The Role of the Transsulfuration Pathway in Non-Alcoholic Fatty Liver Disease
Abstract
:1. Introduction
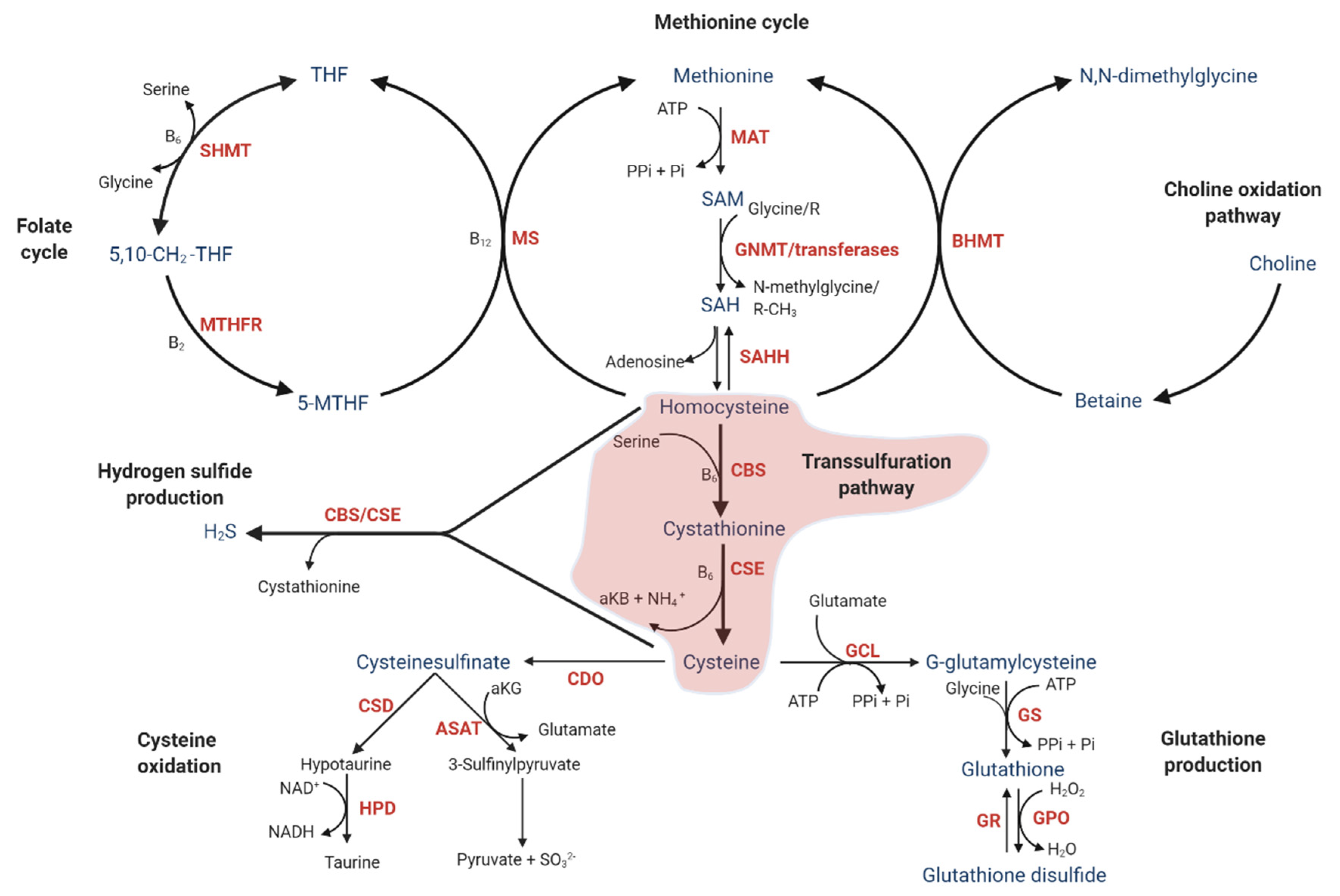
2. Description of the Transsulfuration Pathway
2.1. One-Carbon Metabolism
2.2. Downstream of the Transsulfuration Pathway
2.3. Allosteric and Posttranslational Regulation of the Transsulfuration Pathway Flux
2.4. Importance of Folate and Vitamin B6 and B12 Status in One-Carbon Metabolism and the Transsulfuration Pathway
2.5. Transcriptional Regulation of Enzymes in the Transsulfuration Pathway
2.6. Hydrogen Sulfide Production Is Inherently Coupled to the Transsulfuration Pathway
2.7. Pathways Involving the Conversion of Cysteine to Glutathione, Taurine, and Sulfate
3. Alterations in the Transsulfuration Pathway Linked to NAFLD in Experimental and Animal Models
3.1. Reduced Production of H2S May Be Involved in NAFLD
3.2. Taurine May Have Beneficial Effects in NAFLD Models
3.3. Long-Term Lipotoxicity Leads to Glutathione Depletion and May Be Involved in NAFLD Development
4. The Transsulfuration Pathway in Human NALFD
4.1. Increased Blood Levels of Homocysteine in Human NAFLD
4.2. Genetic Association of the Transsulfuration Pathway with NAFLD
4.3. Reduced H2S Levels May Be Associated with Liver Disease
4.4. Transsulfuration Pathway Metabolite Concentrations and Cofactor Status in Human NAFLD
5. Summary
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neuschwander-Tetri, B.A. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: The central role of nontriglyceride fatty acid metabolites. Hepatology 2010, 52, 774–788. [Google Scholar] [CrossRef]
- Cusi, K. Role of Obesity and Lipotoxicity in the Development of Nonalcoholic Steatohepatitis: Pathophysiology and Clinical Implications. Gastroenterol. 2012, 142, 711–725. [Google Scholar] [CrossRef]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef]
- Wu, D.-D.; Wang, D.-Y.; Li, H.-M.; Guo, J.-C.; Duan, S.-F.; Ji, X.-Y. Hydrogen Sulfide as a Novel Regulatory Factor in Liver Health and Disease. Oxidative Med. Cell. Longev. 2019, 2019, 3831713. [Google Scholar] [CrossRef]
- Li, L.; Zhang, G.-F.; Lee, K.; Lopez, R.; Previs, S.F.; Willard, B.; McCullough, A.; Kasumov, T. A Western diet induced NAFLD in LDLR(-/)(-) mice is associated with reduced hepatic glutathione synthesis. Free. Radic. Biol. Med. 2016, 96, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Kalhan, S.C.; Guo, L.; Edmison, J.; Dasarathy, S.; McCullough, A.J.; Hanson, R.W.; Milburn, M. Plasma metabolomic profile in nonalcoholic fatty liver disease. Metabolism 2011, 60, 404–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, K.; Sagara, M.; Aoki, C.; Tanaka, S.; Aso, Y. Clinical Implication of Plasma Hydrogen Sulfide Levels in Japanese Patients with Type 2 Diabetes. Intern. Med. 2017, 56, 17–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef] [Green Version]
- Kabisch, S.; Bäther, S.; Dambeck, U.; Kemper, M.; Gerbracht, C.; Honsek, C.; Sachno, A.; Pfeiffer, A.F.H. Liver Fat Scores Moderately Reflect Interventional Changes in Liver Fat Content by a Low-Fat Diet but Not by a Low-Carb Diet. Nutrients 2018, 10, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byrne, C.D.; Targher, G. EASL–EASD–EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease: Is universal screening appropriate? Diabetologia 2016, 59, 1141–1144. [Google Scholar] [CrossRef] [Green Version]
- Caussy, C.; Ajmera, V.H.; Puri, P.; Hsu, C.L.-S.; Bassirian, S.; Mgdsyan, M.; Singh, S.; Faulkner, C.; A Valasek, M.; Rizo, E.; et al. Serum metabolites detect the presence of advanced fibrosis in derivation and validation cohorts of patients with non-alcoholic fatty liver disease. Gut 2019, 68, 1884–1892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef]
- Srivastava, A.; Gailer, R.; Tanwar, S.; Trembling, P.; Parkes, J.; Rodger, A.; Suri, D.; Thorburn, D.; Sennett, K.; Morgan, S.; et al. Prospective evaluation of a primary care referral pathway for patients with non-alcoholic fatty liver disease. J. Hepatol. 2019, 71, 371–378. [Google Scholar] [CrossRef] [Green Version]
- Karsdal, M.A.; Henriksen, K.; Nielsen, M.J.; Byrjalsen, I.; Leeming, D.J.; Gardner, S.; Goodman, Z.; Patel, K.; Krag, A.; Christiansen, C.; et al. Fibrogenesis assessed by serological type III collagen formation identifies patients with progressive liver fibrosis and responders to a potential antifibrotic therapy. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G1009–G1017. [Google Scholar] [CrossRef] [Green Version]
- Medici, V.; Peerson, J.M.; Stabler, S.P.; French, S.W.; Gregory, J.F.; Virata, M.C.; Albanese, A.; Bowlus, C.L.; Devaraj, S.; Panacek, E.A.; et al. Impaired homocysteine transsulfuration is an indicator of alcoholic liver disease. J. Hepatol. 2010, 53, 551–557. [Google Scholar] [CrossRef] [Green Version]
- Sarna, L.K.; Siow, Y.L.; O, K. The CBS/CSE system: A potential therapeutic target in NAFLD? Can. J. Physiol. Pharmacol. 2015, 93, 1–11. [Google Scholar] [CrossRef]
- Fiorucci, S.; Distrutti, E. Targeting the transsulfuration-H2S pathway by FXR and GPBAR1 ligands in the treatment of portal hypertension. Pharmacol. Res. 2016, 111, 749–756. [Google Scholar] [CrossRef]
- Ducker, G.S.; Rabinowitz, J.D. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef] [Green Version]
- Pajares, M.A.; Pérez-Sala, D. Betaine homocysteine S-methyltransferase: Just a regulator of homocysteine metabolism? Cell. Mol. Life Sci. 2006, 63, 2792–2803. [Google Scholar] [CrossRef] [Green Version]
- Finkelstein, J.D. Methionine metabolism in mammals. J. Nutr. Biochem. 1990, 1, 228–237. [Google Scholar] [CrossRef]
- Mudd, S.H.; Brosnan, J.T.; Brosnan, M.E.; Jacobs, R.L.; Stabler, S.P.; Allen, R.H.; Vance, D.E.; Wagner, C. Methyl balance and transmethylation fluxes in humans. Am. J. Clin. Nutr. 2007, 85, 19–25. [Google Scholar] [CrossRef]
- Su, X.; E Wellen, K.; Rabinowitz, J.D. Metabolic control of methylation and acetylation. Curr. Opin. Chem. Biol. 2016, 30, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Stipanuk, M.H.; Ueki, I. Dealing with methionine/homocysteine sulfur: Cysteine metabolism to taurine and inorganic sulfur. J. Inherit. Metab. Dis. 2010, 34, 17–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sbodio, J.I.; Snyder, S.H.; Paul, B.D. Regulators of the transsulfuration pathway. Br. J. Pharmacol. 2018, 176, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Ratnam, S.; MacLean, K.N.; Jacobs, R.L.; Brosnan, M.E.; Kraus, J.P.; Brosnan, J.T. Hormonal Regulation of Cystathionine β-Synthase Expression in Liver. J. Biol. Chem. 2002, 277, 42912–42918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Yang, G.; Untereiner, A.; Ju, Y.; Wu, L.; Wang, R. Hydrogen Sulfide Impairs Glucose Utilization and Increases Gluconeogenesis in Hepatocytes. Endocrinology 2013, 154, 114–126. [Google Scholar] [CrossRef] [Green Version]
- Siebert, N.; Cantré, D.; Eipel, C.; Vollmar, B. H2S contributes to the hepatic arterial buffer response and mediates vasorelaxation of the hepatic artery via activation of KATP channels. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G1266–G1273. [Google Scholar] [CrossRef] [Green Version]
- Fiorucci, S.; Antonelli, E.; Mencarelli, A.; Orlandi, S.; Renga, B.; Rizzo, G.; Distrutti, E.; Shah, V.; Morelli, A. The third gas: H2S regulates perfusion pressure in both the isolated and perfused normal rat liver and in cirrhosis. Hepatology 2005, 42, 539–548. [Google Scholar] [CrossRef]
- Prudova, A.; Bauman, Z.; Braun, A.; Vitvitsky, V.; Lu, S.C.; Banerjee, R. S-adenosylmethionine stabilizes cystathionine beta-synthase and modulates redox capacity. Proc. Natl. Acad. Sci. USA 2006, 103, 6489–6494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, W.-N.; Yadav, P.K.; Adamec, J.; Banerjee, R. S-Glutathionylation Enhances Human Cystathionine β-Synthase Activity Under Oxidative Stress Conditions. Antioxid. Redox Signal. 2015, 22, 350–361. [Google Scholar] [CrossRef] [Green Version]
- Finkelstein, J.D. Metabolic regulatory properties of S-adenosylmethionine and S-adenosylhomocysteine. Clin. Chem. Lab. Med. 2007, 45, 1694–1699. [Google Scholar] [CrossRef]
- Singh, S.; Madzelan, P.; Banerjee, R. Properties of an unusual heme cofactor in PLP-dependent cystathionine β-synthase. Nat. Prod. Rep. 2007, 24, 631–639. [Google Scholar] [CrossRef]
- Renga, B.; Bucci, M.; Cipriani, S.; Carino, A.; Monti, M.C.; Zampella, A.; Gargiulo, A.; Bianca, R.D.D.V.; Distrutti, E.; Fiorucci, S. Cystathionine γ-lyase, a H2S-generating enzyme, is a GPBAR1-regulated gene and contributes to vasodilation caused by secondary bile acids. Am. J. Physiol. Hear. Circ. Physiol. 2015, 309, H114–H126. [Google Scholar] [CrossRef] [Green Version]
- Mustafa, A.K.; Gadalla, M.M.; Sen, N.; Kim, S.; Mu, W.; Gazi, S.K.; Barrow, R.K.; Yang, G.; Wang, R.; Snyder, S.H. H2S Signals Through Protein S-Sulfhydration. Sci. Signal. 2009, 2, ra72. [Google Scholar] [CrossRef] [Green Version]
- Lima, C.P.; Davis, S.R.; Mackey, A.D.; Scheer, J.B.; Williamson, J.; Gregory, J.F. Vitamin B-6 deficiency suppresses the hepatic transsulfuration pathway but increases glutathione concentration in rats fed AIN-76A or AIN-93G diets. J. Nutr. 2006, 136, 2141–2147. [Google Scholar] [CrossRef]
- Stabler, S.P.; Sampson, D.A.; Wang, L.-P.; Allen, R.H. Elevations of serum cystathionine and total homocysteine in pyridoxine-, folate-, and cobalamin-deficient rats. J. Nutr. Biochem. 1997, 8, 279–289. [Google Scholar] [CrossRef]
- Gregory, J.F.; DeRatt, B.N.; Rios-Avila, L.; Ralat, M.; Stacpoole, P.W. Vitamin B6 nutritional status and cellular availability of pyridoxal 5′-phosphate govern the function of the transsulfuration pathway’s canonical reactions and hydrogen sulfide production via side reactions. Biochimie 2016, 126, 21–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, M.; Cuskelly, G.J.; Williamson, J.; Toth, J.P.; Gregory, I.J.F. Vitamin B-6 Deficiency in Rats Reduces Hepatic Serine Hydroxymethyltransferase and Cystathionine β-Synthase Activities and Rates of In Vivo Protein Turnover, Homocysteine Remethylation and Transsulfuration. J. Nutr. 2000, 130, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Gregory, J.F.; Park, Y.; Lamers, Y.; Bandyopadhyay, N.; Chi, Y.-Y.; Lee, K.; Kim, S.; Da Silva, V.; Hove, N.; Ranka, S.; et al. Metabolomic Analysis Reveals Extended Metabolic Consequences of Marginal Vitamin B-6 Deficiency in Healthy Human Subjects. PLoS ONE 2013, 8, e63544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rush, E.C.; Katre, P.; Yajnik, C.S. Vitamin B12: One carbon metabolism, fetal growth and programming for chronic disease. Eur. J. Clin. Nutr. 2013, 68, 2–7. [Google Scholar] [CrossRef]
- Lyon, P.; Strippoli, V.; Fang, B.; Cimmino, L. B Vitamins and One-Carbon Metabolism: Implications in Human Health and Disease. Nutrients 2020, 12, 2867. [Google Scholar] [CrossRef]
- Froese, D.S.; Fowler, B.; Baumgartner, M.R. Vitamin B12, folate, and the methionine remethylation cycle-biochemistry, pathways, and regulation. J. Inherit. Metab. Dis. 2019, 42, 673–685. [Google Scholar] [CrossRef] [Green Version]
- Yamada, K.; Kawata, T.; Wada, M.; Isshiki, T.; Onoda, J.; Kawanishi, T.; Kunou, A.; Tadokoro, T.; Tobimatsu, T.; Maekawa, A.; et al. Extremely low activity of methionine synthase in vitamin B-12-deficient rats may be related to effects on coenzyme stabilization rather than to changes in coenzyme induction. J. Nutr. 2000, 130, 1894–1900. [Google Scholar] [CrossRef] [Green Version]
- Shane, B. Folate and Vitamin B12Metabolism: Overview and Interaction with Riboflavin, Vitamin B6, and Polymorphisms. Food Nutr. Bull. 2008, 29, S5–S16. [Google Scholar] [CrossRef] [PubMed]
- Sbodio, J.I.; Snyder, S.H.; Paul, B.D. Golgi stress response reprograms cysteine metabolism to confer cytoprotection in Huntington’s disease. Proc. Natl. Acad. Sci. USA 2018, 115, 780–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renga, B.; Mencarelli, A.; Migliorati, M.; Distrutti, E.; Fiorucci, S. Bile-acid-activated farnesoid X receptor regulates hydrogen sulfide production and hepatic microcirculation. World J. Gastroenterol. 2009, 15, 2097–2108. [Google Scholar] [CrossRef]
- Sen, N.; Paul, B.D.; Gadalla, M.M.; Mustafa, A.K.; Sen, T.; Xu, R.; Kim, S.; Snyder, S.H. Hydrogen Sulfide-Linked Sulfhydration of NF-κB Mediates Its Antiapoptotic Actions. Mol. Cell 2012, 45, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.I.; Boosen, M.; Schaefer, L.; Kozlowska, J.; Eisel, F.; Von Knethen, A.; Beck, M.; Hemeida, R.A.M.; El-Moselhy, M.A.M.; Hamada, F.M.A.; et al. Platelet-derived growth factor-BB induces cystathionine γ-lyase expression in rat mesangial cells via a redox-dependent mechanism. Br. J. Pharmacol. 2012, 166, 2231–2242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hourihan, J.M.; Kenna, J.G.; Hayes, J.D. The Gasotransmitter Hydrogen Sulfide Induces Nrf2-Target Genes by Inactivating the Keap1 Ubiquitin Ligase Substrate Adaptor Through Formation of a Disulfide Bond Between Cys-226 and Cys-613. Antioxid. Redox Signal. 2013, 19, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Steele, M.L.; Fuller, S.; Patel, M.; Kersaitis, C.; Ooi, L.; Münch, G. Effect of Nrf2 activators on release of glutathione, cysteinylglycine and homocysteine by human U373 astroglial cells. Redox Biol. 2013, 1, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Harvey, C.; Thimmulappa, R.; Singh, A.; Blake, D.; Ling, G.; Wakabayashi, N.; Fujii, J.; Myers, A.; Biswal, S. Nrf2-regulated glutathione recycling independent of biosynthesis is critical for cell survival during oxidative stress. Free. Radic. Biol. Med. 2009, 46, 443–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nandi, S.S.; Mishra, P.K. H2S and homocysteine control a novel feedback regulation of cystathionine beta synthase and cystathionine gamma lyase in cardiomyocytes. Sci. Rep. 2017, 7, 3639. [Google Scholar] [CrossRef]
- Jiao, Y.; Lu, Y.; Li, X.-Y. Farnesoid X receptor: A master regulator of hepatic triglyceride and glucose homeostasis. Acta Pharmacol. Sin. 2015, 36, 44–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trauner, M.; Claudel, T.; Fickert, P.; Moustafa, T.; Wagner, M. Bile Acids as Regulators of Hepatic Lipid and Glucose Metabolism. Dig. Dis. 2010, 28, 220–224. [Google Scholar] [CrossRef]
- Luedde, T.; Schwabe, R.F. NF-κB in the liver—linking injury, fibrosis and hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 108–118. [Google Scholar] [CrossRef] [Green Version]
- Murphy, B.; Bhattacharya, R.; Mukherjee, P. Hydrogen sulfide signaling in mitochondria and disease. FASEB J. 2019, 33, 13098–13125. [Google Scholar] [CrossRef] [Green Version]
- Kimura, Y.; Goto, Y.-I.; Kimura, H. Hydrogen Sulfide Increases Glutathione Production and Suppresses Oxidative Stress in Mitochondria. Antioxid. Redox Signal. 2010, 12, 1–13. [Google Scholar] [CrossRef]
- Zhang, D.; Du, J.; Tang, C.; Huang, Y.; Jin, H. H2S-Induced Sulfhydration: Biological Function and Detection Methodology. Front. Pharmacol. 2017, 8, 608. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.W.; Moore, P.K. H2S synthesizing enzymes: Biochemistry and molecular aspects. In Chemistry, Biochemistry and Pharmacology of Hydrogen Sulfide; Handbook of Experimental Pharmacology; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Kabil, O.; Yadav, V.; Banerjee, R. Heme-dependent Metabolite Switching Regulates H2S Synthesis in Response to Endoplasmic Reticulum (ER) Stress. J. Biol. Chem. 2016, 291, 16418–16423. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, R. Catalytic promiscuity and heme-dependent redox regulation of H2S synthesis. Curr. Opin. Chem. Biol. 2017, 37, 115–121. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Xu, C.; Shi, J.; Ding, J.; Wan, X.; Chen, D.; Gao, J.; Li, C.; Zhang, J.; Lin, Y.; et al. Fatty acids promote fatty liver disease via the dysregulation of 3-mercaptopyruvate sulfurtransferase/hydrogen sulfide pathway. Gut 2017, 67, 2169–2180. [Google Scholar] [CrossRef]
- Teskey, G.; Abrahem, R.; Cao, R.; Gyurjian, K.; Islamoglu, H.; Lucero, M.; Martinez, A.; Paredes, E.; Salaiz, O.; Robinson, B.; et al. Glutathione as a Marker for Human Disease. Adv. Clin. Chem. 2018, 87, 141–159. [Google Scholar] [CrossRef] [PubMed]
- Mosharov, E.; Cranford, M.R.; Banerjee, R. The Quantitatively Important Relationship between Homocysteine Metabolism and Glutathione Synthesis by the Transsulfuration Pathway and Its Regulation by Redox Changes†. Biochemistry 2000, 39, 13005–13011. [Google Scholar] [CrossRef]
- Vitvitsky, V.; Mosharov, E.; Tritt, M.; Ataullakhanov, F.; Banerjee, R. Redox regulation of homocysteine-dependent glutathione synthesis. Redox Rep. 2003, 8, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, S.; Kim, H.W. Effects and Mechanisms of Taurine as a Therapeutic Agent. Biomol. Ther. 2018, 26, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Yanagita, T.; Han, S.-Y.; Hu, Y.; Nagao, K.; Kitajima, H.; Murakami, S. Taurine reduces the secretion of apolipoprotein B100 and lipids in HepG2 cells. Lipids Health Dis. 2008, 7, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stipanuk, M.H.; Hirschberger, L.L.; Londono, M.P.; Cresenzi, C.L.; Yu, A.F. The ubiquitin-proteasome system is responsible for cysteine-responsive regulation of cysteine dioxygenase concentration in liver. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E439–E448. [Google Scholar] [CrossRef]
- Jurkowska, H.; Roman, H.B.; Hirschberger, L.L.; Sasakura, K.; Nagano, T.; Hanaoka, K.; Krijt, J.; Stipanuk, M.H. Primary hepatocytes from mice lacking cysteine dioxygenase show increased cysteine concentrations and higher rates of metabolism of cysteine to hydrogen sulfide and thiosulfate. Amino Acids 2014, 46, 1353–1365. [Google Scholar] [CrossRef] [Green Version]
- Niewiadomski, J.; Zhou, J.Q.; Roman, H.B.; Liu, X.; Hirschberger, L.L.; Locasale, J.W.; Stipanuk, M.H. Effects of a block in cysteine catabolism on energy balance and fat metabolism in mice. Ann. N. Y. Acad. Sci. 2016, 1363, 99–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueki, I.; Roman, H.B.; Valli, A.; Fieselmann, K.; Lam, J.; Peters, R.; Hirschberger, L.L.; Stipanuk, M.H. Knockout of the murine cysteine dioxygenase gene results in severe impairment in ability to synthesize taurine and an increased catabolism of cysteine to hydrogen sulfide. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E668–E684. [Google Scholar] [CrossRef] [Green Version]
- Robert, K.; Nehmé, J.; Bourdon, E.; Pivert, G.; Friguet, B.; Delcayre, C.; Delabar, J.; Janel, N. Cystathionine β Synthase Deficiency Promotes Oxidative Stress, Fibrosis, and Steatosis in Mice Liver. Gastroenterology 2005, 128, 1405–1415. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, M.; Osada, J.; Aratani, Y.; Kluckman, K.; Reddick, R.; Malinow, M.R.; Maeda, N. Mice deficient in cystathionine beta-synthase: Animal models for mild and severe homocyst(e)inemia. Proc. Natl. Acad. Sci. USA 1995, 92, 1585–1589. [Google Scholar] [CrossRef] [Green Version]
- Mani, S.; Li, H.; Yang, G.; Wu, L.; Wang, R. Deficiency of cystathionine gamma-lyase and hepatic cholesterol accumulation during mouse fatty liver development. Sci. Bull. 2015, 60, 336–347. [Google Scholar] [CrossRef] [Green Version]
- Grattagliano, I.; Caraceni, P.; Calamita, G.; Ferri, D.; Gargano, I.; Palasciano, G.; Portincasa, P. Severe liver steatosis correlates with nitrosative and oxidative stress in rats. Eur. J. Clin. Investig. 2008, 38, 523–530. [Google Scholar] [CrossRef]
- Dickhout, J.G.; Carlisle, R.E.; Jerome, D.E.; Mohammed-Ali, Z.; Jiang, H.; Yang, G.; Mani, S.; Garg, S.K.; Banerjee, R.; Kaufman, R.J.; et al. Integrated Stress Response Modulates Cellular Redox State via Induction of Cystathionine γ-Lyase. J. Biol. Chem. 2012, 287, 7603–7614. [Google Scholar] [CrossRef] [Green Version]
- Guillén, N.; Navarro, M.A.; Arnal, C.; Noone, E.; Arbonés-Mainar, J.M.; Acín, S.; Surra, J.C.; Muniesa, P.; Roche, H.M.; Osada, J. Microarray analysis of hepatic gene expression identifies new genes involved in steatotic liver. Physiol. Genom. 2009, 37, 187–198. [Google Scholar] [CrossRef] [Green Version]
- Hamelet, J.; DeMuth, K.; Paul, J.-L.; Delabar, J.-M.; Janel, N. Hyperhomocysteinemia due to cystathionine beta synthase deficiency induces dysregulation of genes involved in hepatic lipid homeostasis in mice. J. Hepatol. 2007, 46, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Deng, M.; Su, J.; Lin, Y.; Jia, Z.; Peng, K.; Wang, F.; Yang, T. Specific downregulation of cystathionine β-synthase expression in the kidney during obesity. Physiol. Rep. 2018, 6, e13630. [Google Scholar] [CrossRef] [Green Version]
- Hwang, S.-Y.; Sarna, L.K.; Siow, Y.L.; O, K. High-fat diet stimulates hepatic cystathionine β-synthase and cystathionine γ-lyase expression. Can. J. Physiol. Pharmacol. 2013, 91, 913–919. [Google Scholar] [CrossRef]
- Peh, M.T.; Anwar, A.B.; Ng, D.S.W.; Atan, M.S.B.M.; Kumar, S.D.; Moore, P.K. Effect of feeding a high fat diet on hydrogen sulfide (H2S) metabolism in the mouse. Nitric Oxide 2014, 41, 138–145. [Google Scholar] [CrossRef]
- Bravo, E.; Palleschi, S.; Aspichueta, P.; Buqué, X.; Rossi, B.; Cano, A.; Napolitano, M.; Ochoa, B.; Botham, K.M. High fat diet-induced non alcoholic fatty liver disease in rats is associated with hyperhomocysteinemia caused by down regulation of the transsulphuration pathway. Lipids Health Dis. 2011, 10, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werstuck, G.H.; Lentz, S.R.; Dayal, S.; Hossain, G.S.; Sood, S.K.; Shi, Y.Y.; Zhou, J.; Maeda, N.; Krisans, S.K.; Malinow, M.R.; et al. Homocysteine-induced endoplasmic reticulum stress causes dysregulation of the cholesterol and triglyceride biosynthetic pathways. J. Clin. Investig. 2001, 107, 1263–1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, K.; Zhao, M.; Jiang, H.; Tan, G.; Pan, S.; Sun, X. Role of hydrogen sulfide in hepatic ischemia-reperfusion-induced injury in rats. Liver Transplant. 2009, 15, 1306–1314. [Google Scholar] [CrossRef]
- Tan, G.; Pan, S.; Li, J.; Dong, X.; Kang, K.; Zhao, M.; Jiang, X.; Kanwar, J.R.; Qiao, H.; Jiang, H.; et al. Hydrogen Sulfide Attenuates Carbon Tetrachloride-Induced Hepatotoxicity, Liver Cirrhosis and Portal Hypertension in Rats. PLoS ONE 2011, 6, e25943. [Google Scholar] [CrossRef]
- Luo, Z.-L.; Tang, L.-J.; Wang, T.; Dai, R.-W.; Ren, J.-D.; Cheng, L.; Xiang, K.; Tian, F.-Z. Effects of treatment with hydrogen sulfide on methionine-choline deficient diet-induced non-alcoholic steatohepatitis in rats. J. Gastroenterol. Hepatol. 2013, 29, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zheng, N.; Ziqiang, S.; Cheng, H.; Sun, Z.; Gao, B.; Zhang, Y.; Pang, W.; Huangfu, C.; Ji, S.; et al. Exogenous hydrogen sulfide mitigates the fatty liver in obese mice through improving lipid metabolism and antioxidant potential. Med. Gas Res. 2015, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Cheng, P.; Wang, F.; Chen, K.; Shen, M.; Dai, W.; Xu, L.; Zhang, Y.; Wang, C.; Li, J.; Yang, J.; et al. Hydrogen Sulfide Ameliorates Ischemia/Reperfusion-Induced Hepatitis by Inhibiting Apoptosis and Autophagy Pathways. Mediat. Inflamm. 2014, 2014, 935251. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.-X.; Shen, W.; Sun, H. Effects of nuclear receptor FXR on the regulation of liver lipid metabolism in patients with non-alcoholic fatty liver disease. Hepatol. Int. 2010, 4, 741–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younossi, Z.M.; Ratziu, V.; Loomba, R.; Rinella, M.; Anstee, Q.M.; Goodman, Z.; Bedossa, P.; Geier, A.; Beckebaum, S.; Newsome, P.N.; et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: Interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2019, 394, 2184–2196. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Zhang, J.; Lu, Y.; Wang, R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 2001, 20, 6008–6016. [Google Scholar] [CrossRef] [Green Version]
- Miyazaki, T.; Matsuzaki, Y. Taurine and liver diseases: A focus on the heterogeneous protective properties of taurine. Amino Acids 2014, 46, 101–110. [Google Scholar] [CrossRef]
- Murakami, S.; Ono, A.; Kawasaki, A.; Takenaga, T.; Ito, T. Taurine attenuates the development of hepatic steatosis through the inhibition of oxidative stress in a model of nonalcoholic fatty liver disease in vivo and in vitro. Amino Acids 2018, 50, 1279–1288. [Google Scholar] [CrossRef]
- Jong, C.J.; Azuma, J.; Schaffer, S. Mechanism underlying the antioxidant activity of taurine: Prevention of mitochondrial oxidant production. Amino Acids 2011, 42, 2223–2232. [Google Scholar] [CrossRef] [PubMed]
- Gentile, C.L.; Nivala, A.M.; Gonzales, J.C.; Pfaffenbach, K.T.; Wang, N.; Wei, Y.; Jiang, H.; Orlicky, D.J.; Petersen, D.R.; Pagliassotti, M.J.; et al. Experimental evidence for therapeutic potential of taurine in the treatment of nonalcoholic fatty liver disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R1710–R1722. [Google Scholar] [CrossRef] [PubMed]
- Warskulat, U.; Borsch, E.; Reinehr, R.; Heller-Stilb, B.; Mönnighoff, I.; Buchczyk, D.; Donner, M.; Flögel, U.; Kappert, G.; Soboll, S.; et al. Chronic liver disease is triggered by taurine transporter knockout in the mouse. FASEB J. 2006, 20, 574–576. [Google Scholar] [CrossRef]
- Mardinoglu, A.; Bjornson, E.; Zhang, C.; Klevstig, M.; Söderlund, S.; Ståhlman, M.; Adiels, M.; Hakkarainen, A.; Lundbom, N.; Kilicarslan, M.; et al. Personal model-assisted identification of NAD + and glutathione metabolism as intervention target in NAFLD. Mol. Syst. Biol. 2017, 13, 916. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.C.; Amankwa-Sakyi, M.; Flynn, T.J. Cellular glutathione in fatty liver in vitro models. Toxicol. Vitr. 2011, 25, 1501–1506. [Google Scholar] [CrossRef] [PubMed]
- Haque, J.A.; Mcmahan, R.S.; Campbell, J.S.; Shimizu-Albergine, M.; Wilson, A.M.; Botta, D.; Bammler, T.K.; Beyer, R.P.; Montine, T.J.; Yeh, M.M.; et al. Attenuated progression of diet-induced steatohepatitis in glutathione-deficient mice. Lab. Investig. 2010, 90, 1704–1717. [Google Scholar] [CrossRef]
- Dou, X.; Li, S.; Hu, L.; Ding, L.; Ma, Y.; Ma, W.; Chai, H.; Song, Z. Glutathione disulfide sensitizes hepatocytes to TNFα-mediated cytotoxicity via IKK-β S-glutathionylation: A potential mechanism underlying non-alcoholic fatty liver disease. Exp. Mol. Med. 2018, 50, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinsell, L.W.; Harper, H.A.; Barton, H.C.; Michaels, G.D.; Weiss, H.A. Rate of Disappearance From Plasma of Intravenously Administered Methionine in Patients With Liver Damage. Science 1947, 106, 589–590. [Google Scholar] [CrossRef]
- Horowitz, J.H.; Rypins, E.B.; Henderson, J.; Heymsfield, S.B.; Moffitt, S.D.; Bain, R.P.; Chawla, R.K.; Bleier, J.C.; Daniel, R. Evidence for impairment of transsulfuration pathway in cirrhosis. Gastroenterology 1981, 81, 668–675. [Google Scholar] [CrossRef]
- Marchesini, G.; Bugianesi, E.; Bianchi, G.; Fabbri, A.; Marchi, E.; Zoli, M.; Pisi, E. Defective methionine metabolism in cirrhosis: Relation to severity of liver disease. Hepatology 1992, 16, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Martensson, J.; Foberg, U.; Frydén, A.; Schwartz, M.K.; Sörbo, B.; Weiland, O. Sulfur Amino Acid Metabolism in Hepatobiliary Disorders. Scand. J. Gastroenterol. 1992, 27, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Russmann, S.; Junker, E.; Lauterburg, B.H. Remethylation and transsulfuration of methionine in cirrhosis: Studies with L -[2 H3 -methyl-1-13 C]methionine. Hepatology 2002, 36, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Look, R.R.M.P. Is the Increase in Serum Cystathionine Levels in Patients with Liver Cirrhosis a Consequence of Impaired Homocysteine Transsulfuration at the Level of ?-Cystathionase? Scand. J. Gastroenterol. 2000, 35, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Iino, C.; Endo, T.; Mikami, K.; Kimura, M.; Sawada, N.; Nakaji, S.; Fukuda, S. Changed Amino Acids in NAFLD and Liver Fibrosis: A Large Cross-Sectional Study without Influence of Insulin Resistance. Nutrients 2020, 12, 1450. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Gowda, G.A.N.; Djukovic, D.; Raftery, D. Distinguishing NASH Histological Severity Using a Multiplatform Metabolomics Approach. Metabolites 2020, 10, 168. [Google Scholar] [CrossRef]
- Brochado, M.J.F.; Domenici, F.A.; Martinelli, A.D.L.C.; Zucoloto, S.; Cunha, S.F.D.C.D.; Vannucchi, H. Methylenetetrahydrofolate Reductase Gene Polymorphism and Serum Homocysteine Levels in Nonalcoholic Fatty Liver Disease. Ann. Nutr. Metab. 2013, 63, 193–199. [Google Scholar] [CrossRef]
- De Carvalho, S.C.R.; Muniz, M.T.C.; Siqueira, M.D.V.; Siqueira, E.R.F.; Gomes, A.V.; Silva, K.A.; Bezerra, L.C.L.; D’Almeida, V.; De Oliveira, C.P.M.S.; Pereira, L.M.M.B. Plasmatic higher levels of homocysteine in Non-alcoholic fatty liver disease (NAFLD). Nutr. J. 2013, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Wang, W.; Tang, X.; Chen, R.; Chen, Z.; Lu, Y.; Yuan, H. Association between homocysteine and non-alcoholic fatty liver disease in Chinese adults: A cross-sectional study. Nutr. J. 2016, 15, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulsen, M.; Yesilova, Z.; Bagci, S.; Uygun, A.; Ozcan, A.; Ercin, C.N.; Erdil, A.; Sanisoglu, S.Y.; Cakir, E.; Ates, Y.; et al. Elevated plasma homocysteine concentrations as a predictor of steatohepatitis in patients with non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2005, 20, 1448–1455. [Google Scholar] [CrossRef]
- Jia, G.; Di, F.; Wang, Q.; Shao, J.; Gao, L.; Wang, L.; Li, Q.; Li, N. Non-Alcoholic Fatty Liver Disease Is a Risk Factor for the Development of Diabetic Nephropathy in Patients with Type 2 Diabetes Mellitus. PLoS ONE 2015, 10, e0142808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, Z.; Chen, J.; Ding, C.; Wong, K.; Chen, X.; Pu, L.; Huang, Q.; Chen, X.; Cheng, Z.; Liu, Y.; et al. Association of Hepatic Global DNA Methylation and Serum One-Carbon Metabolites with Histological Severity in Patients with NAFLD. Obesity 2019, 28, 197–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leach, N.V.; Dronca, E.; Vesa, S.C.; Sampelean, D.P.; Craciun, E.C.; Lupsor, M.; Crisan, D.; Tarau, R.; Rusu, R.; Para, I.; et al. Serum homocysteine levels, oxidative stress and cardiovascular risk in non-alcoholic steatohepatitis. Eur. J. Intern. Med. 2014, 25, 762–767. [Google Scholar] [CrossRef]
- Pastore, A.; Alisi, A.; Di Giovamberardino, G.; Crudele, A.; Ceccarelli, S.; Panera, N.; Dionisi-Vici, C.; Nobili, V. Plasma Levels of Homocysteine and Cysteine Increased in Pediatric NAFLD and Strongly Correlated with Severity of Liver Damage. Int. J. Mol. Sci. 2014, 15, 21202–21214. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, S.; Poniachick, J.; Avendaño, M.; Csendes, A.; Burdiles, P.; Smok, G.; Díaz, J.C.; De La Maza, M.P. Serum folate and homocysteine levels in obese females with non-alcoholic fatty liver. Nutrition 2005, 21, 137–141. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Patsiaoura, K.; Katsiki, E.; Zafeiriadou, E.; Deretzi, G.; Zavos, C.; Gavalas, E.; Katsinelos, P.; Mane, V.; et al. Serum homocysteine levels in patients with nonalcoholic fatty liver disease. Ann. Hepatol. 2012, 11, 68–76. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, Y.; Zhang, M.; Wang, Y.; Qin, B. The methylenetetrahydrofolate reductase genotype 677CT and non-alcoholic fatty liver disease have a synergistic effect on the increasing homocysteine levels in subjects from Chongqing, China. Genes Dis. 2019, 6, 88–95. [Google Scholar] [CrossRef]
- Dai, Y.; Zhu, J.; Meng, D.; Yu, C.; Li, Y. Association of homocysteine level with biopsy-proven non-alcoholic fatty liver disease: A meta-analysis. J. Clin. Biochem. Nutr. 2016, 58, 76–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Guan, Y.; Yang, X.; Xia, Z.; Wu, J. Association of Serum Homocysteine Levels with Histological Severity of NAFLD. J. Gastrointest. Liver Dis. 2020, 29, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Hortin, G.L. Homocysteine: Clinical Significance and Laboratory Measurement. Lab. Med. 2006, 37, 551–553. [Google Scholar] [CrossRef]
- Elshorbagy, A.K.; Nurk, E.; Gjesdal, C.G.; Tell, G.S.; Ueland, P.M.; Nygård, O.; Tverdal, A.; E Vollset, S.; Refsum, H. Homocysteine, cysteine, and body composition in the Hordaland Homocysteine Study: Does cysteine link amino acid and lipid metabolism? Am. J. Clin. Nutr. 2008, 88, 738–746. [Google Scholar] [CrossRef] [Green Version]
- Satyanarayana, A.; Balakrishna, N.; Pitla, S.; Reddy, P.Y.; Mudili, S.; Lopamudra, P.; Suryanarayana, P.; Viswanath, K.; Ayyagari, R.; Reddy, G.B. Status of B-Vitamins and Homocysteine in Diabetic Retinopathy: Association with Vitamin-B12 Deficiency and Hyperhomocysteinemia. PLoS ONE 2011, 6, e26747. [Google Scholar] [CrossRef]
- Li, T.; Chen, Y.; Li, J.; Yang, X.; Zhang, H.; Qin, X.; Hu, Y.; Mo, Z. Serum Homocysteine Concentration Is Significantly Associated with Inflammatory/Immune Factors. PLoS ONE 2015, 10, e0138099. [Google Scholar] [CrossRef] [Green Version]
- Ji, Y.; Nordgren, K.K.S.; Chai, Y.; Hebbring, S.J.; Jenkins, G.D.; Abo, R.P.; Peng, Y.; Pelleymounter, L.L.; Moon, I.; Eckloff, B.W.; et al. Human Liver Methionine Cycle: MAT1A and GNMT Gene Resequencing, Functional Genomics, and Hepatic Genotype-Phenotype Correlation. Drug Metab. Dispos. 2012, 40, 1984–1992. [Google Scholar] [CrossRef] [Green Version]
- Frosst, P.; Blom, H.; Milos, R.; Goyette, P.; Sheppard, C.; Matthews, R.; Boers, G.; Heijer, M.D.; Kluijtmans, L.; Heuve, L.V.D.; et al. A candidate genetic risk factor for vascular disease: A common mutation in methylenetetrahydrofolate reductase. Nat. Genet. 1995, 10, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Larrañaga, A.; Saadeh, M.; Hooshmand, B.; Refsum, H.; Smith, A.D.; Marengoni, A.; Vetrano, D.L. Association of Homocysteine, Methionine, and MTHFR 677C>T Polymorphism With Rate of Cardiovascular Multimorbidity Development in Older Adults in Sweden. JAMA Netw. Open 2020, 3, e205316. [Google Scholar] [CrossRef]
- Sun, M.-Y.; Zhang, L.; Shi, S.-L.; Lin, J.-N. Associations between Methylenetetrahydrofolate Reductase (MTHFR) Polymorphisms and Non-Alcoholic Fatty Liver Disease (NAFLD) Risk: A Meta-Analysis. PLoS ONE 2016, 11, e0154337. [Google Scholar] [CrossRef]
- Wang, J.; Huff, A.; Spence, J.D.; Hegele, R. Single nucleotide polymorphism in CTH associated with variation in plasma homocysteine concentration. Clin. Genet. 2004, 65, 483–486. [Google Scholar] [CrossRef]
- Avila, M.A.; Berasain, C.; Torres, L.; Martín-Duce, A.; Corrales, F.J.; Yang, H.; Prieto, J.; Lu, S.C.; Caballería, J.; Rodés, J.; et al. Reduced mRNA abundance of the main enzymes involved in methionine metabolism in human liver cirrhosis and hepatocellular carcinoma. J. Hepatol. 2000, 33, 907–914. [Google Scholar] [CrossRef] [Green Version]
- Ruiz García-Tevijano, E.; Berasain, C.; Rodríguez, J.A.; Corrales, F.J.; Arias, R.; Martín-Duce, A.; Caballería, J.; Mato, J.M.; Avila, M.A. Hyperhomocysteinemia in liver cirrhosis: Mechanisms and role in vascular and hepatic fibrosis. Hypertension 2001, 38, 1217–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, S.K.; Yang, H.; Moylan, C.A.; Pang, H.; Dellinger, A.; Abdelmalek, M.F.; Garrett, M.E.; Ashley–Koch, A.; Suzuki, A.; Tillmann, H.L.; et al. Relationship Between Methylome and Transcriptome in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2013, 145, 1076–1087. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Han, J.; Xiao, L.; Jin, C.-E.; Li, D.-J.; Yang, Z. Role of hydrogen sulfide in portal hypertension and esophagogastric junction vascular disease. World J. Gastroenterol. 2014, 20, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Bull, R.; Rains, J.L.; Bass, P.F.; Levine, S.N.; Reddy, S.; McVie, R.; Bocchini, J.A. Low Levels of Hydrogen Sulfide in the Blood of Diabetes Patients and Streptozotocin-Treated Rats Causes Vascular Inflammation? Antioxid. Redox Signal. 2010, 12, 1333–1337. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, M.; Gooding, K.M.; Whatmore, J.L.; Ball, C.I.; Mawson, D.; Skinner, K.; Tooke, J.E.; Shore, A.C. Adiposity is a major determinant of plasma levels of the novel vasodilator hydrogen sulphide. Diabetologia 2010, 53, 1722–1726. [Google Scholar] [CrossRef] [Green Version]
- Gaggini, M.; Carli, F.; Bugianesi, E.; Gastaldelli, A.; Rosso, C.; Buzzigoli, E.; Marietti, M.; Della Latta, V.; Ciociaro, D.; Abate, M.L.; et al. Altered amino acid concentrations in NAFLD: Impact of obesity and insulin resistance. Hepatology 2018, 67, 145–158. [Google Scholar] [CrossRef] [Green Version]
- García-Cañaveras, J.C.; Donato, M.T.; Castell, J.V.; Lahoz, A. A comprehensive untargeted metabonomic analysis of human steatotic liver tissue by RP and HILIC chromatography coupled to mass spectrometry reveals important metabolic alterations. J. Proteome Res. 2011, 10, 4825–4834. [Google Scholar] [CrossRef]
- Lutchmansingh, F.K.; Hsu, J.W.; Bennett, F.I.; Badaloo, A.V.; McFarlane-Anderson, N.; Gordon-Strachan, G.M.; Wright-Pascoe, R.A.; Jahoor, F.; Boyne, M.S. Glutathione metabolism in type 2 diabetes and its relationship with microvascular complications and glycemia. PLoS ONE 2018, 13, e0198626. [Google Scholar] [CrossRef] [Green Version]
- Honda, Y.; Kessoku, T.; Sumida, Y.; Kobayashi, T.; Kato, T.; Ogawa, Y.; Tomeno, W.; Imajo, K.; Fujita, K.; Yoneda, M.; et al. Efficacy of glutathione for the treatment of nonalcoholic fatty liver disease: An open-label, single-arm, multicenter, pilot study. BMC Gastroenterol. 2017, 17, 96. [Google Scholar] [CrossRef]
- Giustarini, D.; Tsikas, D.; Colombo, G.; Milzani, A.; Dalle-Donne, I.; Fanti, P.; Rossi, R. Pitfalls in the analysis of the physiological antioxidant glutathione (GSH) and its disulfide (GSSG) in biological samples: An elephant in the room. J. Chromatogr. B 2016, 1019, 21–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Wang, L.; Yan, X.; Liu, Q.; Yu, C.; Wei, H.; Li, Y.; Zhang, X.; He, F.; Jiang, Y. A Proton Nuclear Magnetic Resonance Metabonomics Approach for Biomarker Discovery in Nonalcoholic Fatty Liver Disease. J. Proteome Res. 2011, 10, 2797–2806. [Google Scholar] [CrossRef] [PubMed]
- Lake, A.D.; Novak, P.; Shipkova, P.; Aranibar, N.; Robertson, D.; Reily, M.D.; Lu, Z.; Lehman-McKeeman, L.D.; Cherrington, N.J. Decreased hepatotoxic bile acid composition and altered synthesis in progressive human nonalcoholic fatty liver disease. Toxicol. Appl. Pharmacol. 2013, 268, 132–140. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Dzierlenga, A.L.; Lu, Z.; Billheimer, D.D.; Torabzadeh, E.; Lake, A.D.; Li, H.; Novak, P.; Shipkova, P.; Aranibar, N.; et al. Metabolomic profiling distinction of human nonalcoholic fatty liver disease progression from a common rat model. Obesity 2017, 25, 1069–1076. [Google Scholar] [CrossRef] [Green Version]
- Yun, K.U.; Ryu, C.S.; Oh, J.M.; Kim, C.H.; Lee, K.S.; Lee, C.-H.; Lee, H.-S.; Kim, B.-H.; Kim, S.K. Plasma homocysteine level and hepatic sulfur amino acid metabolism in mice fed a high-fat diet. Eur. J. Nutr. 2012, 52, 127–134. [Google Scholar] [CrossRef]
- Ferro, Y.; Carè, I.; Mazza, E.; Provenzano, F.; Colica, C.; Torti, C.; Romeo, S.; Pujia, A.; Montalcini, T. Protein and vitamin B6 intake are associated with liver steatosis assessed by transient elastography, especially in obese individuals. Clin. Mol. Hepatol. 2017, 23, 249–259. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Li, P.; Zhao, Z.-H.; Zhang, Y.; Ma, Z.-M.; Wang, S.-X. Vitamin B6 Prevents Endothelial Dysfunction, Insulin Resistance, and Hepatic Lipid Accumulation in Apoe -/- Mice Fed with High-Fat Diet. J. Diabetes Res. 2016, 2016, 1748065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaimoto, T.; Shibuya, M.; Nishikawa, K.; Maeda, H. High incidence of lipid deposition in the liver of rats fed a diet supplemented with branched-chain amino acids under vitamin B6 deficiency. J. Nutr. Sci. Vitaminol. 2013, 59, 73–78. [Google Scholar] [CrossRef] [Green Version]
- Mayengbam, S.; Raposo, S.; Aliani, M.; House, J.D. Oral exposure to the anti-pyridoxine compound 1-amino d-proline further perturbs homocysteine metabolism through the transsulfuration pathway in moderately vitamin B6 deficient rats. J. Nutr. Biochem. 2015, 26, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Mascolo, E.; Vernì, F. Vitamin B6 and Diabetes: Relationship and Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 3669. [Google Scholar] [CrossRef] [PubMed]


| Enzyme Name | Gene Name | Cofactor | Structure | Tissue Expression | Cellular Location |
|---|---|---|---|---|---|
| Adenosylhomocysteinase (SAHH) | AHCY | NAD+ | Homotetramer 432 amino acids 47.7 kDa | Low tissue specificity. Highly expressed in liver, pancreas and kidney, endocrine tissue, female and male tissue. | Cytoplasma |
| Betaine-homocysteine S methyltransferase (BHMT)—Two genes | BHMT BHMT2 | Zink | Homotetramer 406/363 amino acids 45.0/40.4 kDa | High tissue specificity. Highly expressed in liver, kidney, and urinary tract. | Cytoplasma |
| Cystathionine beta-synthase (CBS) | CBS | Pyridoxal 5′-phosphat (B6) | Homotetramer 551 amino acids 61 kDA | High tissue specificity. Highly expressed in liver and pancreas. Some expression in heart and brain. | Nucleus/cytoplasma |
| Cystathionine gamma-lyase (CSE) | CTH | Pyridoxal 5′-phosphat (B6) | Homotetramer 405 amino acids 44.5 kDa | High tissue specificity. Highly expressed in liver, female tissue and endocrine tissue. Some in the pancreas, brain, and kidneys. | Cytoplasma |
| Cysteine dioxygenase (CDO) | CDO1 | Iron | Monomer 200 amino acids 30.0 kDA | High tissue specificity. Highly expressed in liver and placenta. Some expression in heart, sdipose tissue, brain, and pancreas. | Cytoplasma |
| Cysteine sulfinic acid decarboxylase (CSD) | CSAD | Pyridoxal 5′-phosphat (B6) | Homodimer 493 amino acids 55.0 kDA | Low tissue specificity. Expressed in liver, gastrointestinal (GI) tract, brain female and male tissue, muscle tissue, and adipose tissue. | Cytoplasma |
| Glutathione peroxidase (GPO)—several genes | GPX 1–8 | GPX2—Homotetramer 190 amino acids 22.0 kDa | GPO has low tissue specificity. GPX 2 is highly expressed in the liver, gallbladder and GI tract. | Cytoplasma/mitochondrion | |
| Glutathione reductase (GR) | GSR | FAD | Homodimer 552 amino acids 56.3 kDa | Low tissue specificity. Highly expressed in liver, pancreas, GI tract, endocrine tissue, kidney, female and male tissue. | Cytoplasma/mitochondrion |
| Glutathione synthetase (GS) | GSS | Magnesium | Homodimer 474 amino acids 52.4 kDa | Low tissue specificity. Highly expressed in brain, endocrine tissue, GI tract, kidney and liver. | Cytoplasma |
| Glutamate-cysteine ligase (GCL) | GCLM GCLC | Heterodimer 274 + 252 amino acids 33.7 + 28.1 kDa | Methionine synthase Highly expressed in the liver. | Cytoplasma | |
| Glycine N-methyltransferase (GNMT) | GNMT | Homotetramer 295 amino acids 32.7 kDa | High tissue specificity. Highly expressed in liver and pancreas. Some expression in brain, GI tract, and kidney. | Cytoplasma | |
| Methionine adenosyltransferase (MAT) | MAT1A | Potasium Magnesium | Homodi- and tertramer 395 amino acids 43.6 kDA | High tissue specificity. Highly expressed in liver, pancreas. Some expression lungs and female and male tissue. | Cytoplasma |
| Methionine synthase (MS) | MTR | Cobalamin (B12) Zink | Monomer- and Dimer 1265 amino acids 140.5 kDa | Low tissue specificity. Highly expressed in pancreas, heart, brain, skeletal muscle and placenta. Expressed at lower levels in lung, liver, and kidney. | Cytoplasma |
| Methylenetetrahydrofolate reductase (MTHFR) | MTHFR | FAD | Homodimer 656 amino acids 74.6 kDA | Low tissue specificity. Highly expressed in female and male tissue, GI tract and kidney. Some expression in liver. | Cytoplasma |
| Serine hydroxymethyltransferase (SHMT)—two genes | SHMT1 SHMT2 | Pyridoxal 5′-phosphat (B6) | Homotetramer 486/504 amino acids 53.1/60.0 kDa | High tissue specificity. Highly expressed in liver and kidney. Some expression in lungs, brain, pancreas, and GI tract. | Cytoplasma/mitochondrion |
| Histology | |||||
|---|---|---|---|---|---|
| Study | Steatosis | Fibrosis | Inflammation | Ballooning | NAS |
| Brochado 2013 [111] | No | No | No | NA | NA |
| Gulsen 2005 [114] | NA | Positive | NA | NA | Positive |
| Hirsch 2005 [119] | NA | No | No | NA | No |
| Lai 2020 [116] | Positive | No | No | NA | Positive |
| Pylozos 2012 [120] | No | Negative | Negative (portal) | No | NA |
| Xu 2020 [123] | No | Negative | No | Negative | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Werge, M.P.; McCann, A.; Galsgaard, E.D.; Holst, D.; Bugge, A.; Albrechtsen, N.J.W.; Gluud, L.L. The Role of the Transsulfuration Pathway in Non-Alcoholic Fatty Liver Disease. J. Clin. Med. 2021, 10, 1081. https://doi.org/10.3390/jcm10051081
Werge MP, McCann A, Galsgaard ED, Holst D, Bugge A, Albrechtsen NJW, Gluud LL. The Role of the Transsulfuration Pathway in Non-Alcoholic Fatty Liver Disease. Journal of Clinical Medicine. 2021; 10(5):1081. https://doi.org/10.3390/jcm10051081
Chicago/Turabian StyleWerge, Mikkel Parsberg, Adrian McCann, Elisabeth Douglas Galsgaard, Dorte Holst, Anne Bugge, Nicolai J. Wewer Albrechtsen, and Lise Lotte Gluud. 2021. "The Role of the Transsulfuration Pathway in Non-Alcoholic Fatty Liver Disease" Journal of Clinical Medicine 10, no. 5: 1081. https://doi.org/10.3390/jcm10051081
APA StyleWerge, M. P., McCann, A., Galsgaard, E. D., Holst, D., Bugge, A., Albrechtsen, N. J. W., & Gluud, L. L. (2021). The Role of the Transsulfuration Pathway in Non-Alcoholic Fatty Liver Disease. Journal of Clinical Medicine, 10(5), 1081. https://doi.org/10.3390/jcm10051081






