Crosstalk between Tryptophan Metabolism via Kynurenine Pathway and Carbohydrate Metabolism in the Context of Cardio-Metabolic Risk—Review
Abstract
1. Introduction
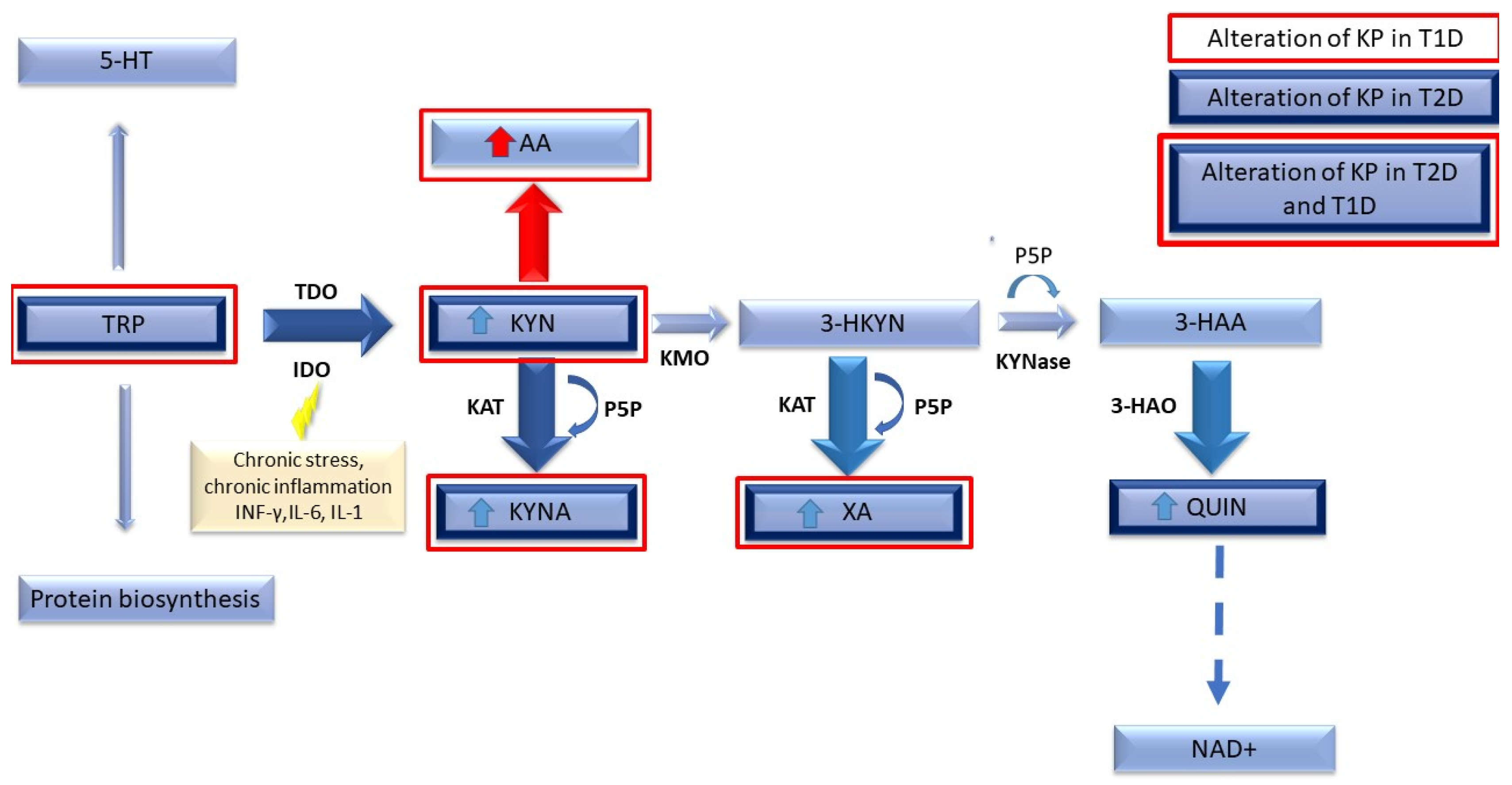
Tryptophan Metabolism via the Kynurenine Pathway
2. Methods
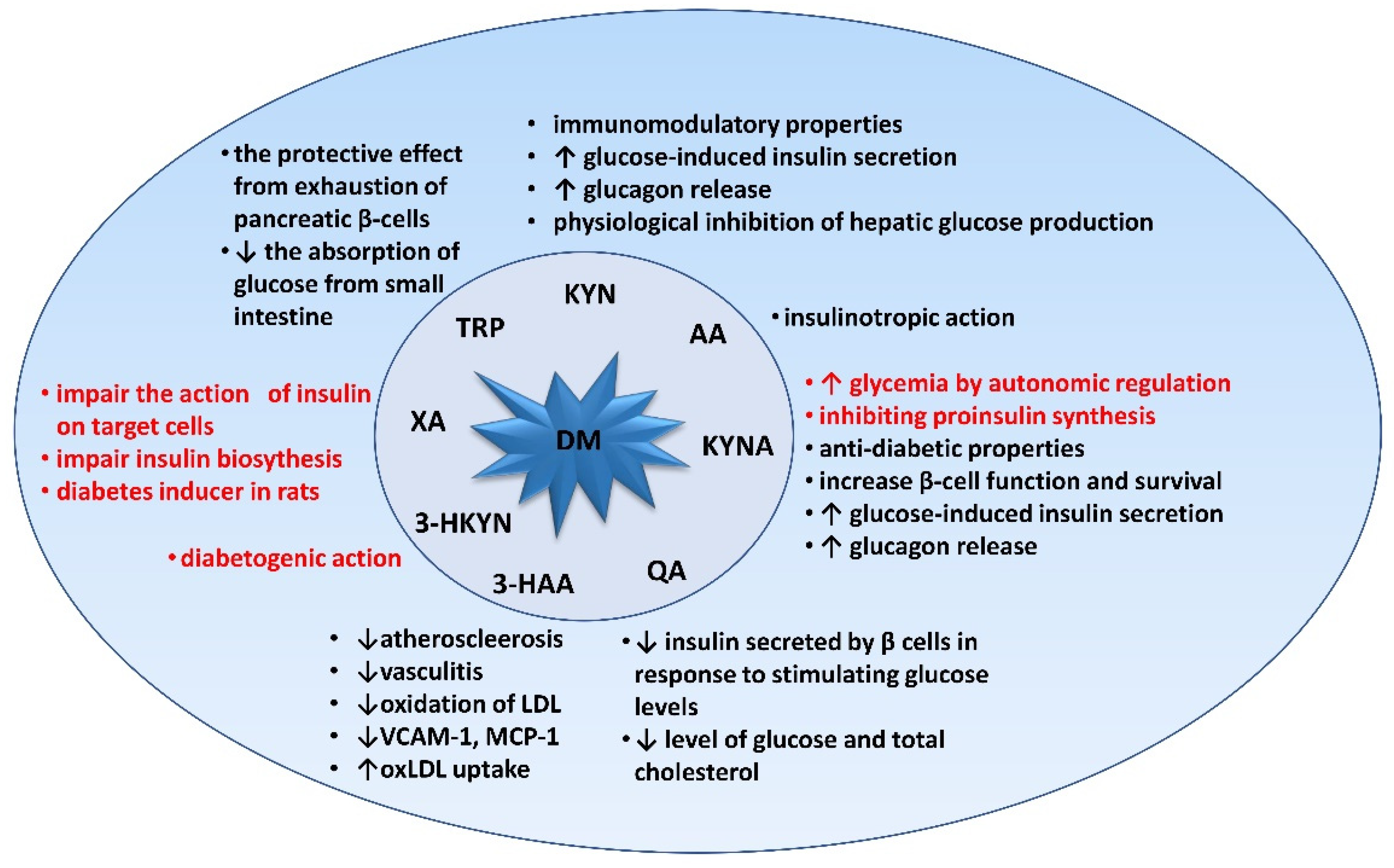
3. Relevance of Tryptophan and Its Metabolites in Carbohydrate Metabolism
3.1. Kynurenine (KYN)
3.2. Kynurenic Acid (KYNA)
3.3. Xanthurenic Acid (XA), 3-hydroxykynurenine (3-HKYN), 3-hydroxyanthranilic Acid (3-HAA), and Quinolinic Acid (QA)
4. Tryptophan Metabolism in Type 1 Diabetes Compared to Type 2 Diabetes
5. Tryptophan Metabolism in Type 2 Diabetes
6. Linking Cardiovascular Diseases to Diabetes and the Kynurenine Pathway
7. Atherosclerosis, Endothelium Dysfunction, and the Pathogenesis of Coronary Artery Disease
7.1. Role of IDO in the Progression of CVD
7.2. The Kynurenine Pathway Links Inflammation, Immune Response to Heart Failure
8. Possible Avenues for Therapeutic Action and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Badawy, A.A. Kynurenine pathway and human systems. Exp. Gerontol. 2020, 129, 110770. [Google Scholar] [CrossRef]
- Badawy, A.A. Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects. Int. J. Tryptophan Res. 2017, 10, 1178646917691938. [Google Scholar] [CrossRef]
- Sas, K.; Szabo, E.; Vecsei, L. Mitochondria, Oxidative Stress and the Kynurenine System, with a Focus on Ageing and Neuroprotection. Molecules 2018, 23, 191. [Google Scholar] [CrossRef] [PubMed]
- Kerner, W.; Bruckel, J.; German Diabetes, A. Definition, classification and diagnosis of diabetes mellitus. Exp. Clin. Endocrinol. Diabetes 2014, 122, 384–386. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Liu, J.J.; Movassat, J.; Portha, B. Emerging role for kynurenines in metabolic pathologies. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 82–90. [Google Scholar] [CrossRef]
- Takikawa, O.; Yoshida, R.; Kido, R.; Hayaishi, O. Tryptophan degradation in mice initiated by indoleamine 2,3-dioxygenase. J. Biol. Chem. 1986, 261, 3648–3653. [Google Scholar] [CrossRef]
- Chen, T.; Zheng, X.; Ma, X.; Bao, Y.; Ni, Y.; Hu, C.; Rajani, C.; Huang, F.; Zhao, A.; Jia, W.; et al. Tryptophan Predicts the Risk for Future Type 2 Diabetes. PLoS ONE 2016, 11, e0162192. [Google Scholar] [CrossRef]
- Yu, E.; Papandreou, C.; Ruiz-Canela, M.; Guasch-Ferre, M.; Clish, C.B.; Dennis, C.; Liang, L.; Corella, D.; Fitó, M.; Razquin, C.; et al. Association of Tryptophan Metabolites with Incident Type 2 Diabetes in the PREDIMED Trial: A Case–Cohort Study. Clin. Chem. 2018, 64, 1211–1220. [Google Scholar] [CrossRef]
- Matsuoka, K.; Kato, K.; Takao, T.; Ogawa, M.; Ishii, Y.; Shimizu, F.; Masuda, J.; Takada, A. Concentrations of various tryptophan metabolites are higher in patients with diabetes mellitus than in healthy aged male adults. Diabetol. Int. 2016, 8, 69–75. [Google Scholar] [CrossRef]
- Law, K.P.; Han, T.-L.; Mao, X.; Zhang, H. Tryptophan and purine metabolites are consistently upregulated in the urinary metabolome of patients diagnosed with gestational diabetes mellitus throughout pregnancy: A longitudinal metabolomics study of Chinese pregnant women part 2. Clin. Chim. Acta 2017, 468, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Rebnord, E.W.; Strand, E.; Midttun, O.; Svingen, G.F.T.; Christensen, M.H.E.; Ueland, P.M.; Mellgren, G.; Njolstad, P.R.; Tell, G.S.; Nygard, O.K.; et al. The kynurenine:tryptophan ratio as a predictor of incident type 2 diabetes mellitus in individuals with coronary artery disease. Diabetologia 2017, 60, 1712–1721. [Google Scholar] [CrossRef] [PubMed]
- Ponter, A.A.; Sève, B.; Morgan, L.M. Intragastric Tryptophan Reduces Glycemia after Glucose, Possibly via Glucose-Mediated Insulinotropic Polypeptide, in Early-Weaned Piglets. J. Nutr. 1994, 124, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.V.; Efanov, A.M.; Fang, X.; Beavers, L.S.; Wang, X.; Wang, J.; Gonzalez Valcarcel, I.C.; Ma, T. GPR142 Controls Tryptophan-Induced Insulin and Incretin Hormone Secretion to Improve Glucose Metabolism. PLoS ONE 2016, 11, e0157298. [Google Scholar] [CrossRef]
- Kuwabara, W.M.T.; Panveloski-Costa, A.C.; Yokota, C.N.F.; Pereira, J.N.B.; Filho, J.M.; Torres, R.P.; Hirabara, S.M.; Curi, R.; Alba-Loureiro, T.C. Comparison of Goto-Kakizaki rats and high fat diet-induced obese rats: Are they reliable models to study Type 2 Diabetes mellitus? PLoS ONE 2017, 12, e0189622. [Google Scholar] [CrossRef] [PubMed]
- Inubushi, T.; Kamemura, N.; Oda, M.; Sakurai, J.; Nakaya, Y.; Harada, N.; Suenaga, M.; Matsunaga, Y.; Ishidoh, K.; Katunuma, N. L-Tryptophan Suppresses Rise in Blood Glucose and Preserves Insulin Secretion in Type-2 Diabetes Mellitus Rats. J. Nutr. Sci. Vitaminol. 2012, 58, 415–422. [Google Scholar] [CrossRef]
- Wittman, J.S. Alteration of Glucose Tolerance by Dietary L-Tryptophan in Rats. J. Nutr. 1976, 106, 631–635. [Google Scholar] [CrossRef]
- Elliott, K.R.F.; Pogson, C.I.; Smith, S.A. Effects of Tryptophan on Gluconeogenesis in the Rat and the Guinea Pig. Biochem. Soc. Trans. 1976, 4, 1048–1049. [Google Scholar] [CrossRef] [PubMed]
- Imai, E.; Shibata, K. Oral Glucose Tolerance and Tryptophan Metabolism in Non-Obese and Non-Insulin-Dependent Diabetic Goto-Kakizaki Rats Fed High-Tryptophan Diets. J. Nutr. Sci. Vitaminol. 2018, 64, 48–55. [Google Scholar] [CrossRef]
- Koopmans, S.J.; Ruis, M.; Dekker, R.; Korte, M. Surplus dietary tryptophan inhibits stress hormone kinetics and induces insulin resistance in pigs. Physiol. Behav. 2009, 98, 402–410. [Google Scholar] [CrossRef]
- Veldhoen, M.; Hirota, K.; Westendorf, A.M.; Buer, J.; Dumoutier, L.; Renauld, J.-C.; Stockinger, B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 2008, 453, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Esser, C. The Aryl Hydrocarbon Receptor in Immunity: Tools and Potential. Methods Mol. Biol. 2016, 239–257. [Google Scholar] [CrossRef]
- Rothhammer, V.; Quintana, F.J. The aryl hydrocarbon receptor: An environmental sensor integrating immune responses in health and disease. Nat. Rev. Immunol. 2019, 19, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Opitz, C.A.; Litzenburger, U.M.; Sahm, F.; Ott, M.; Tritschler, I.; Trump, S.; Schumacher, T.; Jestaedt, L.; Schrenk, D.; Weller, M.; et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 2011, 478, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, H.; Parker, E.; Hamrick, M.W. Kynurenine signaling through the aryl hydrocarbon receptor: Implications for aging and healthspan. Exp. Gerontol. 2020, 130, 110797. [Google Scholar] [CrossRef]
- Vogel, C.F.A.; Goth, S.R.; Dong, B.; Pessah, I.N.; Matsumura, F. Aryl hydrocarbon receptor signaling mediates expression of indoleamine 2,3-dioxygenase. Biochem. Biophys. Res. Commun. 2008, 375, 331–335. [Google Scholar] [CrossRef]
- Yamamoto, T.; Hatabayashi, K.; Arita, M.; Yajima, N.; Takenaka, C.; Suzuki, T.; Takahashi, M.; Oshima, Y.; Hara, K.; Kagawa, K.; et al. Kynurenine signaling through the aryl hydrocarbon receptor maintains the undifferentiated state of human embryonic stem cells. Sci. Signal. 2019, 12, eaaw3306. [Google Scholar] [CrossRef]
- Oxenkrug, G.; Cornicelli, J.; van der Hart, M.; Roeser, J.; Summergrad, P. Kynurenic acid, an aryl hydrocarbon receptor ligand, is elevated in serum of Zucker fatty rats. Integr. Mol. Med. 2016, 3, 761–763. [Google Scholar]
- Gál, E.M.; Sherman, A.D. l-Kynurenine Its synthesis and possible regulatory function in brain. Neurochem. Res. 1980, 5, 223–239. [Google Scholar] [CrossRef]
- Biljes, D.; Hammerschmidt-Kamper, C.; Kadow, S.; Diel, P.; Weigt, C.; Burkart, V.; Esser, C. Impaired glucose and lipid metabolism in ageing aryl hydrocarbon receptor deficient mice. EXCLI J. 2015, 14, 1153–1163. [Google Scholar] [CrossRef]
- Dabir, P.; Marinic, T.E.; Krukovets, I.; Stenina, O.I. Aryl Hydrocarbon Receptor Is Activated by Glucose and Regulates the Thrombospondin-1 Gene Promoter in Endothelial Cells. Circ. Res. 2008, 102, 1558–1565. [Google Scholar] [CrossRef]
- Pelcl, T.; Skrha, J.; Prazny, M.; Vlckova, S.; Fenclova, Z.; Navratil, T.; Malik, J.; Diblik, P.; Zikan, V.; Pelclova, D. Diabetes, Cardiovascular Disorders and 2,3,7,8-Tetrachlorodibenzo-p-Dioxin Body Burden in Czech Patients 50 Years After the Intoxication. Basic Clin. Pharmacol. Toxicol. 2018, 123, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Warner, M.; Mocarelli, P.; Brambilla, P.; Wesselink, A.; Samuels, S.; Signorini, S.; Eskenazi, B. Diabetes, Metabolic Syndrome, and Obesity in Relation to Serum Dioxin Concentrations: The Seveso Women’s Health Study. Environ. Health Perspect. 2013, 121, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, L.V.; Neyens, D.; Ramsay, G.; Taylor, P.M.; Cantrell, D.A. Single cell analysis of kynurenine and System L amino acid transport in T cells. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Fukui, S.; Schwarcz, R.; Rapoport, S.I.; Takada, Y.; Smith, Q.R. Blood?Brain Barrier Transport of Kynurenines: Implications for Brain Synthesis and Metabolism. J. Neurochem. 1991, 56, 2007–2017. [Google Scholar] [CrossRef]
- Møller, S.E. Pharmacokinetics of tryptophan, renal handling of kynurenine and the effect of nicotinamide on its appearance in plasma and urine following L-tryptophan loading of healthy subjects. Eur. J. Clin. Pharmacol. 1981, 21, 137–142. [Google Scholar] [CrossRef]
- Mudry, J.M.; Alm, P.S.; Erhardt, S.; Goiny, M.; Fritz, T.; Caidahl, K.; Zierath, J.R.; Krook, A.; Wallberg-Henriksson, H. Direct effects of exercise on kynurenine metabolism in people with normal glucose tolerance or type 2 diabetes. Diabetes Metab. Res. Rev. 2016, 32, 754–761. [Google Scholar] [CrossRef]
- Favennec, M.; Hennart, B.; Verbanck, M.; Pigeyre, M.; Caiazzo, R.; Raverdy, V.; Verkindt, H.; Leloire, A.; Guillemin, G.J.; Yengo, L.; et al. Post-Bariatric Surgery Changes in Quinolinic and Xanthurenic Acid Concentrations Are Associated with Glucose Homeostasis. PLoS ONE 2016, 11, e0158051. [Google Scholar] [CrossRef]
- Sasaki, N.; Egashira, Y.; Sanada, H. Production of l-tryptophan-derived catabolites in hepatocytes from streptozotocin-induced diabetic rats. Eur. J. Nutr. 2009, 48, 145–153. [Google Scholar] [CrossRef]
- Liu, J.J.; Raynal, S.; Bailbe, D.; Gausseres, B.; Carbonne, C.; Autier, V.; Movassat, J.; Kergoat, M.; Portha, B. Expression of the kynurenine pathway enzymes in the pancreatic islet cells. Activation by cytokines and glucolipotoxicity. Biochim. Biophys. Acta 2015, 1852, 980–991. [Google Scholar] [CrossRef]
- Noto, Y.; Okamoto, H. Inhibition by kynurenine metabolites of proinsulin synthesis in isolated pancreatic islets. Acta Diabetol. Lat. 1978, 15, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Scholz, O.; Welters, A.; Lammert, E. Role of NMDA Receptors in Pancreatic Islets. NMDA Recept. 2017, 121–134. [Google Scholar] [CrossRef]
- Takikawa, O.; Truscott, R.J.W.; Fukao, M.; Miwa, S. Age-Related Nuclear Cataract and Indoleamine 2,3-Dioxygenase-Initiated Tryptophan Metabolism in the Human Lens. Adv. Exp. Med. Biol. 2003, 277–285. [Google Scholar] [CrossRef]
- Agudelo, L.Z.; Ferreira, D.M.S.; Cervenka, I.; Bryzgalova, G.; Dadvar, S.; Jannig, P.R.; Pettersson-Klein, A.T.; Lakshmikanth, T.; Sustarsic, E.G.; Porsmyr-Palmertz, M.; et al. Kynurenic Acid and Gpr35 Regulate Adipose Tissue Energy Homeostasis and Inflammation. Cell Metab. 2018, 27, 378–392 e375. [Google Scholar] [CrossRef]
- Munipally, P.K.; Agraharm, S.G.; Valavala, V.K.; Gundae, S.; Turlapati, N.R. Evaluation of indoleamine 2,3-dioxygenase expression and kynurenine pathway metabolites levels in serum samples of diabetic retinopathy patients. Arch. Physiol. Biochem. 2011, 117, 254–258. [Google Scholar] [CrossRef]
- Buczko, P.; Stokowska, W.; Górska, M.; Kucharewicz, I.; Pawlak, D.; Buczko, W.l. Tryptophan metabolites via kynurenine pathway in saliva of diabetic patients. Dent. Med. Prob. 2006, 43, 21–25. [Google Scholar]
- Preshaw, P.M.; Alba, A.L.; Herrera, D.; Jepsen, S.; Konstantinidis, A.; Makrilakis, K.; Taylor, R. Periodontitis and diabetes: A two-way relationship. Diabetologia 2011, 55, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Murfitt, S.A.; Zaccone, P.; Wang, X.; Acharjee, A.; Sawyer, Y.; Koulman, A.; Roberts, L.D.; Cooke, A.; Griffin, J.L. Metabolomics and Lipidomics Study of Mouse Models of Type 1 Diabetes Highlights Divergent Metabolism in Purine and Tryptophan Metabolism Prior to Disease Onset. J. Proteome Res. 2018, 17, 946–960. [Google Scholar] [CrossRef] [PubMed]
- Oxenkrug, G. Insulin Resistance and Dysregulation of Tryptophan–Kynurenine and Kynurenine–Nicotinamide Adenine Dinucleotide Metabolic Pathways. Mol. Neurobiol. 2013, 48, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Hattori, M.; Kotake, Y.; Kotake, Y. Studies on the urinary excretion of xanthurenic acid in diabetics. Acta Vitam. Enzym. 1984, 6, 221–228. [Google Scholar]
- Rogers, K.S.; Evangelista, S.J. 3-Hydroxykynurenine, 3-Hydroxyanthranilic Acid, and o-Aminophenol Inhibit Leucine-Stimulated Insulin Release from Rat Pancreatic Islets. Exp. Biol. Med. 1985, 178, 275–278. [Google Scholar] [CrossRef]
- Murakami, E.; Kotake, Y. Studies on the Xanthurenic Acid-Insulin Complex. J. Biochem. 1972, 72, 251–259. [Google Scholar] [CrossRef]
- Kotake, Y.; Ueda, T.; Mori, T.; Igaki, S.; Hattori, M. Abnormal tryptophan metabolism and experimental diabetes by xanthurenic acid (XA). Acta Vitam. Enzym. 1975, 29, 236–239. [Google Scholar]
- Meyramov, G.; Korchin, V.; Kocheryzkina, N. Diabetogenic activity of xanturenic acid determined by its chelating properties? Transplant. Proc. 1998, 30, 2682–2684. [Google Scholar] [CrossRef]
- Ikeda, S.; Kotake, Y. Urinary excretion of xanthurenic acid and zinc in diabetes: (3). Occurrence of xanthurenic acid-Zn2+ complex in urine of diabetic patients and of experimentally-diabetic rats. Ital. J. Biochem. 1986, 35, 232–241. [Google Scholar]
- Malina, H.Z.; Richter, C.; Mehl, M.; Hess, O.M. Pathological apoptosis by xanthurenic acid, a tryptophan metabolite: Activation of cell caspases but not cytoskeleton breakdown. BMC Physiol. 2001, 1, 7. [Google Scholar] [CrossRef] [PubMed]
- Kalaska, B.; Ciborowski, M.; Domaniewski, T.; Czyzewska, U.; Godzien, J.; Miltyk, W.; Kretowski, A.; Pawlak, D. Serum metabolic fingerprinting after exposure of rats to quinolinic acid. J. Pharm. Biomed. Anal. 2016, 131, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Masiello, P.; Balestreri, E.; Bacciola, D.; Bergamini, E. Influence of experimental diabetes on brain levels of monoamine neurotransmitters and their precursor amino acids during tryptophan loading. Acta Diabetol. Lat. 1987, 24, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Patterson, A.D.; Bonzo, J.A.; Li, F.; Krausz, K.W.; Eichler, G.S.; Aslam, S.; Tigno, X.; Weinstein, J.N.; Hansen, B.C.; Idle, J.R.; et al. Metabolomics Reveals Attenuation of the SLC6A20 Kidney Transporter in Nonhuman Primate and Mouse Models of Type 2 Diabetes Mellitus. J. Biol. Chem. 2011, 286, 19511–19522. [Google Scholar] [CrossRef]
- Dayer, M.R.; Safari, I.; Dayer, M.S. New Evidence on Hypoglycemic Effect of Quinolinic Acid in Diabetic Rats. Pak. J. Biol. Sci. 2009, 12, 1025–1030. [Google Scholar] [CrossRef][Green Version]
- Schuck, P.F.; Tonin, A.; da Costa Ferreira, G.; Rosa, R.B.; Latini, A.; Balestro, F.; Perry, M.L.S.; Wannmacher, C.M.D.; de Souza Wyse, A.T.; Wajner, M. In vitro effect of quinolinic acid on energy metabolism in brain of young rats. Neurosci. Res. 2007, 57, 277–288. [Google Scholar] [CrossRef]
- Suzuki, T.; De Hartog, M.; Gordon, E.E. Relationship of energy production to gluconeogenesis in renal cortical tubules. J. Cell. Physiol. 1975, 86, 111–119. [Google Scholar] [CrossRef]
- Oxenkrug, G.; der Hart, M.v.; Summergrad, P. Elevated anthranilic acid plasma concentrations in type 1 but not type 2 diabetes mellitus. Integr. Mol. Med. 2015, 2. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kodentsova, V.M.; Vrzhesinskaia, O.A.; Sokol’nikov, A.A.; Alekseeva, I.A.; Spirichev, V.B. Obmen riboflavina i funktsion-al’no sviazannykh s nim vitaminov gruppy Bpri insulinzavisimom sakharnom diabete [Metabolism of riboflavin and B group vitamins functionally bound to it in insulin-dependent diabetes mellitus]. Vopr. Med. Khim. 1993, 39, 33–36. [Google Scholar] [PubMed]
- Galderisi, A.; Pirillo, P.; Moret, V.; Stocchero, M.; Gucciardi, A.; Perilongo, G.; Moretti, C.; Monciotti, C.; Giordano, G.; Baraldi, E. Metabolomics reveals new metabolic perturbations in children with type 1 diabetes. Pediatric Diabetes 2017, 19, 59–67. [Google Scholar] [CrossRef]
- Yeung, A.W.; Terentis, A.C.; King, N.J.; Thomas, S.R. Role of indoleamine 2,3-dioxygenase in health and disease. Clin. Sci. 2015, 129, 601–672. [Google Scholar] [CrossRef]
- Pallotta, M.T.; Orabona, C.; Volpi, C.; Vacca, C.; Belladonna, M.L.; Bianchi, R.; Servillo, G.; Brunacci, C.; Calvitti, M.; Bicciato, S.; et al. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat. Immunol. 2011, 12, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Reizis, B.; Bunin, A.; Ghosh, H.S.; Lewis, K.L.; Sisirak, V. Plasmacytoid Dendritic Cells: Recent Progress and Open Questions. Annu. Rev. Immunol. 2011, 29, 163–183. [Google Scholar] [CrossRef]
- Gilliet, M.; Cao, W.; Liu, Y.-J. Plasmacytoid dendritic cells: Sensing nucleic acids in viral infection and autoimmune diseases. Nat. Rev. Immunol. 2008, 8, 594–606. [Google Scholar] [CrossRef]
- Grohmann, U.; Fallarino, F.; Bianchi, R.; Orabona, C.; Vacca, C.; Fioretti, M.C.; Puccetti, P. A Defect in Tryptophan Catabolism Impairs Tolerance in Nonobese Diabetic Mice. J. Exp. Med. 2003, 198, 153–160. [Google Scholar] [CrossRef]
- Androulidaki, A.; Wachsmuth, L.; Polykratis, A.; Pasparakis, M. Differential role of MyD88 and TRIF signaling in myeloid cells in the pathogenesis of autoimmune diabetes. PLoS ONE 2018, 13, e0194048. [Google Scholar] [CrossRef]
- Orabona, C.; Mondanelli, G.; Pallotta, M.T.; Carvalho, A.; Albini, E.; Fallarino, F.; Vacca, C.; Volpi, C.; Belladonna, M.L.; Berioli, M.G.; et al. Deficiency of immunoregulatory indoleamine 2,3-dioxygenase 1in juvenile diabetes. JCI Insight. 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Anquetil, F.; Mondanelli, G.; Gonzalez, N.; Rodriguez Calvo, T.; Zapardiel Gonzalo, J.; Krogvold, L.; Dahl-Jørgensen, K.; Van den Eynde, B.; Orabona, C.; Grohmann, U.; et al. Loss of IDO1 Expression From Human Pancreatic β-Cells Precedes Their Destruction During the Development of Type 1 Diabetes. Diabetes 2018, 67, 1858–1866. [Google Scholar] [CrossRef] [PubMed]
- Chmiel-Perzyńska, I.; Perzyński, A.; Urbańska, E.M. Experimental diabetes mellitus type 1 increases hippocampal content of kynurenic acid in rats. Pharmacol. Rep. 2014, 66, 1134–1139. [Google Scholar] [CrossRef]
- Rojewska, E.; Ciapała, K.; Piotrowska, A.; Makuch, W.; Mika, J. Pharmacological Inhibition of Indoleamine 2,3-Dioxygenase-2 and Kynurenine 3-Monooxygenase, Enzymes of the Kynurenine Pathway, Significantly Diminishes Neuropathic Pain in a Rat Model. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef]
- Hu, P.; Hunt, N.H.; Arfuso, F.; Shaw, L.C.; Uddin, M.N.; Zhu, M.; Devasahayam, R.; Adamson, S.J.; Benson, V.L.; Chan-Ling, T.; et al. Increased Indoleamine 2,3-Dioxygenase and Quinolinic Acid Expression in Microglia and Müller Cells of Diabetic Human and Rodent Retina. Investig. Opthalmol. Vis. Sci. 2017, 58, 5043. [Google Scholar] [CrossRef]
- Lovejoy, D.; Jacobs, K. Inhibiting the kynurenine pathway in spinal cord injury: Multiple therapeutic potentials? Neural Regen. Res. 2018, 13, 2073. [Google Scholar] [CrossRef]
- Meyramov, G.G.; Meyramova, A.G.A. Diabetogenic Zinc Binding B-Cytotoxic Chemicals: Mechanisms of Action and Methods for Prevention of Diabetes. J. Obes. Eat. Disord. 2016, 2. [Google Scholar] [CrossRef]
- Sarkar, S.A.; Wong, R.; Hackl, S.I.; Moua, O.; Gill, R.G.; Wiseman, A.; Davidson, H.W.; Hutton, J.C. Induction of Indoleamine 2,3-Dioxygenase by Interferon- in Human Islets. Diabetes 2006, 56, 72–79. [Google Scholar] [CrossRef]
- Wolowczuk, I.; Hennart, B.; Leloire, A.; Bessede, A.; Soichot, M.; Taront, S.; Caiazzo, R.; Raverdy, V.; Pigeyre, M.; Guillemin, G.J.; et al. Tryptophan metabolism activation by indoleamine 2,3-dioxygenase in adipose tissue of obese women: An attempt to maintain immune homeostasis and vascular tone. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 303, R135–R143. [Google Scholar] [CrossRef]
- Heilbronn, L.; Campbell, L. Adipose Tissue Macrophages, Low Grade Inflammation and Insulin Resistance in Human Obesity. Curr. Pharm. Des. 2008, 14, 1225–1230. [Google Scholar] [CrossRef] [PubMed]
- Autier, V.; Arbellot, A.; Audet, A.; Moinet, G.; Durbin, P.; Kergoat, M. Implication of Kynurenine Pathway in Glucose Metabolism and Insulin Secretion in Type II Diabetes. Diabetes 2005, 54, A34. [Google Scholar]
- Oxenkrug, G.F. Increased Plasma Levels of Xanthurenic and Kynurenic Acids in Type 2 Diabetes. Mol. Neurobiol. 2015, 52, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Muzik, O.; Burghardt, P.; Yi, Z.; Kumar, A.; Seyoum, B. Successful metformin treatment of insulin resistance is associated with down-regulation of the kynurenine pathway. Biochem. Biophys. Res. Commun. 2017, 488, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Brandacher, G.; Winkler, C.; Aigner, F.; Schwelberger, H.; Schroecksnadel, K.; Margreiter, R.; Fuchs, D.; Weiss, H. Bariatric Surgery Cannot Prevent Tryptophan Depletion Due to Chronic Immune Activation in Morbidly Obese Patients. Obes. Surg. 2006, 16, 541–548. [Google Scholar] [CrossRef]
- Yokoi, N.; Beppu, M.; Yoshida, E.; Hoshikawa, R.; Hidaka, S.; Matsubara, T.; Shinohara, M.; Irino, Y.; Hatano, N.; Seino, S. Identification of putative biomarkers for prediabetes by metabolome analysis of rat models of type 2 diabetes. Metabolomics 2015, 11, 1277–1286. [Google Scholar] [CrossRef]
- Leon, B.M.; Maddox, T.M. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J. Diabetes 2015, 6, 1246–1258. [Google Scholar] [CrossRef]
- Grant, P.J.; Cosentino, F. The 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2019, 40, 3215–3217. [Google Scholar] [CrossRef]
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 2018, 17. [Google Scholar] [CrossRef]
- Dinesh Shah, A.; Langenberg, C.; Rapsomaniki, E.; Denaxas, S.; Pujades-Rodriguez, M.; Gale, C.P.; Deanfield, J.; Smeeth, L.; Timmis, A.; Hemingway, H. Type 2 diabetes and incidence of a wide range of cardiovascular diseases: A cohort study in 1·9 million people. Lancet 2015, 385, S86. [Google Scholar] [CrossRef]
- Valdiglesias, V.; Marcos-Pérez, D.; Lorenzi, M.; Onder, G.; Gostner, J.M.; Strasser, B.; Fuchs, D.; Bonassi, S. Immunological alterations in frail older adults: A cross sectional study. Exp. Gerontol. 2018, 112, 119–126. [Google Scholar] [CrossRef]
- Sorgdrager, F.J.H.; Naudé, P.J.W.; Kema, I.P.; Nollen, E.A.; Deyn, P.P.D. Tryptophan Metabolism in Inflammaging: From Biomarker to Therapeutic Target. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, T.; Strand, E.; Dierkes, J.; Drevon, C.A.; Oyen, J.; Midttun, O.; Ueland, P.M.; Gudbrandsen, O.A.; Pedersen, E.R.; Nygard, O. Associations between intake of fish and n-3 long-chain polyunsaturated fatty acids and plasma metabolites related to the kynurenine pathway in patients with coronary artery disease. Eur. J. Nutr. 2017, 56, 261–272. [Google Scholar] [CrossRef]
- Moffett, J.R.; Namboodiri, M.A.A. Tryptophan and the immune response. Immunol. Cell Biol. 2003, 81, 247–265. [Google Scholar] [CrossRef]
- Schroecksnadel, K.; Frick, B.; Winkler, C.; Fuchs, D. Crucial Role of Interferon-γ and Stimulated Macrophages in Cardiovascular Disease. Curr. Vasc. Pharmacol. 2006, 4, 205–213. [Google Scholar] [CrossRef]
- Rudzite, V.; Sileniece, G.; Liepina, D.; Dalmane, A.; Zirne, R. Impairment of Kynurenine Metabolism in Cardiovascular Disease. Adv. Exp. Med. Biol. 1991, 663–667. [Google Scholar] [CrossRef]
- Wirleitner, B.; Rudzite, V.; Neurauter, G.; Murr, C.; Kalnins, U.; Erglis, A.; Trusinskis, K.; Fuchs, D. Immune activation and degradation of tryptophan in coronary heart disease. Eur. J. Clin. Investig. 2003, 33, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.E.; Astola, N.; Cribbs, A.P.; Goddard, M.E.; Park, I.; Green, P.; Davies, A.H.; Williams, R.O.; Feldmann, M.; Monaco, C. Indoleamine 2,3-dioxygenase-1 is protective in atherosclerosis and its metabolites provide new opportunities for drug development. Proc. Natl. Acad. Sci. USA 2015, 112, 13033–13038. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Mo, J.H.; Gong, X.; Rossetto, C.; Jang, A.; Beck, L.; Elliott, G.I.; Kufareva, I.; Abagyan, R.; Broide, D.H.; et al. 3-Hydroxyanthranilic acid inhibits PDK1 activation and suppresses experimental asthma by inducing T cell apoptosis. Proc. Natl. Acad. Sci. USA 2007, 104, 18619–18624. [Google Scholar] [CrossRef]
- Lee, W.-S.; Lee, S.-M.; Kim, M.-K.; Park, S.-G.; Choi, I.-W.; Choi, I.; Joo, Y.-D.; Park, S.-J.; Kang, S.-W.; Seo, S.-K. The tryptophan metabolite 3-hydroxyanthranilic acid suppresses T cell responses by inhibiting dendritic cell activation. Int. Immunopharmacol. 2013, 17, 721–726. [Google Scholar] [CrossRef]
- Zhang, L.; Ovchinnikova, O.; Jönsson, A.; Lundberg, A.M.; Berg, M.; Hansson, G.K.; Ketelhuth, D.F.J. The tryptophan metabolite 3-hydroxyanthranilic acid lowers plasma lipids and decreases atherosclerosis in hypercholesterolaemic mice. Eur. Heart J. 2012, 33, 2025–2034. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, K.A.; Ovchinnikova, O.; Berg, M.; Baumgartner, R.; Agardh, H.; Pirault, J.; Gistera, A.; Assinger, A.; Laguna-Fernandez, A.; Back, M.; et al. Inhibition of indoleamine 2,3-dioxygenase promotes vascular inflammation and increases atherosclerosis in Apoe-/- mice. Cardiovasc. Res. 2015, 106, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Murr, C.; Zoller, H.; Haun, M.; Widner, B.; Ludescher, C.; Fuchs, D. Modulation of neopterin formation and tryptophan degradation by Th1- and Th2-derived cytokines in human monocytic cells. Clin. Exp. Immunol. 1999, 116, 435–440. [Google Scholar] [CrossRef]
- De Rosa, S.; Cirillo, P.; Pacileo, M.; Petrillo, G.; D’Ascoli, G.-L.; Maresca, F.; Ziviello, F.; Chiariello, M. Neopterin: From Forgotten Biomarker to Leading Actor in Cardiovascular Pathophysiology. Curr. Vasc. Pharmacol. 2011, 9, 188–199. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, G.; Yang, B.; Zhang, B.; Zhang, L.; Ni, Y.; Liu, C.; Luo, Y. Neopterin as a Predictor of Functional Outcome and Mortality in Chinese Patients with Acute Ischemic Stroke. Mol. Neurobiol. 2015, 53, 3939–3947. [Google Scholar] [CrossRef] [PubMed]
- Stążka, J.; Luchowski, P.; Wielosz, M.; Kleinrok, Z.; Urbańska, E.M. Endothelium-dependent production and liberation of kynurenic acid by rat aortic rings exposed to l-kynurenine. Eur. J. Pharmacol. 2002, 448, 133–137. [Google Scholar] [CrossRef]
- Rzeski, W.; Zdzisinska, B.; Rejdak, R.; Kocki, T.; Okuno, E.; Kandefer-Szerszen, M.; Zrenner, E.; Turski, W.A.; Parada-Turska, J.; Wejksza, K. Kynurenic acid production in cultured bovine aortic endothelial cells. Homocysteine is a potent inhibitor. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2004, 369, 300–304. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; McKenzie, G.; Witting, P.K.; Stasch, J.-P.; Hahn, M.; Changsirivathanathamrong, D.; Wu, B.J.; Ball, H.J.; Thomas, S.R.; et al. Kynurenine is an endothelium-derived relaxing factor produced during inflammation. Nat. Med. 2010, 16, 279–285. [Google Scholar] [CrossRef]
- Sakakibara, K.; Feng, G.G.; Li, J.; Akahori, T.; Yasuda, Y.; Nakamura, E.; Hatakeyama, N.; Fujiwara, Y.; Kinoshita, H. Kynurenine causes vasodilation and hypotension induced by activation of KCNQ-encoded voltage-dependent K(+) channels. J. Pharmacol. Sci. 2015, 129, 31–37. [Google Scholar] [CrossRef]
- Fazio, F.; Carrizzo, A.; Lionetto, L.; Damato, A.; Capocci, L.; Ambrosio, M.; Battaglia, G.; Bruno, V.; Madonna, M.; Simmaco, M.; et al. Vasorelaxing Action of the Kynurenine Metabolite, Xanthurenic Acid: The Missing Link in Endotoxin-Induced Hypotension? Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef]
- Ristagno, G.; Latini, R.; Vaahersalo, J.; Masson, S.; Kurola, J.; Varpula, T.; Lucchetti, J.; Fracasso, C.; Guiso, G.; Montanelli, A.; et al. Early activation of the kynurenine pathway predicts early death and long-term outcome in patients resuscitated from out-of-hospital cardiac arrest. Resuscitation 2014, 85, S13. [Google Scholar] [CrossRef]
- Eussen, S.J.P.M.; Ueland, P.M.; Vollset, S.E.; Nygård, O.; Midttun, Ø.; Sulo, G.; Ulvik, A.; Meyer, K.; Pedersen, E.R.; Tell, G.S. Kynurenines as predictors of acute coronary events in the Hordaland Health Study. Int. J. Cardiol. 2015, 189, 18–24. [Google Scholar] [CrossRef]
- Pedersen, E.R.; Tuseth, N.; Eussen, S.J.P.M.; Ueland, P.M.; Strand, E.; Svingen, G.F.T.; Midttun, Ø.; Meyer, K.; Mellgren, G.; Ulvik, A.; et al. Associations of Plasma Kynurenines With Risk of Acute Myocardial Infarction in Patients With Stable Angina Pectoris. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 455–462. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, M.; Ding, Y.; Wang, Q.; Zhang, W.; Song, P.; Zou, M.-H. Activation of NAD(P)H Oxidase by Tryptophan-Derived 3-Hydroxykynurenine Accelerates Endothelial Apoptosis and Dysfunction In Vivo. Circ. Res. 2014, 114, 480–492. [Google Scholar] [CrossRef]
- Reyes Ocampo, J.; Lugo Huitrón, R.; González-Esquivel, D.; Ugalde-Muñiz, P.; Jiménez-Anguiano, A.; Pineda, B.; Pedraza-Chaverri, J.; Ríos, C.; Pérez de la Cruz, V. Kynurenines with Neuroactive and Redox Properties: Relevance to Aging and Brain Diseases. Oxidative Med. Cell. Longev. 2014, 2014, 1–22. [Google Scholar] [CrossRef]
- Platten, M.; Ho, P.P.; Youssef, S.; Fontoura, P.; Garren, H.; Hur, E.M.; Gupta, R.; Lee, L.Y.; Kidd, B.A.; Robinson, W.H.; et al. Treatment of Autoimmune Neuroinflammation with a Synthetic Tryptophan Metabolite. Science 2005, 310, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-G.; Ryu, S.Y.; Jung, I.-H.; Lee, Y.-H.; Kang, K.J.; Lee, M.-R.; Lee, M.-N.; Sonn, S.K.; Lee, J.H.; Lee, H.; et al. Evaluation of VCAM-1 antibodies as therapeutic agent for atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis 2013, 226, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Pae, H.-O.; Oh, G.-S.; Lee, B.-S.; Rim, J.-S.; Kim, Y.-M.; Chung, H.-T. 3-Hydroxyanthranilic acid, one of l-tryptophan metabolites, inhibits monocyte chemoattractant protein-1 secretion and vascular cell adhesion molecule-1 expression via heme oxygenase-1 induction in human umbilical vein endothelial cells. Atherosclerosis 2006, 187, 274–284. [Google Scholar] [CrossRef]
- Baran, H.; Staniek, K.; Kepplinger, B.; Gille, L.; Stolze, K.; Nohl, H. Kynurenic Acid Influences the Respiratory Parameters of Rat Heart Mitochondria. Pharmacology 2001, 62, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Leipnitz, G.; Schumacher, C.; Scussiato, K.; Dalcin, K.B.; Wannmacher, C.M.D.; Wyse, A.T.D.; Dutra-Filho, C.S.; Wajner, M.; Latini, A. Quinolinic acid reduces the antioxidant defenses in cerebral cortex of young rats. Int. J. Dev. Neurosci. 2005, 23, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Kozlova, A.; Frédérick, R. Current state on tryptophan 2,3-dioxygenase inhibitors: A patent review. Expert Opin. Ther. Pat. 2019, 29, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-P.; Song, Y.-L.; Zhu, Z.-M.; Huang, B.; Xiao, Y.-Q.; Luo, D.-Y. Targeting TDO in cancer immunotherapy. Med. Oncol. 2017, 34. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.W.; Connick, J.H. Effects of Quinolinic and Kynurenic Acids on Central Neurons. Adv. Exp. Med. Biol. 1991, 329–336. [Google Scholar] [CrossRef]
- Mizuguchi, T.; Mitaka, T.; Hirata, K.; Nakamura, T.; Mochizuki, Y. Recovery of mRNA Expression of Tryptophan 2,3-Dioxygenase and Serine Dehydratase in Long-Term Cultures of Primary Rat Hepatocytes. J. Biochem. 1996, 120, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Mellor, A.L.; Munn, D.H. Tryptophan Catabolism and Regulation of Adaptive Immunity. J. Immunol. 2003, 170, 5809–5813. [Google Scholar] [CrossRef] [PubMed]
- Sakash, J.B.; Byrne, G.I.; Lichtman, A.; Libby, P. Cytokines Induce Indoleamine 2,3-Dioxygenase Expression in Human Atheroma-Associated Cells: Implications for Persistent Chlamydophila pneumoniae Infection. Infect. Immun. 2002, 70, 3959–3961. [Google Scholar] [CrossRef][Green Version]
- Wang, J.; Simonavicius, N.; Wu, X.; Swaminath, G.; Reagan, J.; Tian, H.; Ling, L. Kynurenic Acid as a Ligand for Orphan G Protein-coupled Receptor GPR35. J. Biol. Chem. 2006, 281, 22021–22028. [Google Scholar] [CrossRef]
- Shahid, F.; Lip, G.Y.H.; Shantsila, E. Role of Monocytes in Heart Failure and Atrial Fibrillation. J. Am. Heart Assoc. 2018, 7. [Google Scholar] [CrossRef]
- Patel, B.; Bansal, S.S.; Ismahil, M.A.; Hamid, T.; Rokosh, G.; Mack, M.; Prabhu, S.D. CCR2+ Monocyte-Derived Infiltrating Macrophages Are Required for Adverse Cardiac Remodeling During Pressure Overload. JACC 2018, 3, 230–244. [Google Scholar] [CrossRef]
- Sager, H.B.; Hulsmans, M.; Lavine, K.J.; Moreira, M.B.; Heidt, T.; Courties, G.; Sun, Y.; Iwamoto, Y.; Tricot, B.; Khan, O.F.; et al. Proliferation and Recruitment Contribute to Myocardial Macrophage Expansion in Chronic Heart Failure. Circ. Res. 2016, 119, 853–864. [Google Scholar] [CrossRef]
- Gullestad, L.; Ueland, T.; Vinge, L.E.; Finsen, A.; Yndestad, A.; Aukrust, P. Inflammatory Cytokines in Heart Failure: Mediators and Markers. Cardiology 2012, 122, 23–35. [Google Scholar] [CrossRef]
- Jones, S.P.; Franco, N.F.; Varney, B.; Sundaram, G.; Brown, D.A.; de Bie, J.; Lim, C.K.; Guillemin, G.J.; Brew, B.J. Expression of the Kynurenine Pathway in Human Peripheral Blood Mononuclear Cells: Implications for Inflammatory and Neurodegenerative Disease. PLoS ONE 2015, 10, e0131389. [Google Scholar] [CrossRef]
- Ueland, P.M.; McCann, A.; Midttun, Ø.; Ulvik, A. Inflammation, vitamin B6 and related pathways. Mol. Asp. Med. 2017, 53, 10–27. [Google Scholar] [CrossRef]
- Lund, A.; Nordrehaug, J.E.; Slettom, G.; Solvang, S.-E.H.; Pedersen, E.K.; Midttun, Ø.; Ulvik, A.; Ueland, P.M.; Nygård, O.; Giil, L.M. Correction: Plasma kynurenines and prognosis in patients with heart failure. PLoS ONE 2020, 15, e0230056. [Google Scholar] [CrossRef]
- Dschietzig, T.B.; Kellner, K.H.; Sasse, K.; Boschann, F.; Klusener, R.; Ruppert, J.; Armbruster, F.P.; Bankovic, D.; Meinitzer, A.; Mitrovic, V.; et al. Plasma Kynurenine Predicts Severity and Complications of Heart Failure and Associates with Established Biochemical and Clinical Markers of Disease. Kidney Blood Press Res. 2019, 44, 765–776. [Google Scholar] [CrossRef]
- Konishi, M.; Ebner, N.; Springer, J.; Schefold, J.C.; Doehner, W.; Dschietzig, T.B.; Anker, S.D.; von Haehling, S. Impact of Plasma Kynurenine Level on Functional Capacity and Outcome in Heart Failure―Results From Studies Investigating Co-morbidities Aggravating Heart Failure (SICA-HF). Circ. J. 2017, 81, 52–61. [Google Scholar] [CrossRef]

| Kynurenine Pathway Metabolite | Effect/Changes | Comments | References |
|---|---|---|---|
| TRP | Across the aging time course increase in the concentration of tryptophan. | The non-obese diabetic (NOD) inbred mouse strain recapitulates the autoimmune nature of T1DM, the NOD-E (transgenic NOD mice that express the I > E heterodimer of the major histocompatibility complex II). | [48] |
| Tryptophan stimulates the release of insulin from β cells of the pancreas and incretin hormones via GPR142 signaling. | Wild-type control (WT) mice in a dose-dependent manner in the presence of 11.1 mM glucose. | [14] | |
| Tryptophan deficiency in the diet can modulate glucose tolerance. | Feeding rats for 14 days with TRP deficient diet caused worse glucose tolerance, and it was reversible after feeding a complete diet. | [17] | |
| Surplus tryptophan in the diet induces insulin resistance. | Feeding pigs for 3 weeks with a high (13.2%) vs. normal (3.4%) TRP large neutral amino acids (LNAA) diet. | [20] | |
| Enhanced TRP disappearance from the bloodstream in diabetic rats after a tryptophan load Impaired acute accumulation of TRP in the diabetic rat brains. | Normal vs. streptozotocin-diabetic rats. | [58] | |
| KYN | “Acute exposure to KYN” enhances glucose-induced insulin secretion. | Normal rat islets and the INS-1 β-cell line. | [40] |
| KYNA | Increase in the concentrations of KYNA observed with the progress of T1D. | NOD vs. NOD-E mice. | [48] |
| Fifty percent higher concentrations of KYNA in the serum from Zucker fatty rats. | Leptin-receptor-deficient Zucker fatty rats (ZFR)(fa/fa) vs. age-matched lean rats (FA/-). | [28] | |
| A 1,8 fold increase in the concentration of KYNA in urine od T2D nonhuman. | Normal vs. spontaneously and naturally diabetic nonhuman primate (rhesus macaques). | [59] | |
| KYNA inhibit the pro-insulin synthesis. | Isolated rat pancreatic islets, KYNA in millimolar concentrations. | [41] | |
| XA | Increases urine excretion of XA as its complex with Zn2+. | Alloxan- and streptozotocin-induced diabetic rats. | [55] |
| Forms complexes with insulin and reduces insulin activity. | XA-induced diabetic rats. | [53] | |
| Inhibits pro-insulin synthesis. | Isolated rat pancreatic islets; XA in millimolar concentrations. | [41] | |
| Induces pancreatic β-cells death. | Damage caused probably via caspase-3 dependent mechanism. | [49] | |
| 3-HKYN3-HAA | Inhibit the leucine stimulated release of insulin at concentrations below 10 mM. | Isolated pancreas islets from rats. | [51] |
| QA | Inhibitory effect of QA on phosphoenolpyruvate carboxykinase activity in diabetic rats; in vivo studies showed that intraperitoneal injection of 300 mg/kg−1 b.wt. initiates reduction of blood glucose level in 1 h after injection, restoring the blood glucose to its normal level at 2 h postinjection and keeping it constant for at least a further 4 h. | Streptozotocin-induced diabetes in rats; in vitro studies in rat’s liver. | [60] |
| QA-induced increase of brain glucose uptake (55%), (14)CO2 generation from glucose, acetate, and citrate was inhibited (up to 60%). QA provokes a mild impairment of brain energy metabolism in vitro. | Thirty-day-old rats. | [61] | |
| STZ-diabetes mellitus causes augmentations of both KYN and QA generations in the liver, indicating the possibility that the immune and neuronal systems of insulin-dependent diabetes mellitus would be influenced by the increased amounts of KYN and QA. | Streptozotocin-induced diabetes in rats; the hepatocytes isolated from the rats were incubated with [5-3H]L-TRP. | [39] | |
| QA expression in the retinas of diabetic rats could contribute to the neuronal degeneration that is characteristic of diabetic retinopathy. | Streptozotocin-induced diabetes in rats. | [62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiluk, M.; Lewkowicz, J.; Pawlak, D.; Tankiewicz-Kwedlo, A. Crosstalk between Tryptophan Metabolism via Kynurenine Pathway and Carbohydrate Metabolism in the Context of Cardio-Metabolic Risk—Review. J. Clin. Med. 2021, 10, 2484. https://doi.org/10.3390/jcm10112484
Kiluk M, Lewkowicz J, Pawlak D, Tankiewicz-Kwedlo A. Crosstalk between Tryptophan Metabolism via Kynurenine Pathway and Carbohydrate Metabolism in the Context of Cardio-Metabolic Risk—Review. Journal of Clinical Medicine. 2021; 10(11):2484. https://doi.org/10.3390/jcm10112484
Chicago/Turabian StyleKiluk, Małgorzata, Janina Lewkowicz, Dariusz Pawlak, and Anna Tankiewicz-Kwedlo. 2021. "Crosstalk between Tryptophan Metabolism via Kynurenine Pathway and Carbohydrate Metabolism in the Context of Cardio-Metabolic Risk—Review" Journal of Clinical Medicine 10, no. 11: 2484. https://doi.org/10.3390/jcm10112484
APA StyleKiluk, M., Lewkowicz, J., Pawlak, D., & Tankiewicz-Kwedlo, A. (2021). Crosstalk between Tryptophan Metabolism via Kynurenine Pathway and Carbohydrate Metabolism in the Context of Cardio-Metabolic Risk—Review. Journal of Clinical Medicine, 10(11), 2484. https://doi.org/10.3390/jcm10112484







