Preliminary Report: Rapid Intraoperative Detection of Residual Glioma Cell in Resection Cavity Walls Using a Compact Fluorescence Microscope
Abstract
:1. Introduction:
2. Materials and Methods
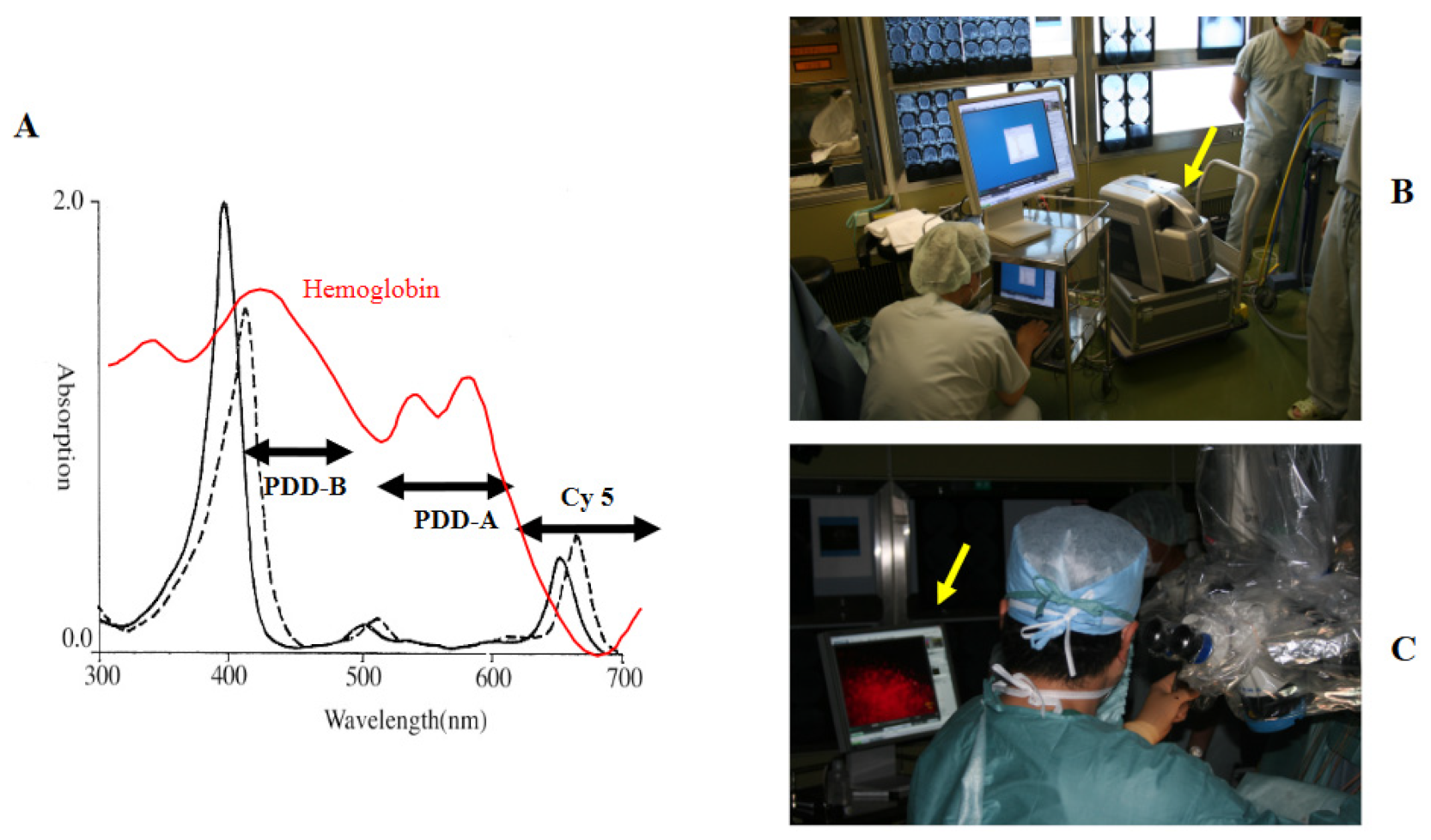
2.1. TPS and Semiconductor Laser
2.2. Intraoperative Photodiagnosis Using a Surgical Microscope
2.3. Compact Fluorescence Microscope
2.4. Real-Time, Intraoperative Fluorescence Cytology
2.4.1. Tissue Preparation
2.4.2. Tissue Assessment by BZ-8000
2.4.3. Analysis of Fluorescence Images
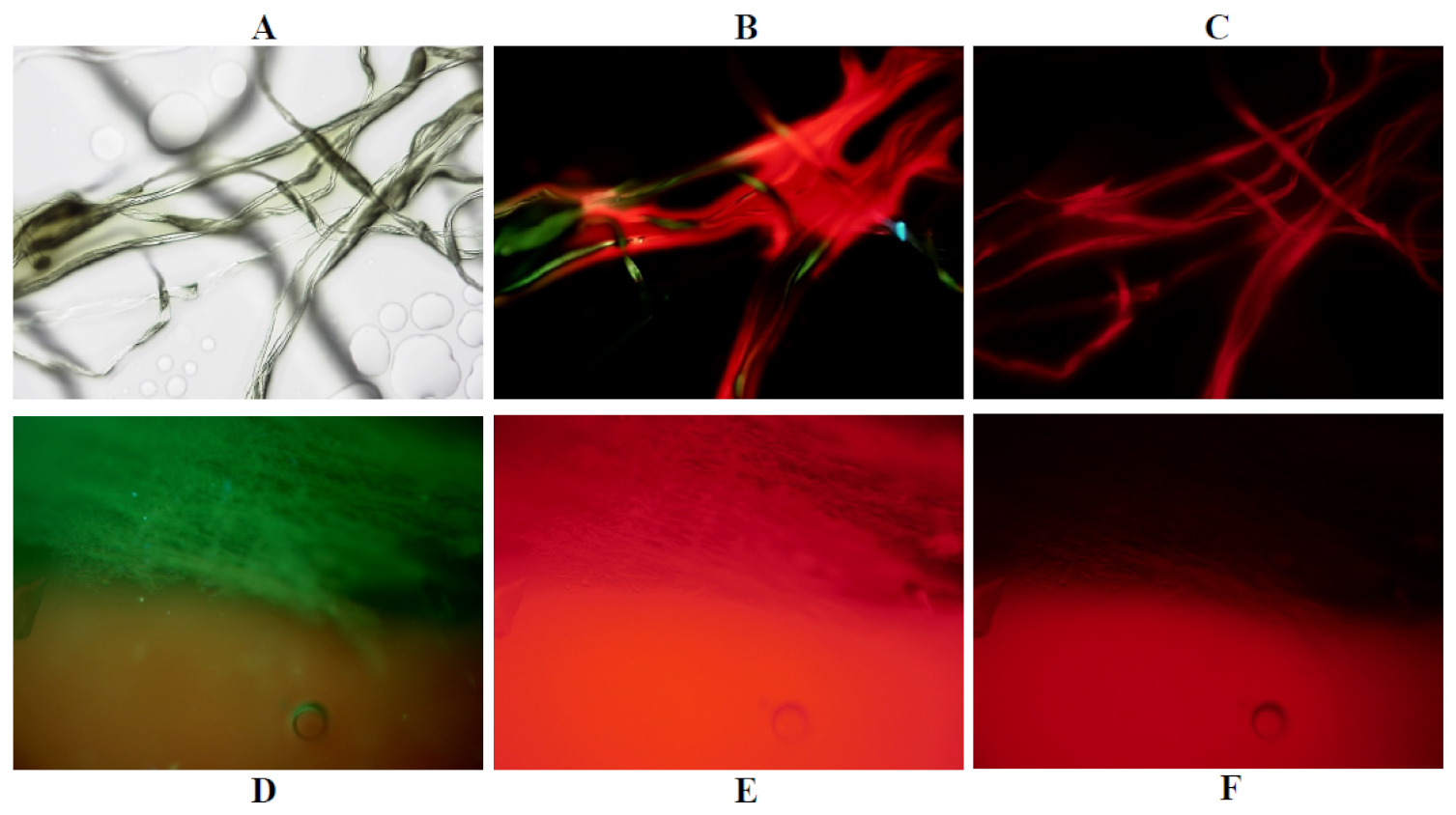
2.5. Phantom Experiments
2.6. Clinical Cases
2.7. Calculation of the Extent of Resection
2.8. Statistical Analysis
3. Results
3.1. Phantom Experiments
3.2. Intraoperative Detection of Infiltrating Tumor Cells Using Fluorescence Microscopy
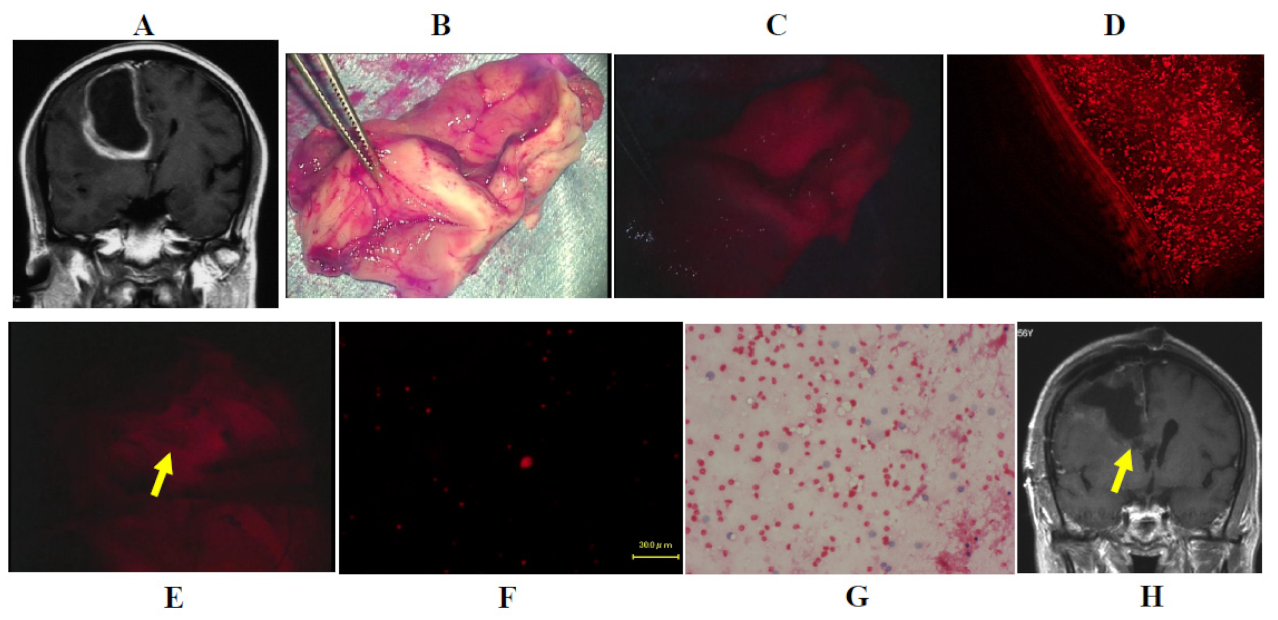
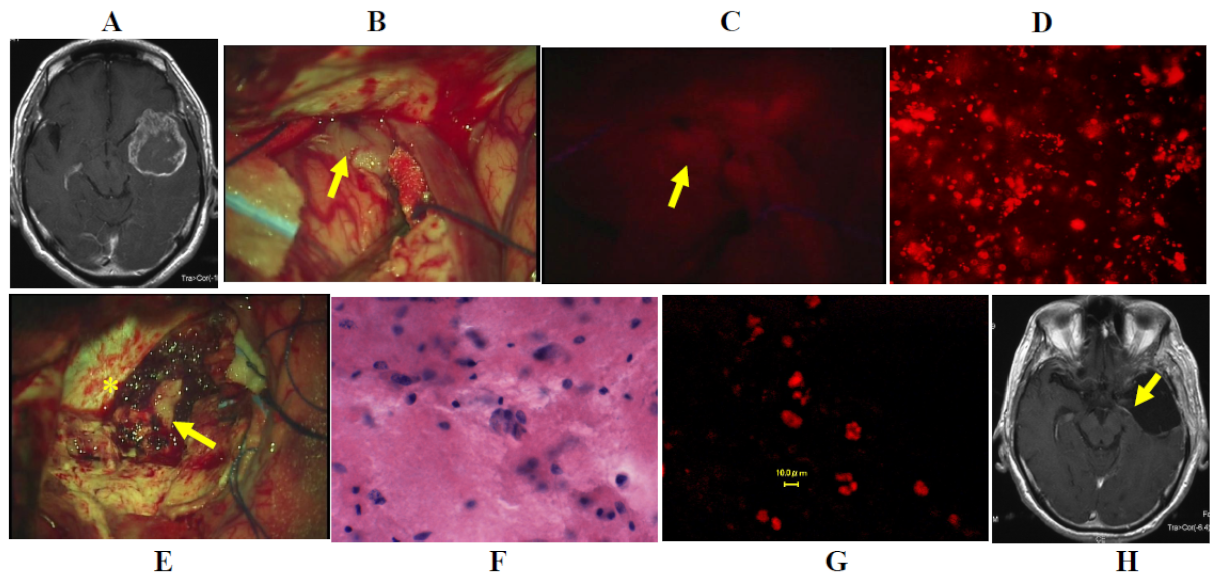
3.3. Illustrative Cases
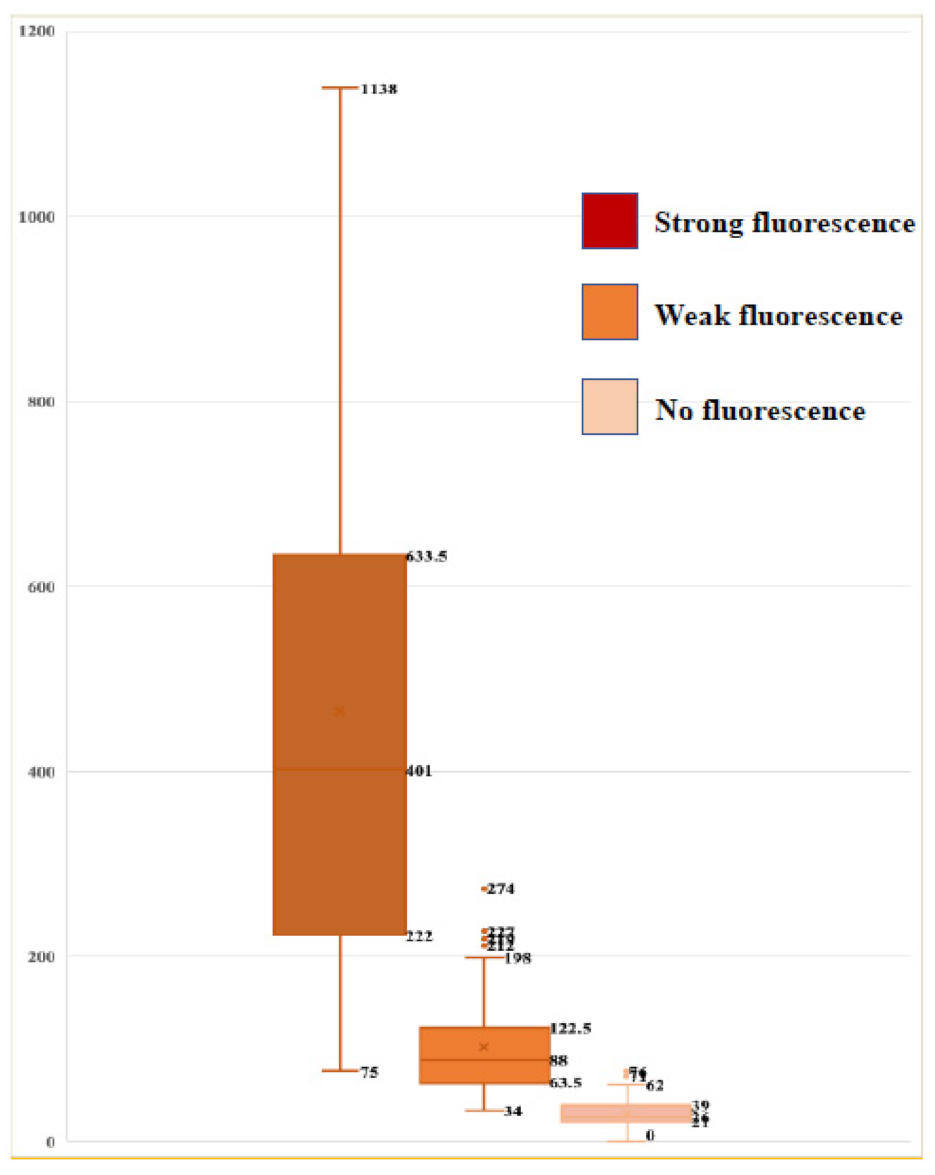
3.4. Association between Fluorescence Intensity on Surgical Microscopy and Number of Cells Emitting Fluorescence from TPS
3.5. Improvement in the Extent of Resection of Malignant Gliomas Using This System
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lacroix, M.; Abi-Said, D.; Fourney, D.R.; Gokaslan, Z.L.; Shi, W.; DeMonte, F.; Sawaya, R. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J. Neurosurg. 2001, 95, 190–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanai, N.; Polley, M.Y.; McDermott, M.W.; Parsa, A.T.; Berger, M.S. An extent of resection threshold for newly diagnoses glioblastomas. J. Neurosurg. 2011, 115, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Chen, I.; Mao, Y. Gross total resection plays a leading role in survival of patients with glioblastoma multiforme. World Neurosurg. 2014, 82, 105–107. [Google Scholar] [CrossRef]
- Grabowski, M.M.; Recinos, P.F.; Nowacki, A.S.; Schroeder, J.L.; Angelov, L.; Barnett, G.H.; Vogelbaum, M.A. Residual tumor volume versus extent of resection: Predictors of survival after surgery of glioblastoma. J. Neurosurg. 2014, 121, 1115–1123. [Google Scholar] [CrossRef]
- Li, Y.M.; Suki, D.; Hess, K.; Sawaya, R. The influence of maximum safe resection of glioblastoma on survival in 1229 patients: Can we do better than gross-total resection? J. Neurosurg. 2016, 124, 977–988. [Google Scholar] [CrossRef] [Green Version]
- Yordanova, Y.N.; Moritz, S.G.; Duffau, H. Awake surgery for WHO II gliomas within “noneloquent” areas in the left dominant hemisphere: Toward a “supratotal” resections. J. Neurosurg. 2011, 115, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.J. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomized controlled multicenter phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Liu, J.T.C.; Meza, D.; Sanai, N. Trends in fluorescence image guided surgery forgliomas. Neurosurgery 2014, 75, 61–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, R.; Cuthbert, H.; Watts, C. Fluorescence-guided surgery in the surgical treatment of gliomas: Past, Present and Future. Cancers 2021, 13, 3508. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Tonn, J.C.; Goetz, C.; Ullrich, W.; Stepp, H.; Bink, A.; Pichlmeier, U. 5-Aminolevulinic acid-derived tumor fluorescence: The diagnostic accuracy of visible fluorescence qualities as corroborated by spectrum and histology and postoperative imaging. Neurosurgery 2014, 74, 310–319. [Google Scholar] [CrossRef] [Green Version]
- Valdes, P.A.; Leblond, F.; Kim, A.; Harris, B.T.; Wilson, B.C.; Fan, X.; Tosteson, T.D.; Hartov, A.; Ji, S.; Erkmen, K.; et al. Quantitative fluorescence in intracranial tumor: Implications for ALA-induced PpIX as an intraoperative biomarker. J. Neurosurg. 2011, 115, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Valdes, P.A.; Leblond, F.; Jacobs, V.L.; Wilson, B.C.; Paulsen, K.D.; Roberts, D.W. Quantitative, spectrally-resolved intraoperative fluorescence imaging. Sci. Rep. 2012, 2, 798. [Google Scholar] [CrossRef]
- Widhalm, G.; Wolfsberger, S.; Minchev, G.; Woehrer, A.; Krssak, M.; Czech, T.; Knosp, E. 5-Aminolevulinic acid is a promising marker for detection of anaplastic foci in diffusely infiltrating gliomas with non-significant contrast enhancement. Cancer 2010, 116, 1545–1552. [Google Scholar] [CrossRef]
- Roberts, D.W.; Valdes, P.A.; Harris, B.T.; Fontaine, K.M.; Hartov, A.; Fan, X.; Paulsen, K.D. Coregistered fluorescence-enhanced tumor resection protoporphyrin IX fluorescence, magnetic resonance imaging enhancement, and neuropathological parameters. J. Neurosurg. 2011, 114, 595–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widhalm, G.; Kiesel, B.; Woehrer, A.; Traub-Weidinger, T.; Preusser, M.; Marosi, C.; Wolfsberger, S. 5-aminolevulinic acid induced fluorescence is a powerful intraoperative marker for precise histopathological grading of gliomas with non-significant contrast-enhancement. PLoS ONE 2013, 8, e76988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsutsumi, M.; Miki, Y.; Akimoto, J.; Haraoka, J.; Aizawa, K.; Hirano, K.; Beppu, M. Photodynamic therapy using talaporfin sodium induces dose-dependent apoptotic cell death in human glioma cell lines. Photodiagnosis. Photodyn. Ther. 2013, 10, 103–110. [Google Scholar] [CrossRef]
- Matsumura, H.; Akimoto, J.; Haraoka, J.; Aizawa, K. Uptake and retention of the mono-L-asparthyl chlorine e6 in experimental glioma. Lasers Med. Sci. 2008, 23, 235–245. [Google Scholar] [CrossRef]
- Namatame, H.; Akimoto, J.; Matsumura, H.; Haraoka, J.; Aizawa, K. Photodynamic therapy of C6-implanted glioma cells in the rat brain employing second-generation photosensitizer talaporfin sodium. Photodiagnosis Photodyn. Ther. 2008, 5, 198–209. [Google Scholar] [CrossRef]
- Akimoto, J.; Fukami, S.; Ichikawa, M.; Mohamed, A.; Kohno, M. Intraoperative photodiagnosis for malignant glioma using photosensitizer talaporfin sodium. Front. Surg. 2019, 6, 12. [Google Scholar] [CrossRef]
- Shimizu, K.; Nitta, M.; Komori, T.; Maruyama, T.; Yasuda, T.; Fujii, Y.; Muragaki, Y. Intraoperative photodynamic diagnosisusing talaporfin sodium simultaneously applied for photodynamic therapyagainst malignant glioma: A prospective clinical study. Front. Neurol. 2018, 9, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akimoto, J.; Haraoka, J.; Aizawa, K. Preliminary clinical report of safety and efficacy of photodynamic therapy using Talaporfin sodium for malignant gliomas. Photodiagnosis Photodyn. Ther. 2008, 5, 198–209. [Google Scholar] [CrossRef]
- Muragaki, Y.; Akimoto, J.; Maruyama, T.; Iseki, H.; Ikuta, S.; Nitta, M.; Kayama, T. Phase II clinical study onintraoperative photodynamic therapy with talaporfin sodium and semiconductor laser in patients with malignant brain tumors. J. Neurosurg. 2013, 119, 845–852. [Google Scholar] [CrossRef]
- Nitta, M.; Muragaki, Y.; Maruyama, T.; Iseki, H.; Komori, T.; Ikuta, S.; Kawamata, T. Role of Photodynamic therapy using talaporfin sodium and a semiconductor laser in patients with newly diagnosed glioblastoma. J. Neurosurg. 2018, 131, 1361–1368. [Google Scholar] [CrossRef]
- Tsurubuchi, T.; Zoboronok, A.; Yamamoto, T.; Nakai, K.; Yoshida, F.; Shirakawa, M.; Matsumura, A. The optimization of fluorescence imaging of brain tumor tissue differentiated from brain edema- In vivo kinetic study of 5-aminolevuinic acid and talaporfin sodium. Photodiagnosis. Photodyn. Ther. 2009, 6, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Wilson, C.B. Glioblastoma; the past, the present, and the future. Clin. Neurosurg. 1992, 38, 32–48. [Google Scholar] [PubMed]
- Bottiroli, G.; Croce, A.C.; Locatelli, D.; Nano, R.; Giombelli, E.; Messina, A.; Benericetti, E. Brain tissue autofluorescence: An aid for intraoperative delineation of tumor resection margins. Cancer Detect. Prev. 1998, 22, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Butte, P.V.; Fang, Q.; Javier, A.; Yong, W.H.; Pikul, B.K.; Black, K.L.; Marcu, L. Intraoperative delineation of primary brain tumors using time-resolved fluorescence. J. Biomed. Opt. 2010, 15, 027008. [Google Scholar] [CrossRef] [Green Version]
- Croce, A.C.; Fiorani, S.; Locatelli, D.; Nano, R.; Ceroni, M.; Tancioni, F.; Bottiroli, G. Diagnostic potential of autofluorecence for an assisted intraoperative delineation of glioblastoma resection margins. Photochem. Photobiol. 2013, 77, 309–318. [Google Scholar] [CrossRef]
- Sanker, T.; Delaney, P.M.; Ryan, R.W.; Eschbacher, J.; Abdelwahab, M.; Nakaji, P.; Preul, M.C. Miniaturized handheld confocal microscopy for neurosurgery; Results in an experimental glioblastoma model. Neurosurgery 2010, 66, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Foster, T.H. In vivo confocal fluorescence imaging of the intratumor distribution of the photosensitizer mono-L-aspartylchlorine-e6. Neoplasia 2008, 10, 429–438. [Google Scholar] [CrossRef] [Green Version]
- Sanai, N.; Snyder, L.A.; Honea, N.J.; Coons, S.W.; Eschbacher, J.M.; Smith, K.A.; Spetzler, R.F. Intraoperative confocal microscopy in the visualization of 5-aminolevulinic acid fluorescence in low grade gliomas. J. Neurosurg. 2011, 115, 740–748. [Google Scholar] [CrossRef]
- Wei, L.; Fujita, Y.; Sanai, N.; Liu, J.T. Toward quantitative neurosurgical guidancewith high-resolution microscopy of 5-aminolevulinic acid-induced protoporphyrin IX. Front. Oncol. 2019, 9, 592. [Google Scholar] [CrossRef] [Green Version]
- Wei, L.; Chen, Y.; Yin, C.; Borwege, S.; Borwege, S.; Sanai, N.; Liu, J.T. Optical-sectioning microscopy of protoporphyrin IX fluorescence in human gliomas. standardization andquantitative comparison with histology. J. Biomed. Opt. 2017, 22, 46005. [Google Scholar] [CrossRef] [PubMed]
- Kantelhardt, S.R.; Leppert, J.; Krajewski, J.; Petkus, N.; Reusche, E.; Tronnier, V.M.; Giese, A. Imaging of brain and brain tumor specimes by time-resolved multiphoton excitation microscopy ex vivo. Neuro-Oncology 2007, 9, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Roberts, D.W.; Olson, J.D.; Evans, L.T.; Kolste, K.K.; Kanick, S.C.; Fan, X.; Paulsen, K.D. Red-light excitation of protoporphyrinIX fluorescence for subsurface tumor detection. J. Neurosurg. 2017, 128, 1690–1697. [Google Scholar] [CrossRef]
- Asai, K.; Sumiyama, Y.; Watanabe, M.; Aizawa, K. Tumor viability using real-time spectral images. Surg. Today 2006, 36, 1075–1084. [Google Scholar] [CrossRef]
- Valdes, P.A.; Jacobs, V.; Harris, B.T.; Wilson, B.C.; Leblond, F.; Paulsen, K.D.; Roberts, D.W. Quantitative fluorescence using 5-aminolevuinic acid-induced protoporphyrin IX biomarkers as a surgical adjunct in low-grade glioma surgery. J. Neurosurg. 2015, 123, 771–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, D.; Hervey-Jumper, S.D.; Chang, S.; Molinaro, A.M.; McDermott, M.W.; Phillips, J.J.; Berger, M.S. A prospective phase II clinical trialof 5-aminolevulinic acid to assess the correlation of intraoperative fluorescenceintensity and degree of histologic cellularity during resection of high-grade gliomas. J. Neurosurg. 2016, 124, 1300–1309. [Google Scholar] [CrossRef] [Green Version]
- Toms, S.A.; Lin, W.C.; Weil, R.J.; Johnson, M.D.; Jansen, E.D.; Mahadevan-Jansen, A. Intraoperative optical spectroscopyidentifies infiltrating glioma margins with high sensitivity. Neurosurgery 2005, 57, ONS-382–ONS-391. [Google Scholar]
- Chung, Y.G.; Schwartz, J.A.; Gardner, C.M.; Sawaya, R.E.; Jacques, S.L. Diagnostic potential of laser-induced autofluorescence emission in brain tissue. J. Korean. Med. Sci. 1997, 12, 135–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kessel, D. Pharmacokinetics of N-asparthyl chlorine-e6 in cancer patients. J. Photochem. Photobiol. B Biol. 1997, 39, 81–83. [Google Scholar] [CrossRef]
- Takada, T.; Tamura, M.; Yamamoto, T.; Matsui, H.; Matsumura, A. Selective accumulation of hematoporphyrin derivative in glioma through proton coupled folate transportor SLCA46A1. J. Clin. Biochem. Nutr. 2014, 54, 26–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akimoto, J.; Fukami, S.; Ichikawa, M.; Nagai, K.; Kohno, M. Preliminary Report: Rapid Intraoperative Detection of Residual Glioma Cell in Resection Cavity Walls Using a Compact Fluorescence Microscope. J. Clin. Med. 2021, 10, 5375. https://doi.org/10.3390/jcm10225375
Akimoto J, Fukami S, Ichikawa M, Nagai K, Kohno M. Preliminary Report: Rapid Intraoperative Detection of Residual Glioma Cell in Resection Cavity Walls Using a Compact Fluorescence Microscope. Journal of Clinical Medicine. 2021; 10(22):5375. https://doi.org/10.3390/jcm10225375
Chicago/Turabian StyleAkimoto, Jiro, Shinjiro Fukami, Megumi Ichikawa, Kenta Nagai, and Michihiro Kohno. 2021. "Preliminary Report: Rapid Intraoperative Detection of Residual Glioma Cell in Resection Cavity Walls Using a Compact Fluorescence Microscope" Journal of Clinical Medicine 10, no. 22: 5375. https://doi.org/10.3390/jcm10225375





