Carbon Molecular Sieve Membranes. ACS Appl. Mater. Interfaces 2018, 10, 20182–20188. [Google Scholar] [CrossRef]
- Xu, R.; Xu, R.; He, L.; Li, L.; Hou, M.; Wang, Y.; Zhang, B.; Liang, C.; Wang, T. Ultraselective carbon molecular sieve membrane for hydrogen purification. J. Energy Chem. 2020, 50, 16–24. [Google Scholar] [CrossRef]
- Thür, R.; Lemmens, V.; Van Havere, D.; van Essen, M.; Nijmeijer, K.; Vankelecom, I.F.J. Tuning 6FDA-DABA membrane performance for CO2 removal by physical densification and decarboxylation cross-linking during simple thermal treatment. J. Membr. Sci. 2020, 610, 118195. [Google Scholar] [CrossRef]
- Karunaweera, C.; Musselman, I.H.; Balkus, K.J.; Ferraris, J.P. Fabrication and characterization of aging resistant carbon molecular sieve membranes for C3 separation using high molecular weight crosslinkable polyimide, 6FDA-DABA. J. Membr. Sci. 2019, 581, 430–438. [Google Scholar] [CrossRef]
- Xu, S.; Zhao, N.; Wu, L.; Kang, S.; Zhang, Z.; Huo, G.; Dai, Z.; Li, N. Carbon molecular sieve gas separation membranes from crosslinkable bromomethylated 6FDA-DAM polyimide. J. Membr. Sci. 2022, 659, 120781. [Google Scholar] [CrossRef]
- Hu, C.-P.; Polintan, C.K.; Tayo, L.L.; Chou, S.-C.; Tsai, H.-A.; Hung, W.-S.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y. The gas separation performance adjustment of carbon molecular sieve membrane depending on the chain rigidity and free volume characteristic of the polymeric precursor. Carbon 2019, 143, 343–351. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, F.; Cornelius, C.J.; Fan, Y. Carbon molecular sieve membranes derived from crosslinkable polyimides for CO2/CH4 and C2H4/C2H6 separations. J. Membr. Sci. 2021, 621, 118785. [Google Scholar] [CrossRef]
- Fu, S.; Fu, S.; Sanders, E.S.; Kulkarni, S.S.; Koros, W.J. Carbon molecular sieve membrane structure–property relationships for four novel 6FDA based polyimide precursors. J. Membr. Sci. 2015, 487, 60–73. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Q.; Xie, Z.; Zhao, B.; Yu, Y.; Liu, Y. Simultaneously enhanced gas permeability, selectivity and aging stability of carbon molecular sieve membranes by the molecule doping of silicon. Carbon 2023, 203, 47–58. [Google Scholar] [CrossRef]
- Park, H.B.; Jung, C.H.; Kim, Y.K.; Nam, S.Y.; Lee, S.Y.; Lee, Y.M. Pyrolytic carbon membranes containing silica derived from poly(imide siloxane): The effect of siloxane chain length on gas transport behavior and a study on the separation of mixed gases. J. Membr. Sci. 2004, 235, 87–98. [Google Scholar] [CrossRef]
- Park, H.B.; Lee, Y.M. Pyrolytic carbon–silica membrane: A promising membrane material for improved gas separation. J. Membr. Sci. 2003, 213, 263–272. [Google Scholar] [CrossRef]
- O’Brien, K.C.; Koros, W.J.; Barbari, T.A. A new technique for the measurement of multicomponent gas transport through polymeric films. J. Membr. Sci. 1986, 29, 229–238. [Google Scholar] [CrossRef]
- Jusoh, N.; Yeong, Y.F.; Lau, K.K.; Shariff, A.M. Fabrication of silanated zeolite T/6FDA-durene composite membranes for CO2/CH4 separation. J. Clean. Prod. 2017, 166, 1043–1058. [Google Scholar] [CrossRef]
- Lin, C.-C.; Chang, C.-B.; Wang, Y.-Z. Preparation and properties of cross-linked sulfonated poly(imide-siloxane) for polymer electrolyte fuel cell application. J. Power Sources 2013, 223, 277–283. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, Q.; Su, X.; Wu, X.; Zhang, X.-G.; Guo, Y.; Li, Z.; Wei, J.; Wang, H.; Zhang, S.; et al. Ingenious Artificial Leaf Based on Covalent Organic Framework Membranes for Boosting CO2 Photoreduction. J. Am. Chem. Soc. 2023, 145, 9520–9529. [Google Scholar] [CrossRef]
- Ku, C.-K.; Lee, Y.-D. Microphase separation in amorphous poly(imide siloxane) segmented copolymers. Polymer 2007, 48, 3565–3573. [Google Scholar] [CrossRef]
- Tai, F.C.; Wei, C.; Chang, S.H.; Chen, W.S. Raman and X-ray diffraction analysis on unburned carbon powder refined from fly ash. J. Raman Spectrosc. 2009, 41, 933–937. [Google Scholar] [CrossRef]
- Nieto-Márquez, A.; Romero, R.; Romero, A.; Valverde, J.L. Carbon nanospheres: Synthesis, physicochemical properties and applications. J. Mater. Chem. 2011, 21, 1664–1672. [Google Scholar] [CrossRef]
- Swaidan, R.; Ma, X.; Litwiller, E.; Pinnau, I. High pressure pure- and mixed-gas separation of CO2/CH4 by thermally-rearranged and carbon molecular sieve membranes derived from a polyimide of intrinsic microporosity. J. Membr. Sci. 2013, 447, 387–394. [Google Scholar] [CrossRef]
- Yin, X.; Wang, J.; Chu, N.; Yang, J.; Lu, J.; Zhang, Y.; Yin, D. Zeolite L/carbon nanocomposite membranes on the porous alumina tubes and their gas separation properties. J. Membr. Sci. 2010, 348, 181–189. [Google Scholar] [CrossRef]
- Yin, X.; Chu, N.; Yang, J.; Wang, J.; Li, Z. Thin zeolite T/carbon composite membranes supported on the porous alumina tubes for CO2 separation. Int. J. Greenh. Gas Control 2013, 15, 55–64. [Google Scholar] [CrossRef]
- Kiyono, M.; Williams, P.J.; Koros, W.J. Effect of polymer precursors on carbon molecular sieve structure and separation performance properties. Carbon 2010, 48, 4432–4441. [Google Scholar] [CrossRef]
- Steel, K.M.; Koros, W.J. An investigation of the effects of pyrolysis parameters on gas separation properties of carbon materials. Carbon 2005, 43, 1843–1856. [Google Scholar] [CrossRef]
- Rungta, M.; Wenz, G.B.; Zhang, C.; Xu, L.; Qiu, W.; Adams, J.S.; Koros, W.J. Carbon molecular sieve structure development and membrane performance relationships. Carbon 2017, 115, 237–248. [Google Scholar] [CrossRef]

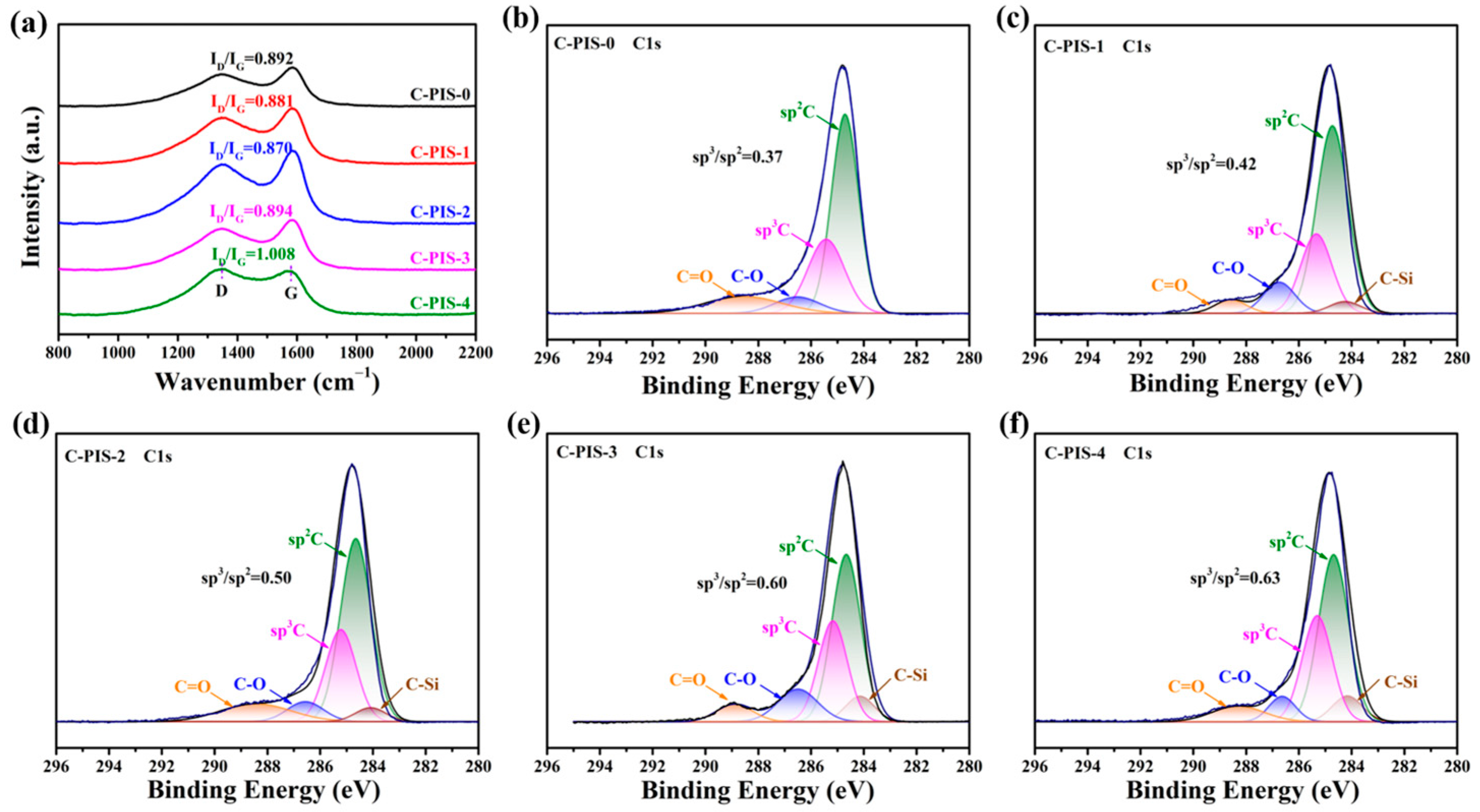
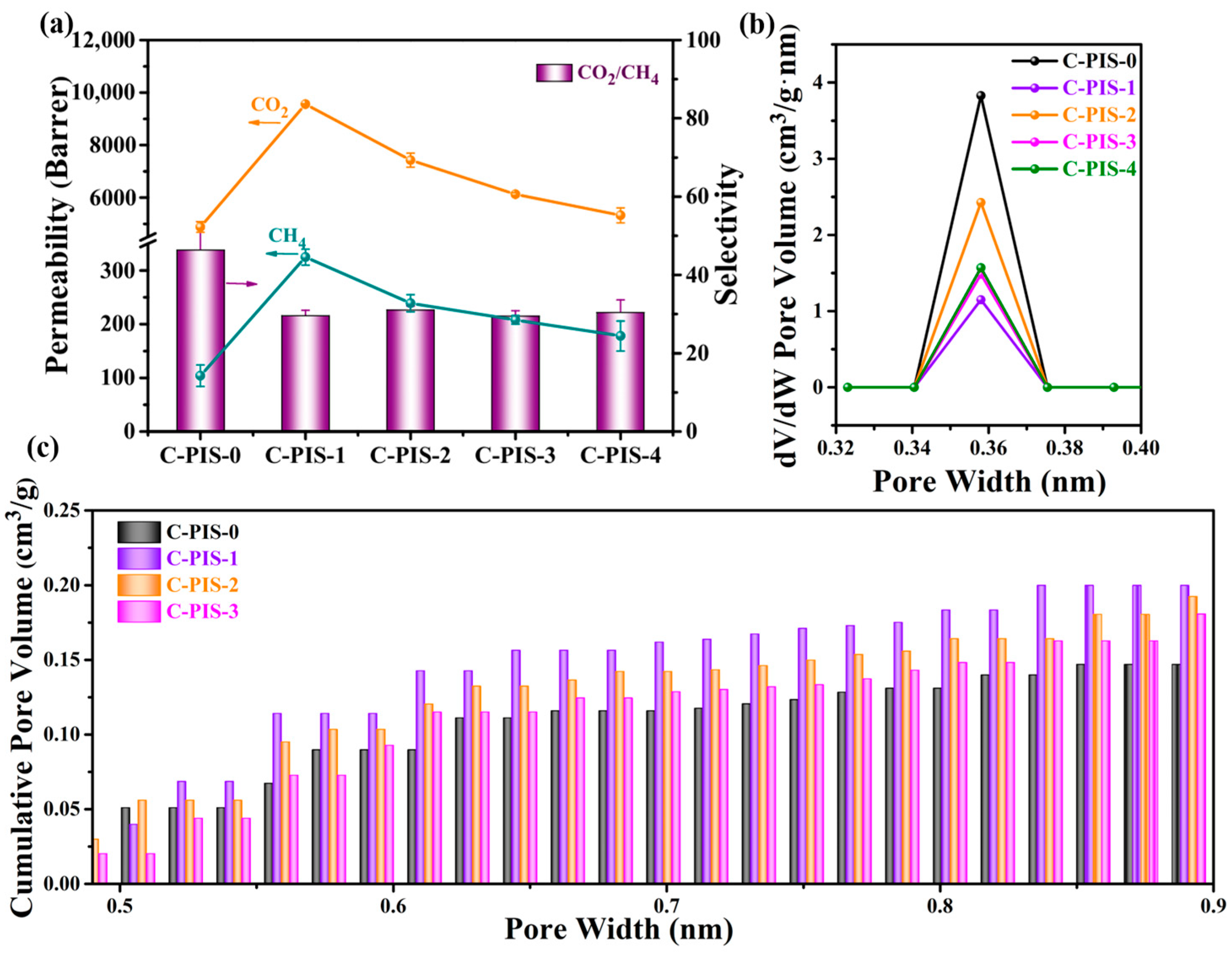
| Sample | Permeability (Barrer) | Ideal Selectivity | |||
|---|---|---|---|---|---|
| CH4 | CO2 | H2 | CO2/CH4 | H2/CH4 | |
| C-PIS-0 | 104 ± 20 | 4880 ± 200 | 6600 ± 200 | 46.9 ± 9.2 | 64 ± 12 |
| C-PIS-1 | 325 ± 15 | 9556 ± 10 | 11,200 ± 330 | 29.4 ± 1.4 | 34.5 ± 1.9 |
| C-PIS-2 | 239 ± 16 | 7420 ± 270 | 7300 ± 330 | 31.1 ± 2.4 | 30.6 ± 2.5 |
| C-PIS-3 | 208 ± 8 | 6124 ± 66 | 7166 ± 59 | 29.4 ± 1.2 | 34.5 ± 1.4 |
| C-PIS-4 | 178 ± 28 | 5320 ± 290 | 6900 ± 300 | 30.0 ± 5.0 | 38.8 ± 6.3 |
| Sample | Permeability (Barrer) | Ideal Selectivity | |||
|---|---|---|---|---|---|
| CH4 | CO2 | H2 | CO2/CH4 | H2/CH4 | |
| C550-PIS-1 | 325 ± 15 | 9556 ± 10 | 11,200 ± 330 | 29.4 ± 1.4 | 34.5 ± 1.9 |
| C600-PIS-1 | 66 ± 2 | 3144 ± 47 | 5560 ± 320 | 47.6 ± 1.6 | 84.3 ± 5.5 |
| C700-PIS-1 | 4.58 ± 0.07 | 402 ± 6 | 1096 ± 28 | 87.8 ± 1.9 | 239.3 ± 7.1 |
| Precursors | Carbonization Temperature (°C) | CO2 Permeability (Barrer) | CH4 Permeability (Barrer) | Selectivity of CO2/CH4 | Ref. |
|---|---|---|---|---|---|
| 6FDA-6FpDA/DABA | 576 | 2610 | 57.3 | 45.55 | [21] |
| 6FDA-6FpDA/DABA | 700 | 771 | 15.6 | 49.42 | [21] |
| 6FDA-6FpDA/DABA | 800 | 221 | 3.99 | 55.39 | [33] |
| PIM-6FDA-OH | 600 | 5450 | 132 | 41.29 | [33] |
| PIM-6FDA-OH | 630 | 2870 | 58 | 49.48 | [33] |
| PIM-6FDA-OH | 800 | 556 | 6 | 92.67 | [33] |
| PFA/CNT/AAO | 600 | 69.57 | / | 116.23 | [15] |
| LC-600-298K | 600 | 572 | 16 | 35.75 | [34] |
| PC-600-298K | 600 | 49.54 | 1.45 | 34.17 | [34] |
| TC-0-2 | 600 | 621 | 21.8 | 28.5 | [35] |
| TC-1-2 | 600 | 253 | 4.9 | 51.6 | [35] |
| TC-2-2 | 600 | 83.1 | 0.85 | 97.3 | [35] |
| TC-3-2 | 600 | 92.5 | 1.04 | 88.9 | [35] |
| 6FDA/BPDA-DAM(1:1) | 550 | 7170 | 247 | 29.03 | [36] |
| 6FDA/BPDA-DAM | 550 | 1280 | 18.5 | 69.19 | [37] |
| 6FDA/BPDA-DAM | 800 | 93.5 | 0.997 | 93.78 | [37] |
| 6FDA/BPDA-DAM | 800 | 94 | 0.77 | 122.08 | [37] |
| 6FDA/BPDA-DAM(1:1) | 675 | 1130 | 16.8 | 67.26 | [38] |
| C-PIS-1 | 550 | 9556 | 325 | 29.4 ± 1.4 | This work |
| C-PIS-1 | 600 | 3144 | 66 | 47.6 ± 1.6 | This work |
| C-PIS-1 | 700 | 402 | 4.58 | 87.8 ± 1.9 | This work |
| C-PIS-2 | 550 | 7421 | 239 | 31.1 ± 2.4 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Guo, H.; Zhao, B.; Yu, Y.; Liu, Y.; Zhang, S. Improved CO2/CH4 Separation in Carbon Molecular Sieve Membranes via Copolymerization of Long-Chain Flexible Structures. Membranes 2025, 15, 128. https://doi.org/10.3390/membranes15050128
Wu Y, Guo H, Zhao B, Yu Y, Liu Y, Zhang S. Improved CO2/CH4 Separation in Carbon Molecular Sieve Membranes via Copolymerization of Long-Chain Flexible Structures. Membranes. 2025; 15(5):128. https://doi.org/10.3390/membranes15050128
Chicago/Turabian StyleWu, Yingxiu, Haiyan Guo, Bingyu Zhao, Yuxiu Yu, Yaodong Liu, and Shouchun Zhang. 2025. "Improved CO2/CH4 Separation in Carbon Molecular Sieve Membranes via Copolymerization of Long-Chain Flexible Structures" Membranes 15, no. 5: 128. https://doi.org/10.3390/membranes15050128
APA StyleWu, Y., Guo, H., Zhao, B., Yu, Y., Liu, Y., & Zhang, S. (2025). Improved CO2/CH4 Separation in Carbon Molecular Sieve Membranes via Copolymerization of Long-Chain Flexible Structures. Membranes, 15(5), 128. https://doi.org/10.3390/membranes15050128






