Remission of Diabetes Following Bariatric Surgery: Plasma Proteomic Profiles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects of Study
2.2. Sample Preparation and Two-Dimensional Differential in Gel Electrophoresis 2D-DIGE
2.3. Enzyme-Linked Immunoassays and Immunenephelometry Assays
2.4. Statistical Analysis
3. Results
3.1. Clinical Variables before and after Surgery
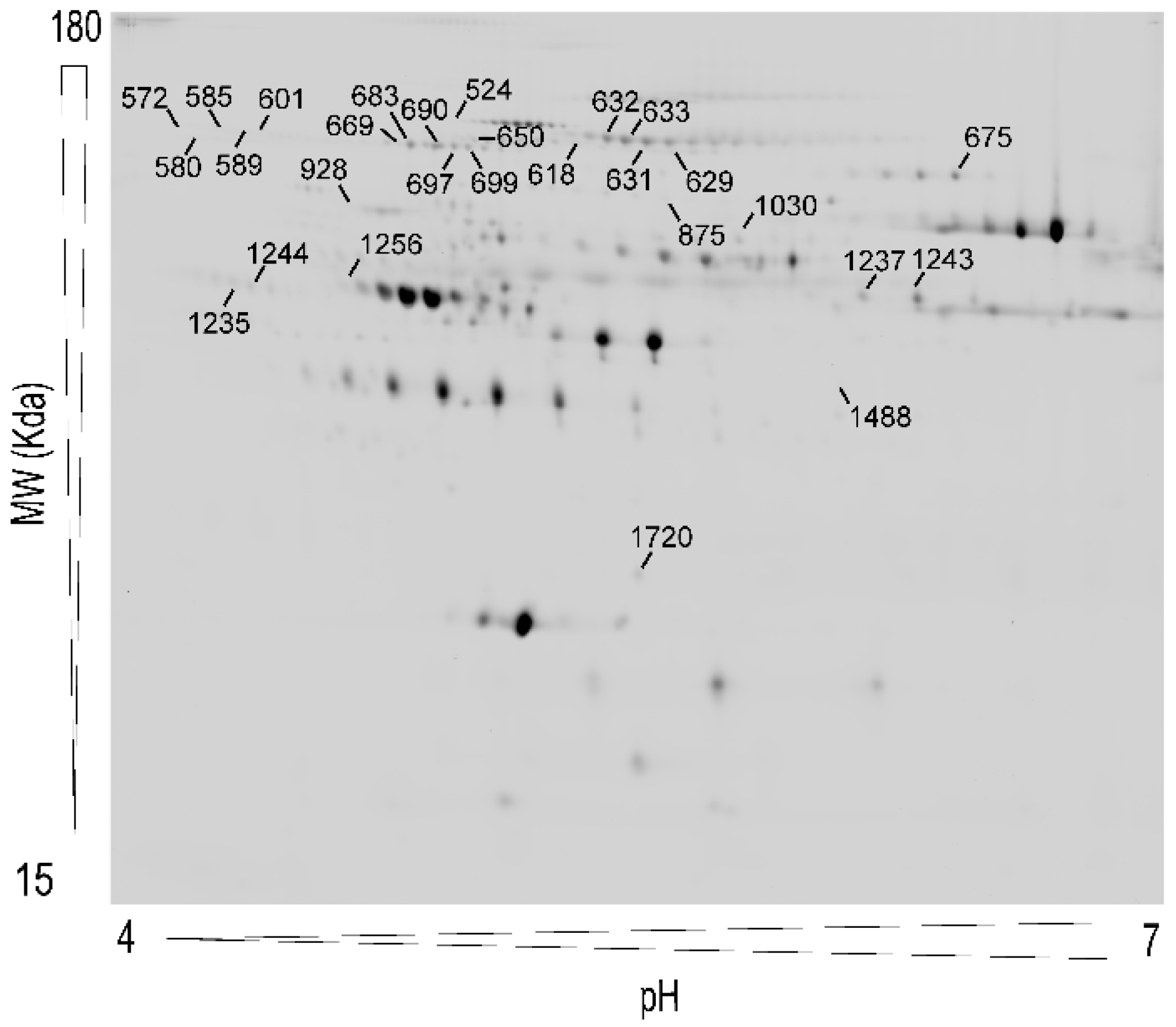
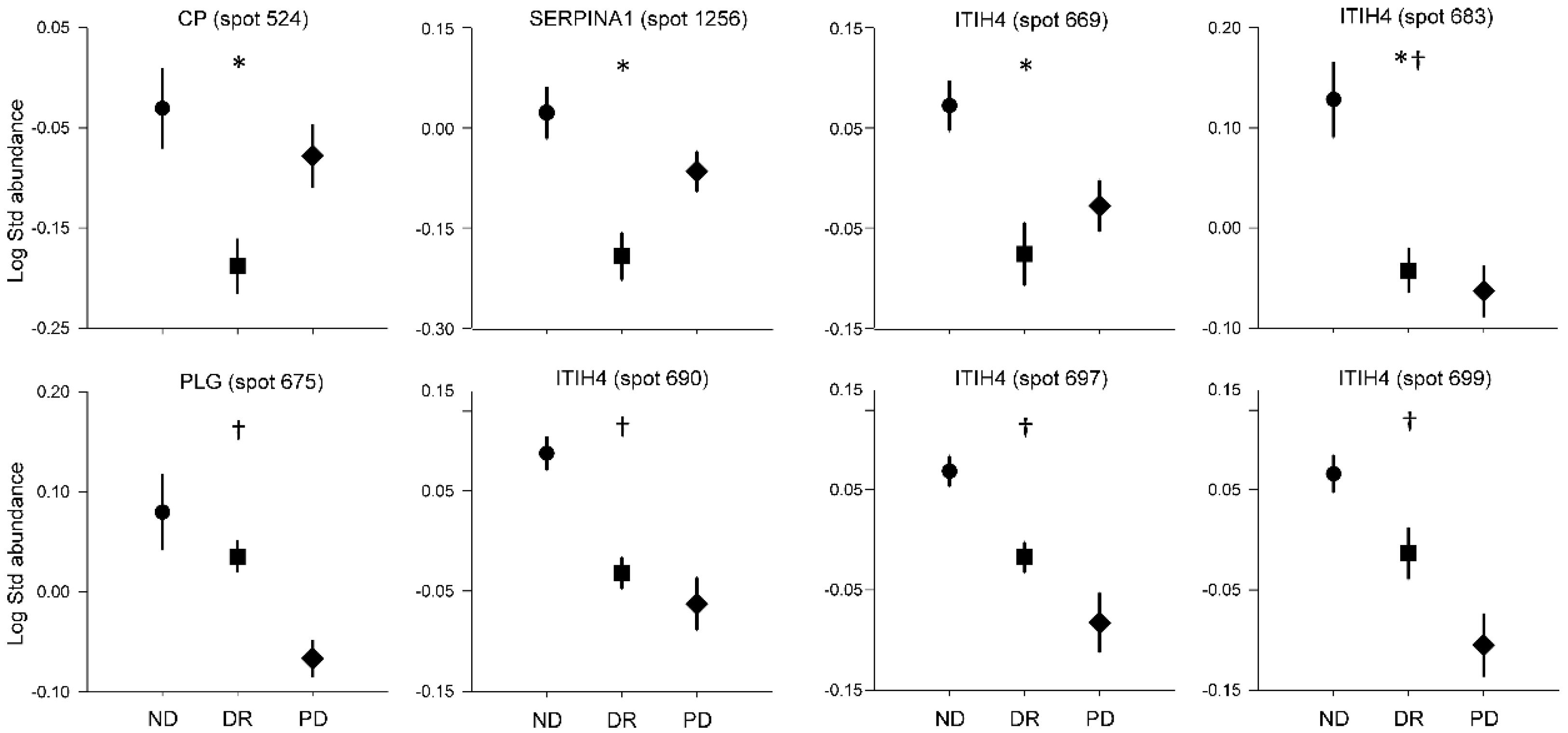
3.2. Analysis of Plasma Proteins Changes in Response to Weight Loss Resulting from Bariatric Surgery
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nguyen, N.T.; Varela, J.E. Bariatric surgery for obesity and metabolic disorders: State of the art. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 160–169. [Google Scholar] [CrossRef]
- Schauer, P.R.; Hanipah, Z.N.; Rubino, F. Metabolic surgery for treating type 2 diabetes mellitus: Now supported by the world’s leading diabetes organizations. Cleve. Clin. J. Med. 2017, 84, S47–S56. [Google Scholar] [CrossRef]
- Han, Y.K.; Jia, Y.; Wang, H.L.; Cao, L.; Zhao, Y.J. Comparative analysis of weight loss and resolution of comorbidities between laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass: A systematic review and meta-analysis based on 18 studies. Int. J. Surg. 2020, 76, 101–110. [Google Scholar] [CrossRef]
- Madadi, F.; Jawad, R.; Mousati, I.; Plaeke, P.; Hubens, G. Remission of type 2 diabetes and sleeve gastrectomy in morbid obesity: A comparative systematic review and meta-analysis. Obes. Surg. 2019, 29, 4066–4076. [Google Scholar] [CrossRef]
- Park, J.Y. Prediction of type 2 diabetes remission after bariatric or metabolic surgery. J. Obes. Metab. Syndr. 2018, 27, 213–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osland, E.; Yunus, R.M.; Khan, S.; Memon, B.; Memon, M.A. Diabetes improvement and resolution following laparoscopic vertical sleeve gastrectomy (LVSG) versus laparoscopic Roux-en-Y gastric bypass (LRYGB) procedures: A systematic review of randomized controlled trials. Surg. Endosc. 2017, 31, 1952–1963. [Google Scholar] [CrossRef] [PubMed]
- Dang, J.T.; Sheppard, C.; Kim, D.; Switzer, N.; Shi, X.; Tian, C.; De Gara, C.; Karmali, S.; Birch, D.W. Predictive factors for diabetes remission after bariatric surgery. Can. J. Surg. 2019, 6, 315–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, T.C.; Pellen, M.G.C.; Sedman, P.C.; Jain, P.K. Preoperative factors predicting remission of type 2 diabetes mellitus after Roux-en-Y gastric bypass surgery for obesity. Obes. Surg. 2010, 20, 1245–1250. [Google Scholar] [CrossRef]
- Davies, N.; O’Sullivan, J.M.; Plank, L.D.; Murphy, R. Gut microbial predictors of type 2 diabetes remission following bariatric surgery. Obes. Surg. 2020, 30, 3536–3548. [Google Scholar] [CrossRef] [PubMed]
- Grenier-Larouche, T.; Carreau, A.M.; Carpentier, A.C. Early metabolic improvement after bariatric surgery: The first steps toward remission of type 2 diabetes. Can. J. Diabetes 2017, 41, 418–425. [Google Scholar] [CrossRef]
- Cummings, D.E.; Rubino, F. Metabolic surgery for the treatment of type 2 diabetes in obese individuals. Diabetologia 2018, 61, 257–264. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Pevida, B.; Escalada, J.; Miras, A.D.; Frühbeck, G. Mechanisms underlying type 2 diabetes remission after metabolic surgery. Front. Endocrinol. 2019, 10, 641. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.-J.; Hur, K.Y.; Lakadawala, M.; Kasama, K.; Wong, S.K.H.; Chen, S.-C.; Lee, Y.-C.; Ser, K.-H. Predicting success of metabolic surgery: Age, body mass index, C-peptide, and duration score. Surg. Obes. Relat. Dis. 2013, 9, 379–384. [Google Scholar] [CrossRef]
- Still, C.D.; Wood, G.C.; Benotti, P.; Petrick, A.T.; Gabrielsen, J.; Strodel, W.E.; Ibele, A.; Seiler, J.; Irving, B.A.; Celaya, M.P.; et al. Preoperative prediction of type 2 diabetes remission after Roux-en-Y gastric bypass surgery: A retrospective cohort study. Lancet Diabetes Endocrinol. 2014, 2, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Moh, M.C.; Cheng, A.; Tan, C.H.; Lim, B.K.; Tan, B.C.; Ng, D.; Sum, C.F.; Subramaniam, T.; Lim, S.C. Metabolic surgery diabetes remission (MDR) score: A new preoperative scoring system for predicting type 2 diabetes remission at 1 year after metabolic surgery in the singapore multi-ethnic asian setting. Obes. Surg. 2020, 30, 3387–3393. [Google Scholar] [CrossRef] [PubMed]
- Aminian, A.; Brethauer, S.A.; Andalib, A.; Nowacki, A.S.; Jimenez, A.; Corcelles, R.; Hanipah, Z.N.; Punchai, S.; Bhatt, D.L.; Kashyap, S.R.; et al. Individualized metabolic surgery score: Procedure selection based on diabetes severity. Ann. Surg. 2017, 266, 650–657. [Google Scholar] [CrossRef]
- Pucci, A.; Tymoszuk, U.; Cheung, W.H.; Makaronidis, J.M.; Scholes, S.; Tharakan, G.; Elkalaawy, M.; Guimaraes, M.; Nora, M.; Hashemi, M.; et al. Type 2 diabetes remission 2 years post Roux-en-Y gastric bypass and sleeve gastrectomy: The role of the weight loss and comparison of DiaRem and DiaBetter scores. Diabet. Med. 2018, 35, 360–367. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association. Standards of medical care in diabetes—2008. Diabetes Care 2008, 31 (Suppl. 1), S12–S54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buse, J.B.; Caprio, S.; Cefalu, W.T.; Ceriello, A.; Del Prato, S.; Inzucchi, S.E.; McLaughlin, S.; Phillips, G.L.; Robertson, R.P.; Rubino, F.; et al. How do we define cure of diabetes? Diabetes Care 2009, 32, 2133–2135. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association. Obesity management for the treatment of type 2 diabetes. Diabetes Care 2018, 41, S65–S72. [Google Scholar] [CrossRef] [Green Version]
- Gastrointestinal surgery for severe obesity: National Institutes of Health Consensus Development Conference Statement. Am. J. Clin. Nutr. 1992, 55, 615S–619S. [CrossRef]
- American Society for Metabolic and Bariatric Surgery. Position statement on global bariatric healthcare. Surg. Obes. Relat. Dis. 2011, 7, 669–671. [Google Scholar] [CrossRef]
- Insenser, M.; Martínez-García, M.A.; Montes, R.; San-Millán, J.L.; Escobar-Morreale, H.F. Proteomic analysis of plasma in the polycystic ovary syndrome identifies novel markers involved in iron metabolism, acute-phase response, and inflammation. J. Clin. Endocrinol. Metab. 2010, 95, 3863–3870. [Google Scholar] [CrossRef] [Green Version]
- Insenser, M.; Martínez-García, M.; Nieto, R.M.; San-Millán, J.L.; Escobar-Morreale, H.F. Impact of the storage temperature on human plasma proteomic analysis: Implications for the use of human plasma collections in research. Proteom. Clin. Appl. 2010, 4, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Dunn, E.J.; Grant, P.J. Type 2 diabetes: An atherothrombotic syndrome. Curr. Mol. Med. 2005, 5, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Faber, D.R.; de Groot, P.G.; Visseren, F.L. Role of adipose tissue in haemostasis, coagulation and fibrinolysis. Obes. Rev. 2009, 10, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Schmatz, R.; Bitencourt, M.R.; Patias, L.D.; Beck, M.; da Alvarez, G.C.; Zanini, D.; Gutierres, J.M.; Diehl, L.N.; Pereira, L.B.; Leal, C.A.; et al. Evaluation of the biochemical, inflammatory and oxidative profile of obese patients given clinical treatment and bariatric surgery. Clin. Chim. Acta 2017, 465, 72–79. [Google Scholar] [CrossRef]
- Hafida, S.; Mirshahi, T.; Nikolajczyk, B.S. The impact of bariatric surgery on inflammation: Quenching the fire of obesity? Curr. Opin. Endocrinol. Diabetes Obes. 2016, 23, 373–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, E.; Unoki-Kubota, H.; Shimizu, Y.; Okamura, T.; Iwata, W.; Kajio, H.; Yamamoto-Honda, R.; Shiga, T.; Yamashita, S.; Tobe, K.; et al. Proteomic analysis of serum biomarkers for prediabetes using the Long-Evans Agouti rat, a spontaneous animal model of type 2 diabetes mellitus. J. Diabetes Investig. 2017, 8, 661–671. [Google Scholar] [CrossRef]
- Feldt, J.; Schicht, M.; Garreis, F.; Welss, J.; Schneider, U.W.; Paulsen, F. Structure, regulation and related diseases of the actin-binding protein gelsolin. Expert Rev. Mol. Med. 2019, 20, e7. [Google Scholar] [CrossRef]
- Spinardi, L.; Witke, W. Gelsolin and diseases. Subcell. Biochem. 2007, 45, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Khatri, N.; Sagar, A.; Peddada, N.; Choudhary, V.; Chopra, B.S.; Garg, V.; Garg, R.; Ashish. Plasma gelsolin levels decrease in diabetic state and increase upon treatment with F-actin depolymerizing versions of gelsolin. J. Diabetes Res. 2014, 2014, 152075. [Google Scholar] [CrossRef]
- Joo, J.I.; Oh, T.S.; Kim, D.H.; Choi, D.K.; Wang, X.; Choi, J.W.; Yun, J.W. Differential expression of adipose tissue proteins between obesity-susceptible and -resistant rats fed a high-fat diet. Proteomics 2011, 11, 1429–1448. [Google Scholar] [CrossRef] [PubMed]
- Krebs, J. Calcium-binding proteins: Cytosolic (annexins, gelsolins, C2-domain proteins). In Encyclopedia of Biological Chemistry; Lennarz, W.J., Lane, M.D., Eds.; Elsevier: New York, NY, USA, 2004; pp. 287–293. [Google Scholar]
- Rehman, A.A.; Ahsan, H.; Khan, F.H. α-2-Macroglobulin: A physiological guardian. J. Cell Physiol. 2013, 228, 1665–1675. [Google Scholar] [CrossRef] [PubMed]
- Mantuano, E.; Mukandala, G.; Li, X.; Campana, W.M.; Gonias, S.L. Molecular dissection of the human alpha2-macroglobulin subunit reveals domains with antagonistic activities in cell signaling. J. Biol. Chem. 2008, 283, 19904–19911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westwood, M.; Aplin, J.D.; Collinge, I.A.; Gill, A.; White, A.; Gibson, J.M. α2-Macroglobulin: A new component in the insulin-like growth factor/insulin-like growth factor binding protein-1 axis. J. Biol. Chem. 2001, 276, 41668–41674. [Google Scholar] [CrossRef] [Green Version]
- Šunderić, M.; Miljuš, G.; Nedić, O. Interaction of insulin-like growth factor-binding protein 2 with α2-macroglobulin in the circulation. Protein J. 2013, 32, 138–142. [Google Scholar] [CrossRef]
- James, K.; Merriman, J.; Gray, R.S.; Duncan, L.J.; Herd, R. Serum alpha 2-macroglobulin levels in diabetes. J. Clin. Pathol. 1980, 33, 163–166. [Google Scholar] [CrossRef] [Green Version]
- Yoshino, S.; Fujimoto, K.; Takada, T.; Kawamura, S.; Ogawa, J.; Kamata, Y.; Kodera, Y.; Shichiri, M. Molecular form and concentration of serum α. Sci. Rep. 2019, 9, 12927. [Google Scholar] [CrossRef]
- De Vries, J.J.; Snoek, C.J.M.; Rijken, D.C.; de Maat, M.P.M. Effects of post-translational modifications of fibrinogen on clot formation, clot structure, and fibrinolysis: A systematic review. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 554–569. [Google Scholar] [CrossRef]
- Sánchez-Navarro, A.; González-Soria, I.; Caldiño-Bohn, R.; Bobadilla, N.A. An integrative view of serpins in health and disease: The contribution of SerpinA3. Am. J. Physiol. Cell Physiol. 2021, 320, C106–C118. [Google Scholar] [CrossRef] [PubMed]
- Mansuy-Aubert, V.; Zhou, Q.L.; Xie, X.; Gong, Z.; Huang, J.-Y.; Khan, A.R.; Aubert, G.; Candelaria, K.; Thomas, S.; Shin, D.-J.; et al. Imbalance between neutrophil elastase and its inhibitor α1-antitrypsin in obesity alters insulin sensitivity, inflammation, and energy expenditure. Cell Metab. 2013, 17, 534–548. [Google Scholar] [CrossRef] [Green Version]
- Kashyap, R.S.; Nayak, A.R.; Deshpande, P.S.; Kabra, D.; Purohit, H.J.; Taori, G.M.; Daginawala, H.F. Inter-α-trypsin inhibitor heavy chain 4 is a novel marker of acute ischemic stroke. Clin. Chim. Acta 2009, 402, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, H.; Matsumoto, I.; Osada, A.; Kurata, I.; Ebe, H.; Tanaka, Y.; Inoue, A.; Umeda, N.; Kondo, Y.; Tsuboi, H.; et al. Identification of novel biomarker as citrullinated inter-alpha-trypsin inhibitor heavy chain 4, specifically increased in sera with experimental and rheumatoid arthritis. Arth. Res. Ther. 2018, 20, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geyer, P.E.; Wewer Albrechtsen, N.J.; Tyanova, S.; Grassl, N.; Iepsen, E.W.; Lundgren, J.; Madsbad, S.; Holst, J.J.; Torekov, S.S.; Mann, M. Proteomics reveals the effects of sustained weight loss on the human plasma proteome. Mol. Syst. Biol. 2016, 12, 901. [Google Scholar] [CrossRef] [PubMed]
- García-Ramírez, M.; Canals, F.; Hernández, C.; Colomé, N.; Ferrer, C.; Carrasco, E.; García-Arumí, J.; Simó, R. Proteomic analysis of human vitreous fluid by fluorescence-based difference gel electrophoresis (DIGE): A new strategy for identifying potential candidates in the pathogenesis of proliferative diabetic retinopathy. Diabetologia 2007, 50, 1294–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, J.; Min, H.; Kim, S.J.; Oh, S.; Kim, K.; Yu, H.G.; Park, T.; Kim, Y. Development of diagnostic biomarkers for detecting diabetic retinopathy at early stages using quantitative proteomics. J. Diabetes Res. 2016, 2016, 6571976. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Cai, L.; Guo, L.; Tsukamoto, Y.; Yutani, C.; Li, X.-A. Accumulation and expression of serum amyloid P component in human atherosclerotic lesions. Atherosclerosis 2010, 211, 90–95. [Google Scholar] [CrossRef] [Green Version]
- Albrechtsen, N.J.W.; Geyer, P.E.; Doll, S.; Treit, P.V.; Bojsen-Moller, K.N.; Martinussen, C.; Jorgensen, N.B.; Torekov, S.S.; Meier, F.; Niu, L.; et al. Plasma proteome profiling reveals dynamics of inflammatory and lipid homeostasis markers after Roux-En-Y gastric bypass surgery. Cell Syst. 2018, 7, 601–612.e3. [Google Scholar] [CrossRef] [Green Version]
- Varela-Rodríguez, B.M.; Juiz-Valiña, P.; Varela, L.; Outeiriño-Blanco, E.; Bravo, S.B.; García-Brao, M.J.; Mena, E.; Noguera, J.F.; Valero-Gasalla, J.; Cordido, F.; et al. Beneficial effects of bariatric surgery-induced by weight loss on the proteome of abdominal subcutaneous adipose tissue. J. Clin. Med. 2020, 9, 213. [Google Scholar] [CrossRef] [Green Version]

| Non-Diabetic Patients | Diabetes Remission | Persistent Diabetes | Group | Surgery | Interaction | ||||
|---|---|---|---|---|---|---|---|---|---|
| Before Surgery (n = 6) | 6 Months (n = 6) | Before Surgery (n = 7) | 6 Months (n = 7) | Before Surgery (n = 4) | 6 Months (n = 4) | p Value | p Value | p Value | |
| Age | 43 ± 10 | NA | 46 ± 8 | NA | 51 ± 5 | NA | 0.359 | NA | NA |
| BMI (kg/m2) | 43.4 ± 4.3 | 30.1 ± 4.6 | 46.5 ± 9.3 | 32.8 ± 5.9 | 44.1 ± 2.2 | 32.8 ± 4.8 | 0.663 | <0.001 | 0.578 |
| Decrease in BMI (kg/m2) | NA | 13.3 ± 3.7 | NA | 13.7 ±3.9 | NA | 11.3 ± 3.8 | 0.578 | NA | NA |
| Excess weight loss (%) | NA | 74 ± 21 | NA | 67 ± 10 | NA | 68 ± 19 | 0.691 | NA | NA |
| Waist (cm) | 127 ± 13 | 101 ± 4 | 128 ± 17 | 110 ± 13 | 127 ± 10 | 110 ± 15 | 0.694 | <0.001 | 0.439 |
| Body fat (%) | 50 ± 4 | 35 ± 7 | 48 ± 4 | 37 ± 3 | 53 ± 4 | 42 ± 9 | 0.363 | <0.001 | 0.617 |
| Glucose (mmol/L) | 5.0 ± 0.6 | 4.3 ± 0.3 | 9.4 ± 3.7 | 4.7 ± 0.5 | 8.0 ± 1.2 | 8.3 ± 2.5 | 0.005 *,† | 0.023 | 0.015 |
| Insulin (pmol/L) | 93 ± 27 | 30 ± 4 | 164 ± 89 | 35 ± 13 | 368 ± 589 | 64 ± 73 | 0.523 | <0.001 | 0.400 |
| HbA1c | 5.6 ± 0.2 | 5.1 ± 0.2 | 7.7 ± 1.4 | 5.6 ± 0.3 | 7.6 ± 0.8 | 7.0 ± 1.3 | 0.001 *,† | <0.001 | 0.023 |
| HOMA-IR | 3.0 ± 1.0 | 0.8 ± 0.1 | 9.3 ± 0.4 | 1.1 ± 0.5 | 17.2 ± 26.3 | 3.1 ± 3.0 | 0.049 † | <0.001 | 0.058 |
| Triglycerides (mmol/L) | 1.0 ± 0.3 | 1.0 ± 0.3 | 3.1 ± 1.9 | 1.1 ± 0.6 | 1.7 ± 0.8 | 1.6 ± 1.0 | 0.079 | 0.007 | 0.003 |
| Total cholesterol (mmol/L) | 5.0 ± 0.8 | 4.4 ± 1.1 | 5.4 ± 1.5 | 4.1 ± 1.0 | 4.4 ± 0.4 | 3.7 ± 0.9 | 0.444 | 0.004 | 0.645 |
| HDL (mmol/L) | 1.4 ± 0.2 | 1.6 ± 0.4 | 1.0 ± 0.3 | 1.2 ± 0.4 | 1.4 ± 0.2 | 1.3 ± 0.2 | 0.030 * | 0.750 | 0.273 |
| LDL (mmol/L) | 3.2 ± 0.7 | 2.2 ± 1.0 | 3.3 ± 0.9 | 2.4 ± 0.9 | 2.3 ± 0.5 | 1.7 ± 0.5 | 0.115 | 0.022 | 0.744 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Insenser, M.; Vilarrasa, N.; Vendrell, J.; Escobar-Morreale, H.F. Remission of Diabetes Following Bariatric Surgery: Plasma Proteomic Profiles. J. Clin. Med. 2021, 10, 3879. https://doi.org/10.3390/jcm10173879
Insenser M, Vilarrasa N, Vendrell J, Escobar-Morreale HF. Remission of Diabetes Following Bariatric Surgery: Plasma Proteomic Profiles. Journal of Clinical Medicine. 2021; 10(17):3879. https://doi.org/10.3390/jcm10173879
Chicago/Turabian StyleInsenser, María, Nuria Vilarrasa, Joan Vendrell, and Héctor F. Escobar-Morreale. 2021. "Remission of Diabetes Following Bariatric Surgery: Plasma Proteomic Profiles" Journal of Clinical Medicine 10, no. 17: 3879. https://doi.org/10.3390/jcm10173879








