Swept Source Optical Coherence Tomography Analysis of the Selected Eye’s Anterior Segment Parameters
Abstract
:1. Introduction
2. Materials and Methods
3. Results
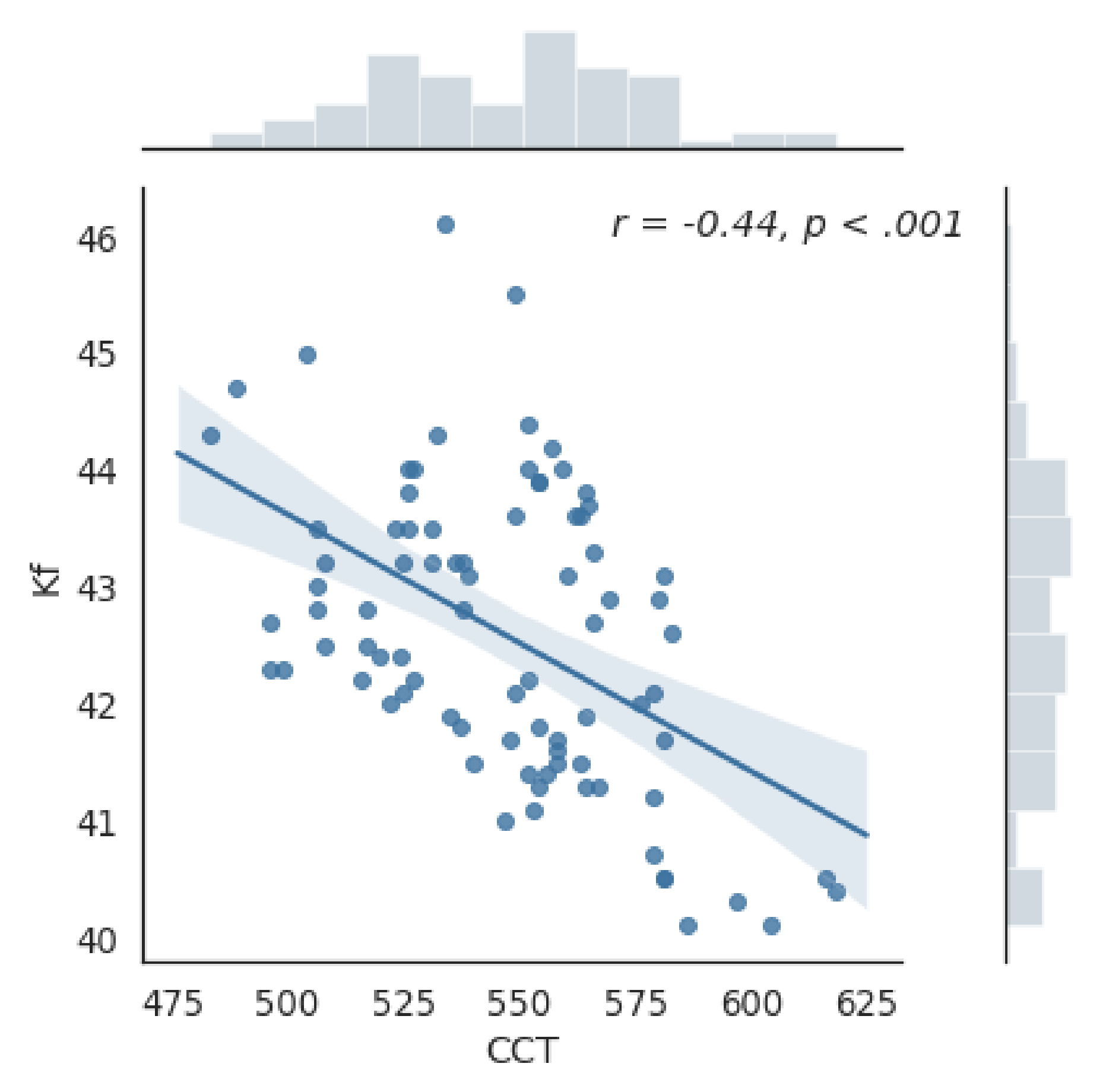
3.1. OCT Parameters of the Cornea
3.2. Pachymetry
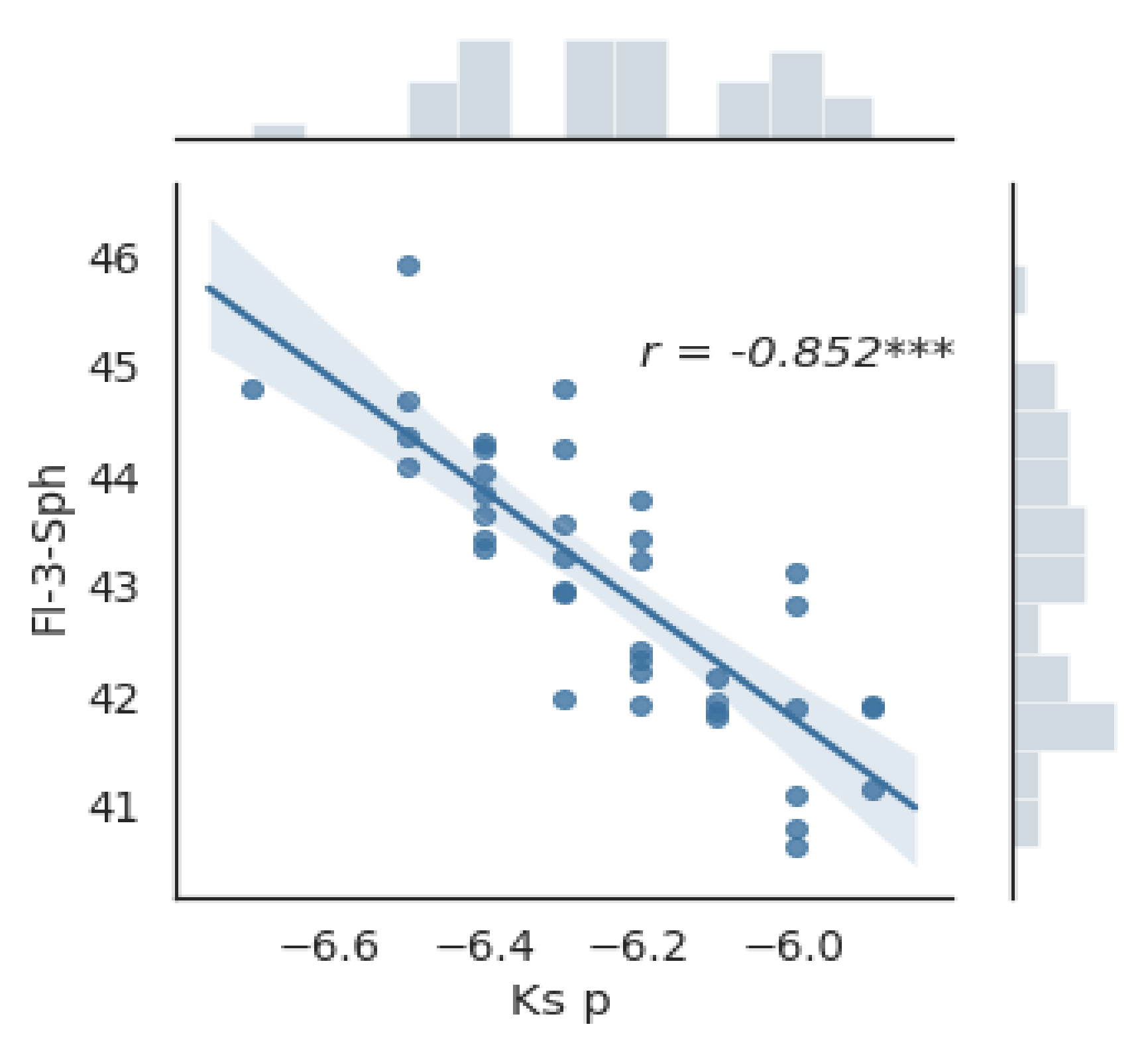
3.3. Fourier Analysis of the Cornea
3.4. Parameters of the Iridocorneal Angle
3.5. Iris Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ks | keratometry steep |
| Kf | keratometry flat |
| pKs | posterior keratometry steep |
| pKf | posterior kerayometry flat |
| CCT | central corneal thickness |
| Fi3(6)-Sph | Fourier index spherical |
| Fi3(6)-Reg | Fourier index regular |
| Fi3(6)-Asy | Fourier index asymmetry |
| Fi3-HO | Furier index Higher Order |
| Tilt | Tilt |
| Decent | Decentralization |
| ACD | Anterior chamber depth |
| ACV | Anterior chamber volume |
| AOD 250,500,750 | Angle opening distance |
| ARA 250,500,750 | Angle recess area |
| TISA 250,500,750 | Trabecular iris space area |
| TIA 250,500,750 | Trabecular iris angle |
| IT 750 | Iris thickness 750 mm |
| IT 2000 | Iris thickness 2000 mm |
| I-Curv | Iris Curvature |
| I-Area | Iris Area |
References
- Pavlin, C.J.; Harasiewicz, K.; Foster, F.S. Ultrasound biomicroscopy of anterior segment structures in normal and glaucomatous eyes. Am. J. Ophthalmol. 1992, 113, 381–389. [Google Scholar] [CrossRef]
- Lee, Y.E.; Joo, C.-K. Assessment of Lens Center Using Optical Coherence Tomography, Magnetic Resonance Imaging, and Photographs of the Anterior Segment of the Eye. Investig. Opthalmol. Vis. Sci. 2015, 56, 5512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radhakrishnan, S.; Rollins, A.M.; Roth, J.E.; Yazdanfar, S.; Westphal, V.; Bardenstein, D.S.; Izatt, J.A. Real-Time Optical Coherence Tomography of the Anterior Segment at 1310 nm. Arch. Ophthalmol. 2001, 119, 1179–1185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabsilber, T.M.; Khoramnia, R.; Auffarth, G.U. Anterior chamber measurements using Pentacam rotating Scheimpflug camera. J. Cataract Refract. Surg. 2006, 32, 456–459. [Google Scholar] [CrossRef]
- Ponce, C.M.P.; Rocha, K.M.; Smith, S.D.; Krueger, R.R. Central and peripheral corneal thickness measured with optical coherence tomography, Scheimpflug imaging, and ultrasound pachymetry in normal, keratoconus-suspect, and post-laser in situ keratomileusis eyes. J. Cataract Refract. Surg. 2009, 35, 1055–1062. [Google Scholar] [CrossRef]
- Wylęgała, E.; Teper, S.; Nowińska, A.K.; Milka, M.; Dobrowolski, D. Anterior segment imaging: Fourier-domain optical coherence tomography versus time-domain optical coherence tomography. J. Cataract Refract. Surg. 2009, 35, 1410–1414. [Google Scholar] [CrossRef]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.B.; E Cornish, E.; Grigg, J.R.; McCluskey, P.J. Anterior segment optical coherence tomography and its clinical applications. Clin. Exp. Optom. 2019, 102, 195–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pujari, A.; Agarwal, D.; Chawla, R.; Kumar, A.; Sharma, N. Intraoperative Optical Coherence Tomography Guided Ocular Surgeries: Critical Analysis of Clinical Role and Future Perspectives. Clin. Ophthalmol. 2020, 14, 2427–2440. [Google Scholar] [CrossRef]
- Wojtkowski, M.; Bajraszewski, T.; Targowski, P.; Kowalczyk, A. Real-time in vivo imaging by high-speed spectral optical coherence tomography. Opt. Lett. 2003, 28, 1745–1747. [Google Scholar] [CrossRef]
- Kaluzny, B.J.; Kałuzny, J.J.; Szkulmowska, A.; Gorczyńska, I.; Szkulmowski, M.; Bajraszewski, T.; Wojtkowski, M.; Targowski, P. Spectral optical coherence tomography: A novel technique for cornea imaging. Cornea 2006, 25, 960–965. [Google Scholar] [CrossRef]
- Yasuno, Y.; Madjarova, V.D.; Makita, S.; Akiba, M.; Morosawa, A.; Chong, C.; Sakai, T.; Chan, K.-P.; Itoh, M.; Yatagai, T. Three-dimensional and high-speed swept-source optical coherence tomography for in vivo investigation of human anterior eye segments. Opt. Express 2005, 13, 10652–10664. [Google Scholar] [CrossRef] [Green Version]
- Szalai, E.; Berta, A.; Hassan, Z.; Módis, L. Reliability and repeatability of swept-source Fourier-domain optical coherence tomography and Scheimpflug imaging in keratoconus. J. Cataract Refract. Surg. 2012, 38, 485–494. [Google Scholar] [CrossRef]
- Fukuda, S.; Kawana, K.; Yasuno, Y.; Oshika, T. Repeatability and reproducibility of anterior ocular biometric measurements with 2-dimensional and 3-dimensional optical coherence tomography. J. Cataract Refract. Surg. 2010, 36, 1867–1873. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Kato, N.; Ishikawa, S.; Ibuki, H.; Yamada, N.; Kimura, I.; Shinoda, K. In vivo crystalline lens measurements with novel swept-source optical coherent tomography: An investigation on variability of measurement. BMJ Open Ophthalmol. 2017, 1, e000058. [Google Scholar] [CrossRef] [Green Version]
- Palazon-Cabanes, A.; Palazon-Cabanes, B.; Rubio-Velazquez, E.; Lopez-Bernal, M.D.; Garcia-Medina, J.J.; Villegas-Perez, M.P. Normative Database for All Retinal Layer Thicknesses Using SD-OCT Posterior Pole Algorithm and the Effects of Age, Gender and Axial Length. J. Clin. Med. 2020, 9, E3317. [Google Scholar] [CrossRef]
- Gali, H.E.; Sella, R.; Afshari, N.A. Cataract grading systems: A review of past and present. Curr. Opin. Ophthalmol. 2019, 30, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Sharif, N.M.; Yazdani, N.; Shahkarami, L.; Moghaddam, H.O.; Ehsaei, A. Analysis of Age, Gender, and Refractive Error-Related Changes of the Anterior Corneal Surface Parameters Using Oculus Keratograph Topography. J. Curr. Ophthalmol. 2020, 32, 263–267. [Google Scholar]
- Xie, X.; Corradetti, G.; Song, A.; Pardeshi, A.; Sultan, W.; Lee, J.Y.; Yu, F.; Zhang, L.; Chen, S.; Chopra, V.; et al. Age- and refraction-related changes in anterior segment anatomical structures measured by swept-source anterior segment OCT. PLoS ONE 2020, 15, e0240110. [Google Scholar] [CrossRef]
- Hua, Y.; Stojanovic, A.; Utheim, T.P.; Chen, X.; Ræder, S.; Huang, J.; Wang, Q. Keratometric Index Obtained by Fourier-Domain Optical Coherence Tomography. PLoS ONE 2015, 10, e0122441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, Y.H.; Kim, H.K.; Sohn, Y.H. Central Corneal Thickness in a Korean Population: The Namil Study. Investig. Opthalmol. Vis. Sci. 2012, 53, 6851–6855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Dong, J.; Wu, Q. Corneal thickness, epithelial thickness and axial length differences in normal and high myopia. BMC Ophthalmol. 2015, 15, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, M.; Chen, A.; Li, Y.; Huang, D. Corneal power measurement with Fourier-domain optical coherence tomography. J. Cataract Refract. Surg. 2010, 36, 2115–2122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanabe, T.; Tomidokoro, A.; Samejima, T.; Miyata, K.; Sato, M.; Kaji, Y.; Oshika, T. Corneal regular and irregular astigmatism assessed by Fourier analysis of video-keratography data in normal and pathologic eyes. Ophthalmology 2004, 111, 752–757. [Google Scholar] [CrossRef]
- Phillips, C.I. Closed-angle glaucoma significance of sectoral variations in angle depth. Br. J. Ophthalmol. 1956, 40, 136–143. [Google Scholar] [CrossRef] [Green Version]
- Kunimatsu, S.; Tomidokoro, A.; Mishima, K.; Takamoto, H.; Tomita, G.; Iwase, A.; Araie, M. Prevalence of appositional angle closure determined by ultrasonic biomicroscopy in eyes with shallow anterior chambers. Ophthalmology 2005, 112, 407–412. [Google Scholar] [CrossRef]
- Narayanaswamy, A.; Sakata, L.M.; He, M.G.; Friedman, D.S.; Chan, Y.-H.; Lavanya, R.; Baskaran, M.; Foster, P.J. Diagnostic Performance of Anterior Chamber Angle Measurements for Detecting Eyes with Narrow Angles: An Anterior Segment OCT Study. Arch. Ophthalmol. 2010, 128, 1321–1327. [Google Scholar] [CrossRef]
- Chan, P.P.-M.; Lai, G.; Chiu, V.; Chong, A.; Yu, M.; Leung, C.K.-S. Anterior chamber angle imaging with swept-source optical coherence tomography: Comparison between CASIAII and ANTERION. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Siguan-Bell, C.S.; Chansangpetch, S.; Perez, C.I.; Kutzscher, A.; Wang, D.; He, M.; Oldenburg, C.; Hee, M.R.; Lin, S.C. Anterior Segment Parameters of Filipino-Americans Compared to Chinese-Americans and Caucasian Americans Using Anterior Segment Optical Coherence Tomography. Transl. Vis. Sci. Technol. 2019, 8, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, K.; Kondo, M.; Takeuchi, M.; Hirano, K. Refractive error and biometrics of anterior segment of eyes of healthy young university students in Japan. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Sakata, L.M.; Lavanya, R.; Friedman, D.S.; Aung, H.T.; Gao, H.; Kumar, R.S.; Foster, P.J.; Aung, T. Comparison of Gonioscopy and Anterior Segment Ocular Coherence Tomography in Detecting Angle Closure in Different Quadrants of the Anterior Chamber Angle. Ophthalmology 2008, 115, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Nongpiur, M.E.; Haaland, B.A.; Friedman, D.S.; Perera, S.A.; He, M.; Foo, L.-L.; Baskaran, M.; Sakata, L.M. Classification algorithms based on anterior segment optical coherence tomography measure-ments for detection of angle closure. Ophthalmology 2013, 120, 48–54. [Google Scholar] [CrossRef]
- Alonso, R.S.; Junior, R.A.; Junior, A.P.; Sakata, L.M.; Ventura, M.P. Glaucoma anterior chamber morphometry based on optical Scheimpflug images. Arq. Bras. Oftalmol. 2010, 73, 497–500. [Google Scholar] [CrossRef] [Green Version]
- Dabasia, P.L.; Edgar, D.F.; Murdoch, I.E.; Lawrenson, J.G. Noncontact Screening Methods for the Detection of Narrow Anterior Chamber Angles. Investig. Opthalmol. Vis. Sci. 2015, 56, 3929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grewal, D.S.; Brar, G.S.; Jain, R.; Grewal, S.P.S. Comparison of Scheimpflug imaging and spectral domain anterior segment optical coherence tomography for detection of narrow anterior chamber angles. Eye 2011, 25, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Kurita, N.; Mayama, C.; Tomidokoro, A.; Aihara, M.; Araie, M. Potential of the Pentacam in Screening for Primary Angle Closure and Primary Angle Closure Suspect. J. Glaucoma 2009, 18, 506–512. [Google Scholar] [CrossRef]
- Li, F.; Zhou, R.; Gao, K.; Jin, L.; Zhang, X. Volumetric parameters-based differentiation of narrow angle from open angle and classification of angle configurations: An SS-OCT study. Br. J. Ophthalmol. 2020, 104, 92–97. [Google Scholar] [CrossRef]

| Parameter | Development of an English Abbreviation | Description of the Parameter | Unit |
|---|---|---|---|
| Cornea Parameters | |||
| Ks | Keratometry steep | steep meridian value of keratometry | (D)—diopter |
| Kf | KKe Keratometry flat | flat meridian value of keratometry | (D)—diopter |
| pKs | Posterior keratometry steep | value of the steep meridian of the posterior curvature of the cornea | (D)—diopter |
| pKf | Posterior keratometry flat | value of the flat meridian of the posterior curvature of the cornea | (D)—diopter |
| CCT | Central corneal thickness | corneal thickness in the axis of the examination (central) | (µm)—micrometers |
| Fi3(6)-Sph | Fourier index spherical | the spherical refractive power component of the cornea obtained by Fourier analysis of the topographic data of the cornea with a diameter of 3, (6) mm. Corresponds to the zero-order component in Fourier analysis | (D)—diopter |
| Fi3(6)-Reg | Fourier index regular | the component of regular corneal refractive power astigmatism obtained by Fourier analysis of corneal topographic data with a diameter of 3, (6) mm. Corresponds to the second order component in the Fourier analysis | (D)—diopter |
| Fi3(6)-Asy | Fourier index assymetry | asymmetric refractive power component of the cornea obtained by Fourier analysis of corneal topographic data with a diameter of 3, (6) mm. Corresponds to the first-order component in Fourier analysis | (D)—diopter |
| Fi3-HO | Fourier index Higher Order | higher order irregularities—calculated by combining tertiary and higher components in Fourier analysis | (D)—diopter |
| Lens parameters | |||
| Tilt | Tilt | the tilt of the lens axis in relation to the vision axis | (°)—degrees |
| Decent | Decentralization | the decentration of the lens in relation to the visual axis | (mm)—millimeter |
| Filtration angle parameters | |||
| ACD | Anterior chamber depth | Anterior chamber depth | (mm)—millimeter |
| ACV | Anterior chamber volume | Anterior chamber volume | (mm3)—cubic millimeter |
| AOD 250,500,750 | Angle opening distance | angle opening distance—the distance between two points located on the posterior surface of the cornea and the front surface of the iris at a distance of 250,500,750 µm from the peak of the filtration angle | (mm)—millimeter |
| ARA 250,500,750 | Angle recess area | the recess-side area of the filtration angle delimited by a section defining AOD250,500,750 | (mm2)—square millimeter |
| TISA 250,500,750 | Trabecular Iris space area | the area obtained by subtracting from the ARA250 surface the area of the cavity of the angle delimited by the segment joining the spur of the sclera from its mirror point determined on the iris | (mm2)—square millimeter |
| TIA 250,500,750 | Trabecular Iris angle | angle defined by 3 points—peak of the filtration angle, and points AOD250,500,750 | (°)—degrees |
| Study Group (n = 166) | Women (n = 92) | Men (n = 74) | Average Difference | p | ||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Mean ± Standard Deviation | IC 95 | Mean ± Standard Deviation | IC 95 | Mean ± Standard Deviation | IC95 | ||
| Ks(D) | 43.63±1.3 | 43.34–43.92 | 44.09 ±1.2 | 43.73–44.44 | 43.07 ±1.23 | 42.65–43.47 | 1.02 | <0.0001* |
| Kf(D) | 42.59 ± 1.27 | 42.32–42.87 | 43.1 ±1.14 | 42.76–43.44 | 41.96 ±1.16 | 41.57–42.34 | 1.14 | <0.0001* |
| CCT (µm) | 546.65 ± 28.69 | 540–553 | 540.87 ±21.52 | 534–547 | 553.84 ± 34.64 | 542–565 | 12.9 | 0.04 * |
| ACD (mm) | 3.1 ± 0.31 | 3.03–3.17 | 3.04 ±0.25 | 2.96–3.11 | 3.18 ±0.36 | 3.06–3.3 | 0.14 | 0.04 * |
| ACV (mm3) | 154.6 ± 37.42 | 134.4–178.3 | 146.7 ± 34.67 | 123.5–164.6 | 167.5 ± 41.45 | 140.1 – 182.8 | 20.3 | 0.11 * |
| FI-3-Sph (D) | 43.09 ± 1.27 | 42.81–43.37 | 43.58 ±1.16 | 43.24–43.93 | 42.48 ±1.15 | 42.1–42.86 | 1.1 | <0.0001 * |
| FI-6-Sph (D] | 42.91 ± 1.25 | 42.64–43.19 | 43.40 ±1.13 | 43.06–43.73 | 42.32 ±1.44 | 41.93–42.7 | 1.08 | <0.0001 * |
| TILT (°) | 5.08 ± 1.2 | 4.82–5.35 | 4.9 ±1.02 | 4.59–5.2 | 5.31 ± 1.37 | 4.85–5.75 | 0.41 | 0.13 * |
| Comparison of the results of the non—normally distributed parameters of the anterior segment in the studied groups | ||||||||
| Study group (n = 166) | Women (n = 92) | Men (n = 74) | p | |||||
| Parameter | Median | Interquartile range | Median | Interquartile range | Median | Interquartile range | ||
| pKs(D) | −6.3 | (−6.4)–(−6.1) | -6.3 | (−6.5)–(−6.2) | −6.2 | (−6.3)–(−6.0) | <0.001** | |
| pKf (D) | −5.9 | (−6.1)–(−5.8) | -5.95 | (−6.1)–(−5.9) | −5.8 | (−6.0)–(−5.7) | <0.01 ** | |
| FI-3-Reg (D) | 0.48 | 0.33–0.65 | 0.485 | 0.34–0.63 | 0.47 | 0.28–0.69 | 0.8 ** | |
| FI-3-Asy (D) | 0.22 | 0.14–0.31 | 0.24 | 0.16–0.30 | 0.20 | 0.14–0.31 | 0.53 ** | |
| FI-3-HO (D) | 0.15 | 0.12–0.17 | 0.14 | 0.11–0.17 | 0.15 | 0.13–0.18 | 0.267 ** | |
| FI-6-Reg (D) | 0.47 | .32–0.67 | 0.46 | 0.36–0.63 | 0.53 | 0.31–0.68 | 0.68 ** | |
| FI-6-Asy (D) | 0.31 | 0.20–0.40 | 0.33 | 0.23–0.40 | 0.23 | 0.17–0.36 | 0.19 ** | |
| FI-6-HO (D) | 0.16 | 0.14–0.19 | 0.16 | 0.13–0.20 | 0.16 | 0.15–0.18 | 0.48 ** | |
| Decent (mm) | 0.18 | 0.14–0.27 | 0.17 | 0.13–0.23 | 0.19 | 0.16–0.31 | 0.16 ** | |
| Parameter. | Mean ± Standard Deviation | IC95 | Mean ± Standard Deviation | IC95 | Mean ± Standard Deviation | IC95 | Mean ± Standard Deviation | IC95 |
|---|---|---|---|---|---|---|---|---|
| 0° | 90° | 180° | 270° | |||||
| 250 µm | ||||||||
| AOD (mm) | 0.39 ± 0.19 | 0.34–0.43 | 0.38 ± 0.13 | 0.33–0.43 | 0.39 ± 0.15 | 0.36–0.42 | 0.46 ± 0.2 | 0.40–0.52 |
| ARA (mm2) | 0.1 ± 0.05 | 0.09–0.12 | 0.09 ± 0.04 | 0.08–0.11 | 0.1 ± 0.04 | 0.09–0.11 | 0.13 ± 0.07 | 0.11–0.15 |
| TISA (mm2) | 0.08 ± 0.04 | 0.07–0.09 | 0.08 ± 0.03 | 0.07–0.09 | 0.08 ± 0.03 | 0.07–0.09 | 0.10 ± 0.04 | 0.08–0.11 |
| TIA (°) | 52.4 ± 13.73 | 47.4–57.3 | 50.6 ± 14.37 | 47.5–53.6 | 52.1 ± 13.96 | 49.1–55.1 | 54.6 ± 14.66 | 50.6–58.5 |
| 500 µm | ||||||||
| AOD (mm) | 0.57 ± 0.25 | 0.51–0.62 | 0.53 ± 0.18 | 0.46–0.6 | 0.56 ± 0.21 | 0.51–0.61 | 0.65 ± 0.27 | 0.58–0.73 |
| ARA (mm2) | 0.22 ± 0.11 | 0.19–0.24 | 0.21 ± 0.07 | 0.18–0.24 | 0.22 ± 0.09 | 0.2–0.24 | 0.27 ± 0.13 | 0.23–0.3 |
| TISA (mm2) | 0.2 ± 0.09 | 0.18–0.22 | 0.19 ± 0.06 | 0.17–0.22 | 0.2 ± 0.07 | 0.18–0.22 | 0.24 ± 0.1 | 0.21–0.26 |
| TIA (°) | 44.3 ± 12.1 | 41.6–46.8 | 43.7 ± 11.8 | 39.5–48.0 | 44.9 ± 11.8 | 42.3–47.4 | 47.9 ± 13.8 | 44.2–51.6 |
| 750 µm | ||||||||
| AOD (mm) | 0.76 ± 0.31 | 0.69–0.82 | 0.69 ± 0.22 | 0.61–0.77 | 0.76 ± 0.27 | 0.7–0.82 | 0.86 ± 0.31 | 0.78–0.95 |
| ARA (mm2) | 0.39 ± 0.17 | 0.35–0.43 | 0.36 ± 0.12 | 0.32–0.41 | 0.39 ± 0.14 | 0.35–0.42 | 0.46 ± 0.2 | 0.4–0.51 |
| TISA (mm2) | 0.37 ± 0.16 | 0.33–0.4 | 0.35 ± 0.11 | 0.3–0.39 | 0.37 ± 0.13 | 0.34–0.4 | 0.43 ± 0.17 | 0.38–0.47 |
| TIA (°) | 42.15 ± 11.3 | 39.7–44.6 | 40.6 ± 10.1 | 37.0–44.3 | 42.7 ± 10.8 | 40.3–45.0 | 45.7 ± 12.1 | 42.5–48.9 |
| Iris parameters | ||||||||
| IT 750 (mm) | 0.37 ± 0.07 | 0.35–0.39 | 0.37 ± 0.08 | 0.34–0.4 | 0.36 ± 0.06 | 0.35–0.38 | 0.36 ± 0.06 | 0.35–0.39 |
| IT 2000 (mm) | 0.42 ± 0.07 | 0.4–0.44 | 0.49 ± 0.08 | 0.46–0.52 | 0.41 ± 0.07 | 0.39–0.43 | 0.41 ± 0.07 | 0.39–0.42 |
| I-Curv(mm) | 0.042 ± 0.11 | 0.01–0.06 | 0.048 ± 0.09 | 0.01–0.09 | 0.054 ± 0.1 | 0.03–0.07 | 0.046 ± 0.09 | 0.02–0.07 |
| I-Area (mm2) | 1.29 ± 0.17 | 1.25–1.32 | 1.39 ± 0.15 | 1.32–1.46 | 1.25 ± 0.17 | 1.21–1.29 | 1.26 ± 0.16 | 1.22–1.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dembski, M.; Nowińska, A.; Ulfik-Dembska, K.; Wylęgała, E. Swept Source Optical Coherence Tomography Analysis of the Selected Eye’s Anterior Segment Parameters. J. Clin. Med. 2021, 10, 1094. https://doi.org/10.3390/jcm10051094
Dembski M, Nowińska A, Ulfik-Dembska K, Wylęgała E. Swept Source Optical Coherence Tomography Analysis of the Selected Eye’s Anterior Segment Parameters. Journal of Clinical Medicine. 2021; 10(5):1094. https://doi.org/10.3390/jcm10051094
Chicago/Turabian StyleDembski, Michał, Anna Nowińska, Klaudia Ulfik-Dembska, and Edward Wylęgała. 2021. "Swept Source Optical Coherence Tomography Analysis of the Selected Eye’s Anterior Segment Parameters" Journal of Clinical Medicine 10, no. 5: 1094. https://doi.org/10.3390/jcm10051094
APA StyleDembski, M., Nowińska, A., Ulfik-Dembska, K., & Wylęgała, E. (2021). Swept Source Optical Coherence Tomography Analysis of the Selected Eye’s Anterior Segment Parameters. Journal of Clinical Medicine, 10(5), 1094. https://doi.org/10.3390/jcm10051094







