Effect of SSRIs on Resting-State Functional Brain Networks in Adolescents with Major Depressive Disorder
Abstract
:1. Introduction
2. Material and Methods
2.1. Participants
2.2. Pre- and Post-Treatment Clinical Assessment
2.3. Image Acquisition and Processing
2.4. Resting-State Functional Connectivity and Network Construction
2.5. Graph-Theoretic Analysis
2.5.1. Graph Measures
- : an undirected graph where N is the set of nodes and E is the collection of existing connections/links;
- : the connection between node i and j;
- : the existence of a connection . if the link is connected, otherwise ;
- : the functional connectivity of a connection ;
- : the collection of modules of the graph where m is a module;
- : the module that node i belongs to.
Clustering Coefficient
Characteristic Path Length
Smallworldness
Degree
Local Efficiency
Modules
Participation Coefficient
Betweenness Centrality
2.5.2. Graph Measure AUCs
2.6. Statistical Analyses
3. Results
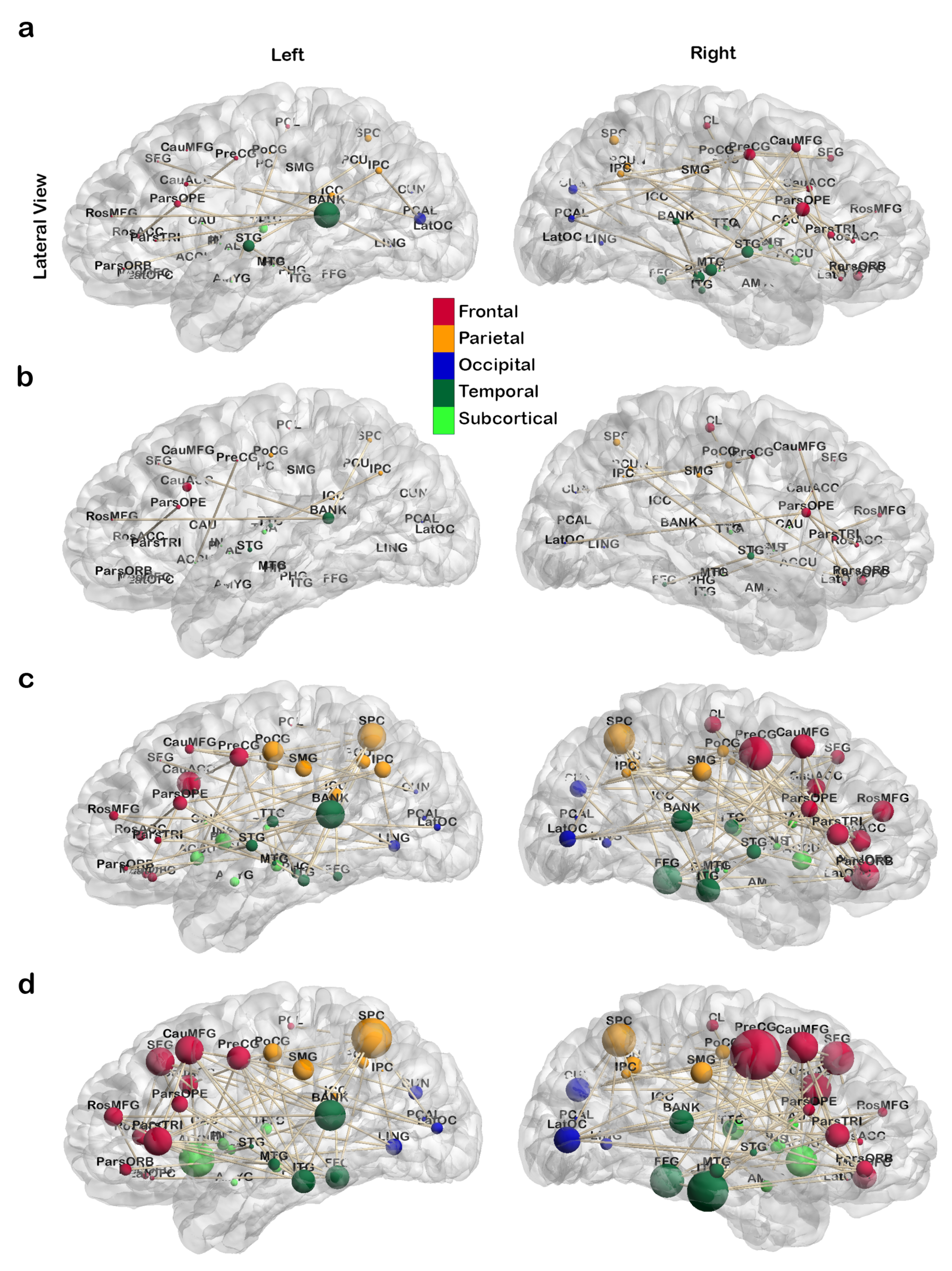
3.1. Treatment-Related Changes in Network Topology
3.1.1. Global Topological Metrics
3.1.2. Local Topological Metrics
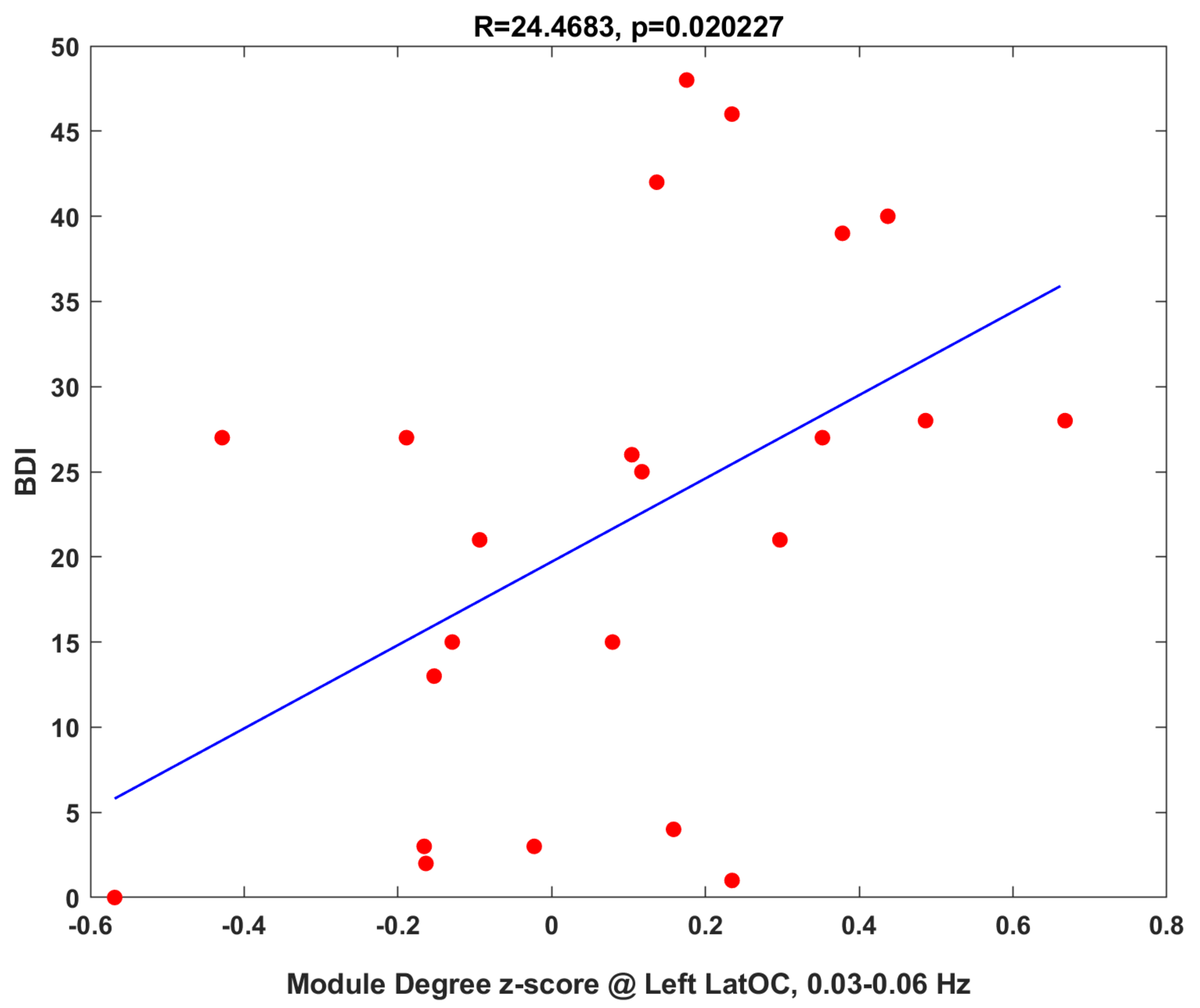
3.1.3. Clinical Correlations
3.2. Treatment-Related Changes in Functional Connectivity in Four Frequency Bands
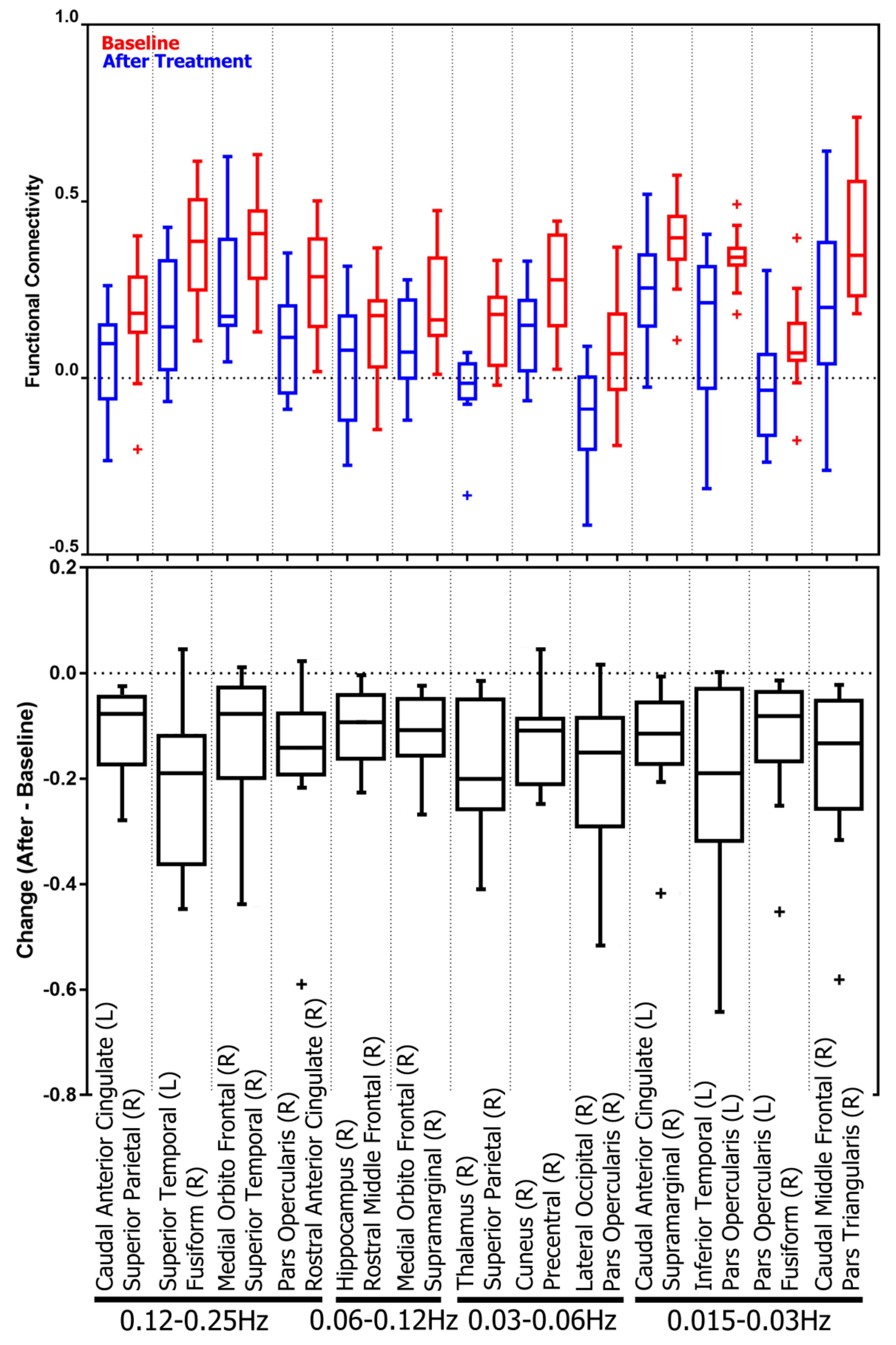
3.2.1. Subnetwork Analysis
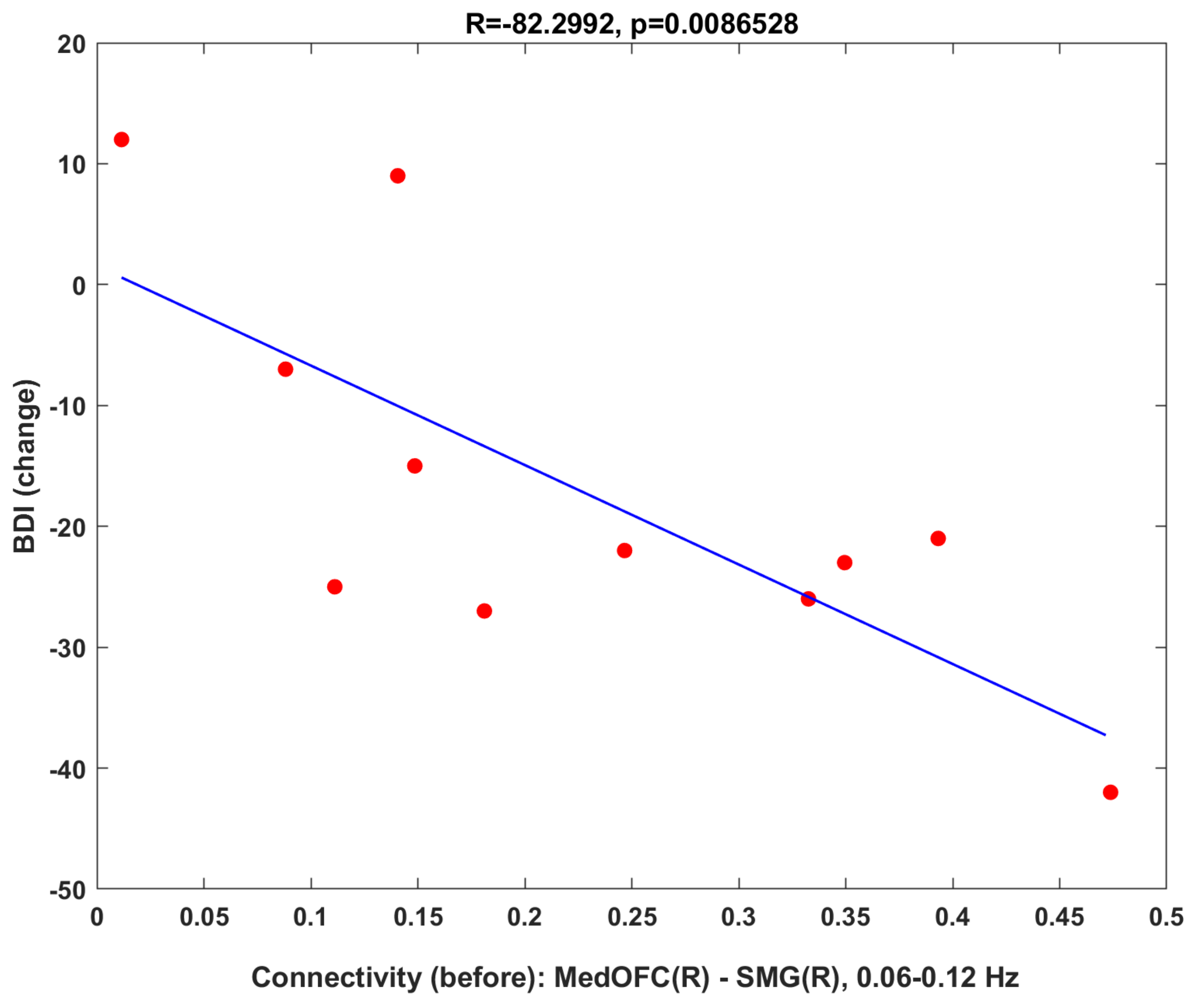
3.2.2. Clinical Correlation: Baseline Connectivity Predicts Treatment Changes
4. Discussion
4.1. Treatment Impact on Network Topology and Functional Connectivity
4.1.1. Global Topology
4.1.2. Regional Changes and Correlation to Literature
4.1.3. Frequency Dependency in Regional Changes
4.1.4. Functional Connectivity
4.2. Methodological Considerations
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kessler, R.C.; Amminger, G.P.; Aguilar-Gaxiola, S.; Alonso, J.; Lee, S.; Ustun, T.B. Age of onset of mental disorders: A review of recent literature. Curr. Opin. Psychiatry 2007, 20, 359–364. [Google Scholar] [CrossRef]
- Giedd, J.N.; Blumenthal, J.; Jeffries, N.O.; Castellanos, F.X.; Liu, H.; Zijdenbos, A.; Paus, T.; Evans, A.C.; Rapoport, J.L. Brain development during childhood and adolescence: A longitudinal MRI study. Nat. Neurosci. 1999, 2, 861–863. [Google Scholar] [CrossRef]
- Blakemore, S.J.; Choudhury, S. Development of the adolescent brain: Implications for executive function and social cognition. J. Child Psychol. Psychiatry 2006, 47, 296–312. [Google Scholar] [CrossRef]
- Rockville, M. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings; NSDUH Series H-48, HHS Publication No. (SMA) 14-4863; Substance Abuse and Mental Health Services Administration: Rockville, MD, USA, 2014. [Google Scholar]
- Avenevoli, S.; Swendsen, J.; He, J.P.; Burstein, M.; Merikangas, K.R. Major depression in the National Comorbidity Survey–Adolescent Supplement: Prevalence, correlates, and treatment. J. Am. Acad. Child Adolesc. Psychiatry 2015, 54, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Naicker, K.; Galambos, N.L.; Zeng, Y.; Senthilselvan, A.; Colman, I. Social, demographic, and health outcomes in the 10 years following adolescent depression. J. Adolesc. Health 2013, 52, 533–538. [Google Scholar] [CrossRef]
- Kennard, B.D.; Silva, S.G.; Tonev, S.; Rohde, P.; Hughes, J.L.; Vitiello, B.; Kratochvil, C.J.; Curry, J.F.; Emslie, G.J.; Reinecke, M.; et al. Remission and recovery in the Treatment for Adolescents with Depression Study (TADS): Acute and long-term outcomes. J. Am. Acad. Child Adolesc. Psychiatry 2009, 48, 186–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koolschijn, P.C.M.; van Haren, N.E.; Lensvelt-Mulders, G.J.; Hulshoff Pol, H.E.; Kahn, R.S. Brain volume abnormalities in major depressive disorder: A meta-analysis of magnetic resonance imaging studies. Hum. Brain Mapp. 2009, 30, 3719–3735. [Google Scholar] [CrossRef] [PubMed]
- Bremner, J.D.; Narayan, M.; Anderson, E.R.; Staib, L.H.; Miller, H.L.; Charney, D.S. Hippocampal volume reduction in major depression. Am. J. Psychiatry 2000, 157, 115–118. [Google Scholar] [CrossRef]
- Lorenzetti, V.; Allen, N.B.; Fornito, A.; Yücel, M. Structural brain abnormalities in major depressive disorder: A selective review of recent MRI studies. J. Affect. Disord. 2009, 117, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kieseppä, T.; Eerola, M.; Mäntylä, R.; Neuvonen, T.; Poutanen, V.P.; Luoma, K.; Tuulio-Henriksson, A.; Jylhä, P.; Mantere, O.; Melartin, T.; et al. Major depressive disorder and white matter abnormalities: A diffusion tensor imaging study with tract-based spatial statistics. J. Affect. Disord. 2010, 120, 240–244. [Google Scholar] [CrossRef]
- Korgaonkar, M.S.; Grieve, S.M.; Koslow, S.H.; Gabrieli, J.D.; Gordon, E.; Williams, L.M. Loss of white matter integrity in major depressive disorder: Evidence using tract-based spatial statistical analysis of diffusion tensor imaging. Hum. Brain Mapp. 2011, 32, 2161–2171. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Huang, X.; Wu, Q.; Yang, C.; Kuang, W.; Du, M.; Lui, S.; Yue, Q.; Chan, R.C.; Kemp, G.J.; et al. Is depression a disconnection syndrome? Meta-analysis of diffusion tensor imaging studies in patients with MDD. J. Psychiatry Neurosci. JPN 2013, 38, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Korgaonkar, M.S.; Fornito, A.; Williams, L.M.; Grieve, S.M. Abnormal structural networks characterize major depressive disorder: A connectome analysis. Biol. Psychiatry 2014, 76, 567–574. [Google Scholar] [CrossRef]
- Zou, K.; Huang, X.; Li, T.; Gong, Q.; Li, Z.; Ou-yang, L.; Deng, W.; Chen, Q.; Li, C.; Ding, Y.; et al. Alterations of white matter integrity in adults with major depressive disorder: A magnetic resonance imaging study. J. Psychiatry Neurosci. JPN 2008, 33, 525–530. [Google Scholar]
- Greicius, M.D.; Flores, B.H.; Menon, V.; Glover, G.H.; Solvason, H.B.; Kenna, H.; Reiss, A.L.; Schatzberg, A.F. Resting-state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol. Psychiatry 2007, 62, 429–437. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, J.P.; Etkin, A.; Furman, D.J.; Lemus, M.G.; Johnson, R.F.; Gotlib, I.H. Functional neuroimaging of major depressive disorder: A meta-analysis and new integration of baseline activation and neural response data. Am. J. Psychiatry 2012, 169, 693–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaiser, R.H.; Andrews-Hanna, J.R.; Wager, T.D.; Pizzagalli, D.A. Large-scale network dysfunction in major depressive disorder: A meta-analysis of resting-state functional connectivity. JAMA Psychiatry 2015, 72, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Cullen, K.R.; Westlund, M.K.; Klimes-Dougan, B.; Mueller, B.A.; Houri, A.; Eberly, L.E.; Lim, K.O. Abnormal amygdala resting-state functional connectivity in adolescent depression. JAMA Psychiatry 2014, 71, 1138–1147. [Google Scholar] [CrossRef] [PubMed]
- Sen, B.; Cullen, K.R.; Parhi, K.K. Classification of Adolescent Major Depressive Disorder via Static and Dynamic Connectivity. IEEE J. Biomed. Health Inform. 2020. [Google Scholar] [CrossRef]
- Cullen, K.R.; Klimes-Dougan, B.; Vu, D.P.; Westlund Schreiner, M.; Mueller, B.A.; Eberly, L.E.; Camchong, J.; Westervelt, A.; Lim, K.O. Neural correlates of antidepressant treatment response in adolescents with major depressive disorder. J. Child Adolesc. Psychopharmacol. 2016, 26, 705–712. [Google Scholar] [CrossRef] [Green Version]
- Klimes-Dougan, B.; Schreiner, M.W.; Thai, M.; Gunlicks-Stoessel, M.; Reigstad, K.; Cullen, K.R. Neural and neuroendocrine predictors of pharmacological treatment response in adolescents with depression: A preliminary study. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 81, 194–202. [Google Scholar] [CrossRef]
- Victor, T.A.; Furey, M.L.; Fromm, S.J.; Öhman, A.; Drevets, W.C. Relationship between amygdala responses to masked faces and mood state and treatment in major depressive disorder. Arch. Gen. Psychiatry 2010, 67, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Xu, J.; Arnone, D.; Nie, B.; Yu, H.; Jiang, H.; Bai, Y.; Luo, C.; Campbell, R.; Shan, B.; et al. Resting-state brain alteration after a single dose of SSRI administration predicts 8-week remission of patients with major depressive disorder. Psychol. Med. 2017, 47, 438–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.H.; Suckling, J.; Ooi, C.; Fu, C.H.; Williams, S.C.; Walsh, N.D.; Mitterschiffthaler, M.T.; Pich, E.M.; Bullmore, E. Functional coupling of the amygdala in depressed patients treated with antidepressant medication. Neuropsychopharmacology 2008, 33, 1909–1918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, C.H.; Williams, S.C.; Cleare, A.J.; Brammer, M.J.; Walsh, N.D.; Kim, J.; Andrew, C.M.; Pich, E.M.; Williams, P.M.; Reed, L.J.; et al. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: A prospective, event-related functional magnetic resonance imaging study. Arch. Gen. Psychiatry 2004, 61, 877–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassett, D.S.; Bullmore, E.T.; Meyer-Lindenberg, A.; Apud, J.A.; Weinberger, D.R.; Coppola, R. Cognitive fitness of cost-efficient brain functional networks. Proc. Natl. Acad. Sci. USA 2009, 106, 11747–11752. [Google Scholar] [CrossRef] [Green Version]
- Bullmore, E.; Sporns, O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009, 10, 186–198. [Google Scholar] [CrossRef]
- Rubinov, M.; Sporns, O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage 2010, 52, 1059–1069. [Google Scholar] [CrossRef]
- Van Wijk, B.C.; Stam, C.J.; Daffertshofer, A. Comparing brain networks of different size and connectivity density using graph theory. PLoS ONE 2010, 5, e13701. [Google Scholar] [CrossRef]
- Chu, S.H.; Parhi, K.K.; Lenglet, C. Function-specific and Enhanced Brain Structural Connectivity Mapping via Joint Modeling of Diffusion and Functional MRI. Sci. Rep. 2018, 8, 4741. [Google Scholar] [CrossRef] [Green Version]
- Achard, S.; Salvador, R.; Whitcher, B.; Suckling, J.; Bullmore, E. A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. J. Neurosci. 2006, 26, 63–72. [Google Scholar] [CrossRef]
- He, Y.; Chen, Z.J.; Evans, A.C. Small-world anatomical networks in the human brain revealed by cortical thickness from MRI. Cereb. Cortex 2007, 17, 2407–2419. [Google Scholar] [CrossRef] [Green Version]
- Salvador, R.; Suckling, J.; Coleman, M.R.; Pickard, J.D.; Menon, D.; Bullmore, E. Neurophysiological architecture of functional magnetic resonance images of human brain. Cereb. Cortex 2005, 15, 1332–1342. [Google Scholar] [CrossRef] [Green Version]
- Stam, C.J. Functional connectivity patterns of human magnetoencephalographic recordings: A ‘small-world’ network? Neurosci. Lett. 2004, 355, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Strogatz, S.H. Exploring complex networks. Nature 2001, 410, 268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassett, D.S.; Nelson, B.G.; Mueller, B.A.; Camchong, J.; Lim, K.O. Altered resting state complexity in schizophrenia. Neuroimage 2012, 59, 2196–2207. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Liang, M.; Zhou, Y.; He, Y.; Hao, Y.; Song, M.; Yu, C.; Liu, H.; Liu, Z.; Jiang, T. Disrupted small-world networks in schizophrenia. Brain 2008, 131, 945–961. [Google Scholar] [CrossRef] [Green Version]
- Lynall, M.E.; Bassett, D.S.; Kerwin, R.; McKenna, P.J.; Kitzbichler, M.; Muller, U.; Bullmore, E. Functional connectivity and brain networks in schizophrenia. J. Neurosci. 2010, 30, 9477–9487. [Google Scholar] [CrossRef] [Green Version]
- van den Heuvel, M.P.; Mandl, R.C.; Stam, C.J.; Kahn, R.S.; Pol, H.E.H. Aberrant frontal and temporal complex network structure in schizophrenia: A graph theoretical analysis. J. Neurosci. 2010, 30, 15915–15926. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Chen, Z.; Evans, A. Structural insights into aberrant topological patterns of large-scale cortical networks in Alzheimer’s disease. J. Neurosci. 2008, 28, 4756–4766. [Google Scholar] [CrossRef]
- Sanz-Arigita, E.J.; Schoonheim, M.M.; Damoiseaux, J.S.; Rombouts, S.A.; Maris, E.; Barkhof, F.; Scheltens, P.; Stam, C.J. Loss of ‘small-world’ networks in Alzheimer’s disease: Graph analysis of FMRI resting-state functional connectivity. PLoS ONE 2010, 5, e13788. [Google Scholar] [CrossRef] [Green Version]
- Stam, C.; De Haan, W.; Daffertshofer, A.; Jones, B.; Manshanden, I.; van Cappellen van Walsum, A.M.; Montez, T.; Verbunt, J.; De Munck, J.; Van Dijk, B.; et al. Graph theoretical analysis of magnetoencephalographic functional connectivity in Alzheimer’s disease. Brain 2008, 132, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Supekar, K.; Menon, V.; Rubin, D.; Musen, M.; Greicius, M.D. Network analysis of intrinsic functional brain connectivity in Alzheimer’s disease. PLoS Comput. Biol. 2008, 4, e1000100. [Google Scholar] [CrossRef]
- Leistedt, S.J.; Linkowski, P. Brain, networks, depression, and more. Eur. Neuropsychopharmacol. 2013, 23, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, J.; Wu, Q.; Kuang, W.; Huang, X.; He, Y.; Gong, Q. Disrupted brain connectivity networks in drug-naive, first-episode major depressive disorder. Biol. Psychiatry 2011, 70, 334–342. [Google Scholar] [CrossRef]
- Ye, M.; Yang, T.; Qing, P.; Lei, X.; Qiu, J.; Liu, G. Changes of functional brain networks in major depressive disorder: A graph theoretical analysis of resting-state fMRI. PLoS ONE 2015, 10, e0133775. [Google Scholar] [CrossRef] [Green Version]
- Kaufman, J.; Birmaher, B.; Brent, D.; Rao, U.; Flynn, C.; Moreci, P.; Williamson, D.; Ryan, N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry 1997, 36, 980–988. [Google Scholar] [CrossRef]
- Poznanski, E. Children’s depression rating scale-revised (September 1984). Psychopharmacol. Bull. 1985, 21, 979–989. [Google Scholar]
- Beck, A.T.; Steer, R.A.; Brown, G.K. Beck depression inventory-II. San Antonio 1996, 78, 490–498. [Google Scholar]
- Osman, A.; Kopper, B.A.; Barrios, F.; Gutierrez, P.M.; Bagge, C.L. Reliability and validity of the Beck depression inventory–II with adolescent psychiatric inpatients. Psychol. Assess. 2004, 16, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, D.; O’Hara, M.W.; Simms, L.J.; Kotov, R.; Chmielewski, M.; McDade-Montez, E.A.; Gamez, W.; Stuart, S. Development and validation of the Inventory of Depression and Anxiety Symptoms (IDAS). Psychol. Assess. 2007, 19, 253. [Google Scholar] [CrossRef] [Green Version]
- Watson, D.; O’Hara, M.W.; Chmielewski, M.; McDade-Montez, E.A.; Koffel, E.; Naragon, K.; Stuart, S. Further validation of the IDAS: Evidence of convergent, discriminant, criterion, and incremental validity. Psychol. Assess. 2008, 20, 248. [Google Scholar] [CrossRef]
- Watson, D.; O’Hara, M.W.; Naragon-Gainey, K.; Koffel, E.; Chmielewski, M.; Kotov, R.; Stasik, S.M.; Ruggero, C.J. Development and validation of new anxiety and bipolar symptom scales for an expanded version of the IDAS (the IDAS-II). Assessment 2012, 19, 399–420. [Google Scholar] [CrossRef]
- Poznanski, E.O.; Mokros, H.B. Children’s Depression Rating Scale, Revised (CDRS-R); Western Psychological Services: Los Angeles, CA, USA, 1996. [Google Scholar]
- Fischl, B. FreeSurfer. Neuroimage 2012, 62, 774–781. [Google Scholar] [CrossRef] [Green Version]
- Jenkinson, M.; Smith, S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001, 5, 143–156. [Google Scholar] [CrossRef]
- Jenkinson, M.; Bannister, P.; Brady, M.; Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002, 17, 825–841. [Google Scholar] [CrossRef]
- Andersson, J.L.; Jenkinson, M.; Smith, S. Non-linear registration, aka Spatial normalisation FMRIB technical report TR07JA2. FMRIB Anal. Group Univ. Oxf. 2007, 2, 1–21. [Google Scholar]
- Glover, G.H.; Li, T.Q.; Ress, D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn. Reson. Med. 2000, 44, 162–167. [Google Scholar] [CrossRef]
- Power, J.D.; Barnes, K.A.; Snyder, A.Z.; Schlaggar, B.L.; Petersen, S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 2012, 59, 2142–2154. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; Cullen, K.R.; Mueller, B.; Schreiner, M.W.; Lim, K.O.; Schulz, S.C.; Parhi, K.K. Network analysis of functional brain connectivity in borderline personality disorder using resting-state fMRI. NeuroImage Clin. 2016, 11, 302–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skidmore, F.; Korenkevych, D.; Liu, Y.; He, G.; Bullmore, E.; Pardalos, P.M. Connectivity brain networks based on wavelet correlation analysis in Parkinson fMRI data. Neurosci. Lett. 2011, 499, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Nason, G.P.; Silverman, B.W. The stationary wavelet transform and some statistical applications. In Wavelets and Statistics; Springer: New York, NY, USA, 1995; pp. 281–299. [Google Scholar]
- Shensa, M.J. The discrete wavelet transform: Wedding the a trous and Mallat algorithms. IEEE Trans. Signal Process. 1992, 40, 2464–2482. [Google Scholar] [CrossRef] [Green Version]
- Watts, D.J.; Strogatz, S.H. Collective dynamics of ‘small-world’ networks. Nature 1998, 393, 440. [Google Scholar] [CrossRef]
- Guimera, R.; Amaral, L.A.N. Cartography of complex networks: Modules and universal roles. J. Stat. Mech. Theory Exp. 2005, 2005, P02001. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Chow, S.C.; Shao, J.; Wang, H.; Lokhnygina, Y. Sample Size Calculations in Clinical Research; Chapman and Hall/CRC: Boca Raton, FL, USA, 2017. [Google Scholar]
- Rosner, B. Fundamentals of Biostatistics; Cengage Learning: Boston, MA, USA, 2010. [Google Scholar]
- Zalesky, A.; Fornito, A.; Bullmore, E.T. Network-based statistic: Identifying differences in brain networks. Neuroimage 2010, 53, 1197–1207. [Google Scholar] [CrossRef]
- Meskaldji, D.E.; Ottet, M.C.; Cammoun, L.; Hagmann, P.; Meuli, R.; Eliez, S.; Thiran, J.P.; Morgenthaler, S. Adaptive strategy for the statistical analysis of connectomes. PLoS ONE 2011, 6, e23009. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Achard, S.; Bullmore, E. Efficiency and cost of economical brain functional networks. PLoS Comput. Biol. 2007, 3, e17. [Google Scholar] [CrossRef]
- Nutt, D.J.; Forshall, S.; Bell, C.; Rich, A.; Sandford, J.; Nash, J.; Argyropoulos, S. Mechanisms of action of selective serotonin reuptake inhibitors in the treatment of psychiatric disorders. Eur. Neuropsychopharmacol. 1999, 9, S81–S86. [Google Scholar] [CrossRef]
- Li, W.; Ward, B.D.; Liu, X.; Chen, G.; Jones, J.L.; Antuono, P.G.; Li, S.J.; Goveas, J.S. Disrupted small world topology and modular organisation of functional networks in late-life depression with and without amnestic mild cognitive impairment. J. Neurol. Neurosurg. Psychiatry 2014, 86, 1097–1105. [Google Scholar] [CrossRef]
- Sendi, M.S.E.; Zendehrouh, E.; Sui, J.; Fu, Z.; Zhi, D.; Lv, L.; Ma, X.; Ke, Q.; Li, X.; Wang, C.; et al. Aberrant dynamic functional connectivity of default mode network predicts symptom severity in major depressive disorder. Brain Connect. 2021. [Google Scholar] [CrossRef]
- Shi, Y.; Li, J.; Feng, Z.; Xie, H.; Duan, J.; Chen, F.; Yang, H. Abnormal functional connectivity strength in first-episode, drug-naive adult patients with major depressive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 97, 109759. [Google Scholar] [CrossRef]
- Brakowski, J.; Spinelli, S.; Dörig, N.; Bosch, O.G.; Manoliu, A.; Holtforth, M.G.; Seifritz, E. Resting state brain network function in major depression–depression symptomatology, antidepressant treatment effects, future research. J. Psychiatr. Res. 2017, 92, 147–159. [Google Scholar] [CrossRef]
- Yao, Z.; Zou, Y.; Zheng, W.; Zhang, Z.; Li, Y.; Yu, Y.; Zhang, Z.; Fu, Y.; Shi, J.; Zhang, W.; et al. Structural alterations of the brain preceded functional alterations in major depressive disorder patients: Evidence from multimodal connectivity. J. Affect. Disord. 2019, 253, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Chen, V.C.H.; Shen, C.Y.; Liang, S.H.Y.; Li, Z.H.; Hsieh, M.H.; Tyan, Y.S.; Lu, M.L.; Lee, Y.; McIntyre, R.S.; Weng, J.C. Assessment of brain functional connectome alternations and correlation with depression and anxiety in major depressive disorders. PeerJ 2017, 5, e3147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.L.; Du, M.Y.; Chen, T.L.; Chen, Z.Q.; Huang, X.Q.; Luo, Y.; Zhao, Y.J.; Kumar, P.; Gong, Q.Y. Neural correlates during working memory processing in major depressive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2015, 56, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Barch, D.M.; Sheline, Y.I.; Csernansky, J.G.; Snyder, A.Z. Working memory and prefrontal cortex dysfunction: Specificity to schizophrenia compared with major depression. Biol. Psychiatry 2003, 53, 376–384. [Google Scholar] [CrossRef]
- Ye, T.; Peng, J.; Nie, B.; Gao, J.; Liu, J.; Li, Y.; Wang, G.; Ma, X.; Li, K.; Shan, B. Altered functional connectivity of the dorsolateral prefrontal cortex in first-episode patients with major depressive disorder. Eur. J. Radiol. 2012, 81, 4035–4040. [Google Scholar] [CrossRef]
- Na, K.S.; Won, E.; Kang, J.; Chang, H.S.; Yoon, H.K.; Tae, W.S.; Kim, Y.K.; Lee, M.S.; Joe, S.H.; Kim, H.; et al. Brain-derived neurotrophic factor promoter methylation and cortical thickness in recurrent major depressive disorder. Sci. Rep. 2016, 6, 21089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, H.; Zuo, C.; Zhang, H.; Jiao, F.; Zhang, B.; Tang, W.; Geng, D.; Guan, Y.; Shi, S. Regional cerebral metabolism alterations affect resting-state functional connectivity in major depressive disorder. Quant. Imaging Med. Surg. 2018, 8, 910–924. [Google Scholar] [CrossRef]
- Steffens, D.C.; Wang, L.; Pearlson, G.D. Functional connectivity predictors of acute depression treatment outcome. Int. Psychogeriatr. 2019, 31, 1831–1835. [Google Scholar] [CrossRef]
- Shimizu, Y.; Yoshimoto, J.; Toki, S.; Takamura, M.; Yoshimura, S.; Okamoto, Y.; Yamawaki, S.; Doya, K. Toward probabilistic diagnosis and understanding of depression based on functional MRI data analysis with logistic group LASSO. PLoS ONE 2015, 10, e0123524. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Qiu, M.; Ding, L.; Mellor, D.; Li, G.; Shen, T.; Peng, D. Intrinsic gray-matter connectivity of the brain in major depressive disorder. J. Affect. Disord. 2019, 251, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Alders, G.L.; Davis, A.D.; MacQueen, G.; Strother, S.C.; Hassel, S.; Zamyadi, M.; Sharma, G.B.; Arnott, S.R.; Downar, J.; Harris, J.K.; et al. Escitalopram ameliorates differences in neural activity between healthy comparison and major depressive disorder groups on an fMRI Emotional conflict task: A CAN-BIND-1 study. J. Affect. Disord. 2020, 264, 414–424. [Google Scholar] [CrossRef]
- Schreiner, M.W.; Klimes-Dougan, B.; Cullen, K.R. Neural Correlates of Suicidality in Adolescents with Major Depression: Resting-State Functional Connectivity of the Precuneus and Posterior Cingulate Cortex. Suicide Life-Threat. Behav. 2019, 49, 899–913. [Google Scholar] [CrossRef] [PubMed]
- Smoski, M.J.; Rittenberg, A.; Dichter, G.S. Major depressive disorder is characterized by greater reward network activation to monetary than pleasant image rewards. Psychiatry Res. Neuroimaging 2011, 194, 263–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, C.; Zhou, J.; Ma, H.; Tan, Y.; Wu, X.; Guan, C.; Qiao, H.; Li, J.; Zhong, Y.; Wang, C.; et al. Abnormal resting state activity of left middle occipital gyrus and its functional connectivity in female patients with major depressive disorder. BMC Psychiatry 2018, 18, 270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alders, G.L.; Davis, A.D.; MacQueen, G.; Strother, S.C.; Hassel, S.; Zamyadi, M.; Sharma, G.B.; Arnott, S.R.; Downar, J.; Harris, J.K.; et al. Reduced accuracy accompanied by reduced neural activity during the performance of an emotional conflict task by unmedicated patients with major depression: A CAN-BIND fMRI study. J. Affect. Disord. 2019, 257, 765–773. [Google Scholar] [CrossRef]
- Chen, C.H.; Ridler, K.; Suckling, J.; Williams, S.; Fu, C.H.; Merlo-Pich, E.; Bullmore, E. Brain imaging correlates of depressive symptom severity and predictors of symptom improvement after antidepressant treatment. Biol. Psychiatry 2007, 62, 407–414. [Google Scholar] [CrossRef]
- Latora, V.; Marchiori, M. Efficient behavior of small-world networks. Phys. Rev. Lett. 2001, 87, 198701. [Google Scholar] [CrossRef] [Green Version]
- Seminowicz, D.; Mayberg, H.; McIntosh, A.; Goldapple, K.; Kennedy, S.; Segal, Z.; Rafi-Tari, S. Limbic–frontal circuitry in major depression: A path modeling metanalysis. Neuroimage 2004, 22, 409–418. [Google Scholar] [CrossRef]
- Rubin-Falcone, H.; Zanderigo, F.; Thapa-Chhetry, B.; Lan, M.; Miller, J.M.; Sublette, M.E.; Oquendo, M.A.; Hellerstein, D.J.; McGrath, P.J.; Stewart, J.W.; et al. Pattern recognition of magnetic resonance imaging-based gray matter volume measurements classifies bipolar disorder and major depressive disorder. J. Affect. Disord. 2018, 227, 498–505. [Google Scholar] [CrossRef]
- Goghari, V.M.; Smith, G.N.; Honer, W.G.; Kopala, L.C.; Thornton, A.E.; Su, W.; MacEwan, G.W.; Lang, D.J. Effects of eight weeks of atypical antipsychotic treatment on middle frontal thickness in drug-naive first-episode psychosis patients. Schizophr. Res. 2013, 149, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Yücel, M.; Lorenzetti, V.; Walterfang, M.; Kawasaki, Y.; Whittle, S.; Suzuki, M.; Pantelis, C.; Allen, N.B. An MRI study of the superior temporal subregions in patients with current and past major depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.H.; Tian, K.; Wang, D.F.; Wang, Y.; Cheung, E.F.; Xie, G.R.; Chan, R.C. Anhedonia correlates with abnormal functional connectivity of the superior temporal gyrus and the caudate nucleus in patients with first-episode drug-naive major depressive disorder. J. Affect. Disord. 2017, 218, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Cullen, K.R.; Gee, D.G.; Klimes-Dougan, B.; Gabbay, V.; Hulvershorn, L.; Mueller, B.A.; Camchong, J.; Bell, C.J.; Houri, A.; Kumra, S.; et al. A preliminary study of functional connectivity in comorbid adolescent depression. Neurosci. Lett. 2009, 460, 227–231. [Google Scholar] [CrossRef] [Green Version]
- Siegle, G.J.; Thompson, W.; Carter, C.S.; Steinhauer, S.R.; Thase, M.E. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: Related and independent features. Biol. Psychiatry 2007, 61, 198–209. [Google Scholar] [CrossRef]
- Brody, A.L.; Barsom, M.W.; Bota, R.G.; Saxena, S. Prefrontal–subcortical and limbic circuit mediation of major depressive disorder. Semin. Clin. Neuropsychiatry 2001, 6, 102–112. [Google Scholar] [CrossRef]
- Li, L.; Ma, N.; Li, Z.; Tan, L.; Liu, J.; Gong, G.; Shu, N.; He, Z.; Jiang, T.; Xu, L. Prefrontal white matter abnormalities in young adult with major depressive disorder: A diffusion tensor imaging study. Brain Res. 2007, 1168, 124–128. [Google Scholar] [CrossRef]
- Circuits, F.S. Frontal-subcortical circuits and neuropsychiatric disorders. Neurosciences 1994, 6, 358–370. [Google Scholar]
- Koenig, J.; Schreiner, M.W.; Klimes-Dougan, B.; Ubani, B.; Mueller, B.A.; Lim, K.O.; Kaess, M.; Cullen, K.R. Increases in orbitofrontal cortex thickness following antidepressant treatment are associated with changes in resting state autonomic function in adolescents with major depression–Preliminary findings from a pilot study. Psychiatry Res. Neuroimaging 2018, 281, 35–42. [Google Scholar] [CrossRef]
- Mayberg, H.S. Limbic-cortical dysregulation: A proposed model of depression. J. Neuropsychiatry Clin. Neurosci. 1997, 9, 471–481. [Google Scholar]
- Phillips, M.L.; Drevets, W.C.; Rauch, S.L.; Lane, R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol. Psychiatry 2003, 54, 515–528. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, X.; Xiao, J.; Liao, J.; Zhong, M.; Wang, W.; Yao, S. Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biol. Psychiatry 2012, 71, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liu, L.; Friston, K.J.; Shen, H.; Wang, L.; Zeng, L.L.; Hu, D. A treatment-resistant default mode subnetwork in major depression. Biol. Psychiatry 2013, 74, 48–54. [Google Scholar] [CrossRef]
- Ochsner, K.N.; Gross, J.J. The neural architecture of emotion regulation. Handb. Emot. Regul. 2007, 1, 87–109. [Google Scholar]
- Phelps, E.A.; LeDoux, J.E. Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron 2005, 48, 175–187. [Google Scholar] [CrossRef] [Green Version]
- Drevets, W.C. Neuroimaging abnormalities in the amygdala in mood disorders. Ann. N. Y. Acad. Sci. 2003, 985, 420–444. [Google Scholar] [CrossRef] [PubMed]
- Canli, T.; Cooney, R.E.; Goldin, P.; Shah, M.; Sivers, H.; Thomason, M.E.; Whitfield-Gabrieli, S.; Gabrieli, J.D.; Gotlib, I.H. Amygdala reactivity to emotional faces predicts improvement in major depression. Neuroreport 2005, 16, 1267–1270. [Google Scholar] [CrossRef]
- Lui, S.; Wu, Q.; Qiu, L.; Yang, X.; Kuang, W.; Chan, R.C.; Huang, X.; Kemp, G.J.; Mechelli, A.; Gong, Q. Resting-state functional connectivity in treatment-resistant depression. Am. J. Psychiatry 2011, 168, 642–648. [Google Scholar] [CrossRef]
- Drevets, W.C.; Price, J.L.; Furey, M.L. Brain structural and functional abnormalities in mood disorders: Implications for neurocircuitry models of depression. Brain Struct. Funct. 2008, 213, 93–118. [Google Scholar] [CrossRef] [Green Version]
- Sheline, Y.I.; Barch, D.M.; Donnelly, J.M.; Ollinger, J.M.; Snyder, A.Z.; Mintun, M.A. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: An fMRI study. Biol. Psychiatry 2001, 50, 651–658. [Google Scholar] [CrossRef]
- Partiot, A.; Grafman, J.; Sadato, N.; Wachs, J.; Hallett, M. Brain activation during the generation of non-emotional and emotional plans. Neuroreport 1995, 6, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Beauregard, M.; Leroux, J.M.; Bergman, S.; Arzoumanian, Y.; Beaudoin, G.; Bourgouin, P.; Stip, E. The functional neuroanatomy of major depression: An fMRI study using an emotional activation paradigm. Neuroreport 1998, 9, 3253–3258. [Google Scholar] [CrossRef]
- Awata, S.; Ito, H.; Konno, M.; Ono, S.; Kawashima, R.; Fukuda, H.; Sato, M. Regional cerebral blood flow abnormalities in late-life depression: Relation to refractoriness and chronification. Psychiatry Clin. Neurosci. 1998, 52, 97–105. [Google Scholar] [CrossRef]
- Brody, A.L.; Saxena, S.; Stoessel, P.; Gillies, L.A.; Fairbanks, L.A.; Alborzian, S.; Phelps, M.E.; Huang, S.C.; Wu, H.M.; Ho, M.L.; et al. Regional brain metabolic changes in patients with major depression treated with either paroxetine or interpersonal therapy: Preliminary findings. Arch. Gen. Psychiatry 2001, 58, 631–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumari, V.; Mitterschiffthaler, M.T.; Teasdale, J.D.; Malhi, G.S.; Brown, R.G.; Giampietro, V.; Brammer, M.J.; Poon, L.; Simmons, A.; Williams, S.C.; et al. Neural abnormalities during cognitive generation of affect in treatment-resistant depression. Biol. Psychiatry 2003, 54, 777–791. [Google Scholar] [CrossRef]
- Lubar, J.F.; Congedo, M.; Askew, J.H. Low-resolution electromagnetic tomography (LORETA) of cerebral activity in chronic depressive disorder. Int. J. Psychophysiol. 2003, 49, 175–185. [Google Scholar] [CrossRef] [Green Version]
- Buzsáki, G.; Draguhn, A. Neuronal oscillations in cortical networks. Science 2004, 304, 1926–1929. [Google Scholar] [CrossRef] [Green Version]
- Zuo, X.N.; Di Martino, A.; Kelly, C.; Shehzad, Z.E.; Gee, D.G.; Klein, D.F.; Castellanos, F.X.; Biswal, B.B.; Milham, M.P. The oscillating brain: Complex and reliable. Neuroimage 2010, 49, 1432–1445. [Google Scholar] [CrossRef] [Green Version]
- Xue, S.W.; Li, D.; Weng, X.C.; Northoff, G.; Li, D.W. Different neural manifestations of two slow frequency bands in resting functional magnetic resonance imaging: A systemic survey at regional, interregional, and network levels. Brain Connect. 2014, 4, 242–255. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wang, S.; Zhang, X.; Wang, Z.; Tian, X.; He, Y. Abnormal amplitude of low-frequency fluctuations of intrinsic brain activity in Alzheimer’s disease. J. Alzheimer’s Dis. 2014, 40, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Wang, X.; Wang, W.; Liu, J.; Qiu, J. Frequency-dependent alterations in regional homogeneity in major depression. Behav. Brain Res. 2016, 306, 13–19. [Google Scholar] [CrossRef]
- Gawryluk, J.R.; Mazerolle, E.L.; D’Arcy, R.C. Does functional MRI detect activation in white matter? A review of emerging evidence, issues, and future directions. Front. Neurosci. 2014, 8, 239. [Google Scholar] [CrossRef] [Green Version]
- Grajauskas, L.A.; Frizzell, T.; Song, X.; D’Arcy, R.C. White matter fMRI activation cannot be treated as a nuisance regressor: Overcoming a historical blind spot. Front. Neurosci. 2019, 13, 1024. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Huang, Y.; Bailey, S.K.; Gao, Y.; Cutting, L.E.; Rogers, B.P.; Newton, A.T.; Gore, J.C. Detection of synchronous brain activity in white matter tracts at rest and under functional loading. Proc. Natl. Acad. Sci. USA 2018, 115, 595–600. [Google Scholar] [CrossRef] [Green Version]
- Mayberg, H.S. Positron emission tomography imaging in depression: A neural systems perspective. Neuroimaging Clin. 2003, 13, 805–815. [Google Scholar] [CrossRef]
- Mulders, P.C.; van Eijndhoven, P.F.; Schene, A.H.; Beckmann, C.F.; Tendolkar, I. Resting-state functional connectivity in major depressive disorder: A review. Neurosci. Biobehav. Rev. 2015, 56, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Genovese, C.R.; Lazar, N.A.; Nichols, T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 2002, 15, 870–878. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.; Glover, G.H. Time–frequency dynamics of resting-state brain connectivity measured with fMRI. Neuroimage 2010, 50, 81–98. [Google Scholar] [CrossRef] [Green Version]
- Hindriks, R.; Adhikari, M.H.; Murayama, Y.; Ganzetti, M.; Mantini, D.; Logothetis, N.K.; Deco, G. Can sliding-window correlations reveal dynamic functional connectivity in resting-state fMRI? Neuroimage 2016, 127, 242–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Button, K.S.; Ioannidis, J.P.; Mokrysz, C.; Nosek, B.A.; Flint, J.; Robinson, E.S.; Munafò, M.R. Power failure: Why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 2013, 14, 365–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawilowsky, S.S. New effect size rules of thumb. J. Mod. Appl. Stat. Methods 2009, 8, 597–599. [Google Scholar] [CrossRef]
- Szucs, D.; Ioannidis, J.P. Empirical assessment of published effect sizes and power in the recent cognitive neuroscience and psychology literature. PLoS Biol. 2017, 15, e2000797. [Google Scholar] [CrossRef] [PubMed]




| Baseline | Post Treatment | |
|---|---|---|
| Gender | 3 male, 9 female | |
| Handedness | 11 right, 1 left | |
| IQ (mean ± SD) | ||
| Age (years, mean ± SD) | ||
| BDI-II (mean ± SD) | ||
| Duration of illness (years, mean ± SD) | ||
| No. | Region of Interest | Abbr. | No. | Region of Interest | Abbr. | No. | Region of Interest | Abbr. |
|---|---|---|---|---|---|---|---|---|
| 1 | Banks superior temporal sulcus | BANK | 14 | Parahippocampal gyrus | PHG | 27 | Superior parietal cortex | SPC |
| 2 | Caudal anterior cingulate cortex | CauACC | 15 | Paracentral lobule | PCL | 28 | Superior temporal gyrus | STG |
| 3 | Caudal middle frontal gyrus | CauMFG | 16 | Pars opercularis | ParsOPE | 29 | Supramarginal gyrus | SMG |
| 4 | Cuneus cortex | CUN | 17 | Pars orbitalis | ParsORB | 30 | Transverse temporal cortex | TTC |
| 5 | Fusiform gyrus | FFG | 18 | Pars triangularis | ParsTRI | 31 | Insula | INS |
| 6 | Inferior parietal cortex | IPC | 19 | Pericalcarine cortex | PCAL | 32 | Thalamus | THA |
| 7 | Inferior temporal gyrus | ITG | 20 | Postcentral gyrus | PoCG | 33 | Caudate | CAU |
| 8 | Isthmus-cingulate cortex | ICC | 21 | Posterior cingulate cortex | PCC | 34 | Putamen | PUT |
| 9 | Lateral occipital cortex | LatOC | 22 | Precentral gyrus | PreCG | 35 | Pallidum | PAL |
| 10 | Lateral orbitalfrontal cortex | LatOFC | 23 | Precuneus gyrus | PCUN | 36 | Hippocampus | HIP |
| 11 | Lingual gyrus | LING | 24 | Rostral anterior cingulate cortex | RosACC | 37 | Amygdala | AMYG |
| 12 | Medial orbitalfrontal cortex | MedOFC | 25 | Rostral middle frontal gyrus | RosMFG | 38 | Accumbens | ACCU |
| 13 | Middle temporal gyrus | MTG | 26 | Superior frontal gyrus | SFG |
| Frequency Band | Brain Region | Network Measure | Uncorrected p-Value | Effect Size | Power |
|---|---|---|---|---|---|
| 0.12–0.25 Hz | Right caudal middle frontal gyrus | Local Efficiency | 0 | −1.2021 | 0.8376 |
| Clustering Coefficient | 0 | −1.1443 | 0.8004 | ||
| 0.06–0.12 Hz | Left pars triangularis | Participation Coefficient | 0 | 0.7271 | 0.4291 |
| 0.03–0.06 Hz | Right precentral gyrus | Betweenness Centrality | 0.000244 | −1.8910 | 0.9962 |
| Left lateral occipital cortex | Within-Module-Degree Z-score | 0.000244 | −0.8587 | 0.5571 | |
| 0.015–0.03 Hz | –None– | ||||
| Brain Region | Clustering Coefficient | Local Efficiency | ||||
|---|---|---|---|---|---|---|
| p-Value | Effect Size | Power | p-Value | Effect Size | Power | |
| Right caudal middle frontal gyrus | 0 | −1.1443 | 0.8004 | 0 | −1.2021 | 0.8376 |
| Left caudal middle frontal gyrus | 0.0371 | −0.6342 | 0.3424 | 0.0247 | −0.5485 | 0.2693 |
| Right superior temporal gyrus | 0.0037 | −1.3222 | 0.8995 | 0.0081 | −1.2372 | 0.8578 |
| Left superior temporal gyrus | 0.0161 | −0.9318 | 0.6264 | 0.0105 | −1.1316 | 0.7916 |
| Right rostral middle frontal gyrus | 0.0369 | −0.7923 | 0.4924 | 0.0427 | −0.8007 | 0.5006 |
| Left rostral middle frontal gyrus | 0.0271 | −0.8621 | 0.5603 | 0.0437 | −0.8181 | 0.5175 |
| Left pars triangularis | 0.0139 | −0.7683 | 0.4690 | 0.0195 | −0.7555 | 0.4565 |
| Left Putamen | 0.0269 | −0.6604 | 0.3662 | 0.0078 | −0.7819 | 0.4823 |
| Right superior frontal gyrus | 0.0427 | −0.6444 | 0.3517 | 0.0151 | −0.7948 | 0.4948 |
| 0.12–0.25 Hz | 0.06–0.12 Hz | 0.03–0.06 Hz | 0.015–0.03 Hz | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brain Region | p-Value | Effect Size | Power | p-Value | Effect Size | Power | p-Value | Effect Size | Power | p-Value | Effect Size | Power |
| Right AMYG | 0.0229 | 0.9426 | 0.6364 | 0.0251 | 0.9588 | 0.6513 | 0.0471 | 0.8203 | 0.5197 | 0.0310 | 0.8350 | 0.5340 |
| Right LING | 0.0396 | 0.6730 | 0.3779 | 0.0093 | 0.9946 | 0.6830 | 0.0439 | 0.6990 | 0.4022 | 0.2832 | 0.2845 | 0.1072 |
| Left IPC | 0.0134 | −0.7909 | 0.4910 | 0.0095 | −0.8019 | 0.5017 | 0.0493 | −0.6442 | 0.3514 | 0.1904 | −0.3872 | 0.1577 |
| Right CauACC | 0.0195 | −0.5995 | 0.3119 | 0.0061 | −0.7787 | 0.4791 | 0.0750 | −0.5659 | 0.2834 | 0.0764 | −0.5485 | 0.2693 |
| Right PCL | 0.0134 | 1.0429 | 0.7240 | 0.0376 | 0.8733 | 0.5711 | 0.0732 | 0.5933 | 0.3066 | 0.1189 | 0.3374 | 0.1311 |
| Right ACCU | 0.0117 | 0.7705 | 0.4711 | 0.0364 | 0.4711 | 0.2110 | 0.2185 | 0.1645 | 0.0688 | 0.4985 | 0.0057 | 0.0500 |
| Left PCAL | 0.0098 | 0.6922 | 0.3958 | 0.0483 | 0.5589 | 0.2777 | 0.0942 | 0.5722 | 0.2887 | 0.1550 | 0.4514 | 0.1976 |
| Left MTG | 0.0435 | 0.7227 | 0.4249 | 0.0598 | 0.6256 | 0.3347 | 0.0962 | 0.5306 | 0.2551 | 0.1958 | 0.3376 | 0.1312 |
| Left PCL | 0.0273 | 0.8818 | 0.5792 | 0.0701 | 0.6791 | 0.3835 | 0.1899 | 0.3893 | 0.1589 | 0.2307 | 0.2698 | 0.1013 |
| Left STG | 0.0305 | −0.5878 | 0.3018 | 0.0959 | −0.4065 | 0.1690 | 0.4817 | −0.0101 | 0.0501 | 0.0911 | −0.3201 | 0.1228 |
| Right SMG | 0.2937 | −0.1640 | 0.0687 | 0.0376 | −0.5360 | 0.2594 | 0.0562 | −0.5787 | 0.2941 | 0.1162 | −0.4814 | 0.2183 |
| Right ParsOPE | 0.0581 | −0.4317 | 0.1846 | 0.0259 | −0.8706 | 0.5685 | 0.0474 | −0.8409 | 0.5398 | 0.2766 | −0.2684 | 0.1008 |
| Right CauMFG | 0.1460 | −0.4486 | 0.1957 | 0.0713 | −0.6097 | 0.3207 | 0.0154 | −0.8278 | 0.5270 | 0.0735 | −0.5341 | 0.2578 |
| Left SMG | 0.2910 | −0.1788 | 0.0722 | 0.0596 | −0.3989 | 0.1645 | 0.0439 | −0.5430 | 0.2649 | 0.0854 | −0.4743 | 0.2133 |
| Right PreCG | 0.0981 | −0.5977 | 0.3103 | 0.0935 | −0.5981 | 0.3107 | 0.0488 | −0.7695 | 0.4702 | 0.0364 | −0.8346 | 0.5336 |
| Left AMYG | 0.1047 | 0.4765 | 0.2149 | 0.0735 | 0.4982 | 0.2305 | 0.0425 | 0.5281 | 0.2531 | 0.0288 | 0.6769 | 0.3815 |
| Right ICC | 0.2344 | 0.2565 | 0.0963 | 0.1973 | 0.3520 | 0.1385 | 0.1230 | 0.4846 | 0.2206 | 0.0374 | 0.7931 | 0.4932 |
| Right LatOC | 0.4194 | 0.0826 | 0.0547 | 0.4849 | 0.0191 | 0.0503 | 0.2109 | −0.2854 | 0.1076 | 0.0144 | −0.6771 | 0.3817 |
| Right SPC | 0.1628 | −0.4106 | 0.1715 | 0.0867 | −0.4923 | 0.2262 | 0.0715 | −0.5109 | 0.2399 | 0.0317 | −0.6434 | 0.3507 |
| Left LatOC | 0.3459 | −0.1780 | 0.0721 | 0.3745 | −0.1399 | 0.0636 | 0.2603 | −0.2623 | 0.0985 | 0.0188 | −0.6102 | 0.3212 |
| Left MedOFC | 0.2705 | 0.1984 | 0.0775 | 0.1609 | 0.3556 | 0.1404 | 0.1006 | 0.3966 | 0.1632 | 0.0330 | 0.6721 | 0.3770 |
| Left SPC | 0.4883 | 0.0102 | 0.0501 | 0.3472 | −0.1433 | 0.0642 | 0.1157 | −0.4680 | 0.2089 | 0.0186 | −0.7846 | 0.4849 |
| Right ITG | 0.0442 | −0.5813 | 0.2963 | −0.0867 | 0.3706 | 0.1484 | −0.0696 | 0.4827 | 0.2193 | 0.0061 | −0.7844 | 0.4847 |
| Frequency Band | Connection | Uncorrected p-Value | Effect Size | Power | |
|---|---|---|---|---|---|
| Region 1 | Region 2 | ||||
| 0.12–0.25 Hz | Right fusiform gyrus | Left superior temporal gyrus | 0 | −1.3560 | 0.9133 |
| Right rostral anterior cingulate cortex | Right pars opercularis | 0 | −1.0312 | 0.7143 | |
| Right superior temporal gyrus | Right medial orbitofrontal cortex | 0 | −0.8439 | 0.5427 | |
| Right superior parietal cortex | Left caudal anterior cingulate cortex | 0 | −0.7088 | 0.4116 | |
| Right inferior temporal gyrus | Left banks superior temporal sulcus | 0.0002441 | −1.0550 | 0.7338 | |
| Right rostral anterior cingulate cortex | Left pars opercularis | 0.0002441 | −1.0415 | 0.7228 | |
| 0.06–0.12 Hz | Right medial orbitofrontal cortex | Right supramarginal gyrus | 0 | −0.8198 | 0.5192 |
| Right hippocampus | Right rostral middle frontal gyrus | 0 | −0.5818 | 0.2968 | |
| Right fusiform gyrus | Left superior temporal gyrus | 0.0002441 | −1.1454 | 0.8012 | |
| Right pars opercularis | Right rostral anterior cingulate cortex | 0.0002441 | −0.9197 | 0.6151 | |
| Right rostral anterior cingulate cortex | Left pars opercularis | 0.0004883 | −0.9616 | 0.6538 | |
| Right precentral gyrus | Left caudal anterior cingulate cortex | 0.0004883 | −0.8591 | 0.5575 | |
| 0.03–0.06 Hz | Right thalamus | Right superior parietal cortex | 0 | −1.5722 | 0.9707 |
| Right cuneus cortex | Right precentral gyrus | 0 | −0.8869 | 0.5841 | |
| Right lateral occipital cortex | Right pars opercularis | 0 | −1.2856 | 0.8828 | |
| Right thalamus | Left superior parietal cortex | 0.0002441 | −1.6582 | 0.9822 | |
| 0.015–0.03 Hz | Left caudal anterior cingulate cortex | Right supramarginal gyrus | 0 | −0.9047 | 0.6010 |
| Left inferior temporal gyrus | Left pars opercularis | 0 | −1.2272 | 0.8522 | |
| Left pars opercularis | Right fusiform gyrus | 0 | −0.8613 | 0.5595 | |
| Right caudal middle frontal gyrus | Right pars triangularis | 0 | −0.8023 | 0.5021 | |
| Left fusiform gyrus | Right precentral gyrus | 0.0002441 | −1.2953 | 0.8874 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, S.-H.; Parhi, K.K.; Westlund Schreiner, M.; Lenglet, C.; Mueller, B.A.; Klimes-Dougan, B.; Cullen, K.R. Effect of SSRIs on Resting-State Functional Brain Networks in Adolescents with Major Depressive Disorder. J. Clin. Med. 2021, 10, 4322. https://doi.org/10.3390/jcm10194322
Chu S-H, Parhi KK, Westlund Schreiner M, Lenglet C, Mueller BA, Klimes-Dougan B, Cullen KR. Effect of SSRIs on Resting-State Functional Brain Networks in Adolescents with Major Depressive Disorder. Journal of Clinical Medicine. 2021; 10(19):4322. https://doi.org/10.3390/jcm10194322
Chicago/Turabian StyleChu, Shu-Hsien, Keshab K. Parhi, Melinda Westlund Schreiner, Christophe Lenglet, Bryon A. Mueller, Bonnie Klimes-Dougan, and Kathryn R. Cullen. 2021. "Effect of SSRIs on Resting-State Functional Brain Networks in Adolescents with Major Depressive Disorder" Journal of Clinical Medicine 10, no. 19: 4322. https://doi.org/10.3390/jcm10194322






