Development and Validation of the Pain and State of Health Inventory (PHI): Application for the Perioperative Setting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Development Process
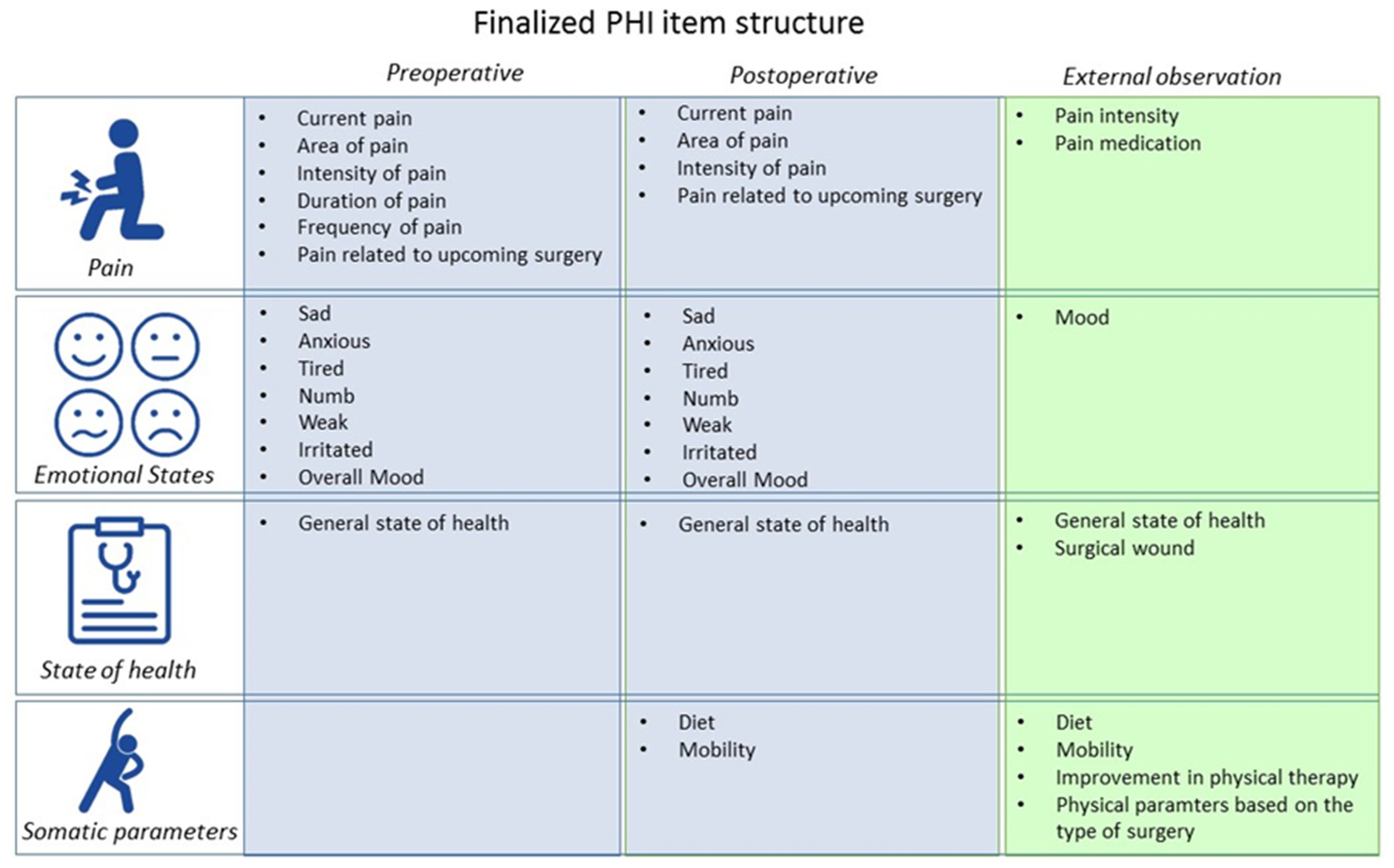
2.1.1. Conceptual Structure
2.1.2. Pre- and Postoperative Pain
2.1.3. State of Health
2.1.4. Somatic Parameters
2.1.5. Application of the Inventory
2.1.6. Preliminary Studies to Test the First Editions
2.1.7. Finalized PHI
2.2. Validation
2.2.1. Participants
2.2.2. Study Design and Materials
2.2.3. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Acceptance of the Inventory
3.3. Pain
3.3.1. Painful Areas
3.3.2. Pain Intensity, Frequency, and Duration
3.4. Factor Loading Pre- and Postoperatively for Emotional States
3.5. Sensitivity to Changes
3.6. Internal Validity
3.6.1. Pain
3.6.2. Emotional State
3.6.3. General State
3.6.4. Somatic Parameters
4. Discussion
4.1. Strengths of the Study
4.2. Limitations of the Study
4.3. Future Research Steps
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, C.L.; Rowlingson, A.J.; Partin, A.W.; Kalish, M.A.; Courpas, G.E.; Walsh, P.C.; Fleisher, L.A. Correlation of postoperative pain to quality of recovery in the immediate postoperative period. Reg. Anesth. Pain Med. 2005, 30, 516–522. [Google Scholar] [CrossRef]
- Wu, C.L.; Richman, J.M. Postoperative pain and quality of recovery. Curr. Opin. Anaesthesiol. 2004, 17, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.H.; A McGlynn, E.; Cleary, P.D. Quality of health care. Part 2: Measuring quality of care. J. Head Trauma Rehabil. 1997, 12, 101–102. [Google Scholar] [CrossRef]
- Kluivers, K.B.; Riphagen, I.; Vierhout, M.E.; Brölmann, H.A.; De Vet, H.C. Systematic review on recovery specific quality-of-life instruments. Surgery 2008, 143, 206–215. [Google Scholar] [CrossRef]
- Myles, P.S.; Hunt, J.O.; Nightingale, C.E.; Fletcher, H.; Beh, T.; Tanil, D.; Nagy, A.; Rubinstein, A.; Ponsford, J.L. Development and psychometric testing of a quality of recovery score after general anesthesia and surgery in adults. Anesth. Analg. 1999, 88, 83–90. [Google Scholar] [PubMed]
- Wu, C.L.; Fleisher, L.A. Outcomes research in regional anesthesia and analgesia. Anesth. Analg. 2000, 91, 1232–1242. [Google Scholar] [PubMed]
- Myles, P.S. More than just morbidity and mortality—Quality of recovery and long-term functional recovery after surgery. Anaesthesia 2020, 75 (Suppl. 1), e143–e150. [Google Scholar] [CrossRef]
- Bisgaard, T.; Rosenberg, J.; Kehlet, H. From acute to chronic pain after laparoscopic cholecystectomy: A prospective follow-up analysis. Scand. J. Gastroenterol. 2005, 40, 1358–1364. [Google Scholar] [CrossRef]
- Perkins, F.M.; Kehlet, H. Chronic Pain as an Outcome of Surgery. Anesthesiology 2000, 93, 1123–1133. [Google Scholar] [CrossRef] [Green Version]
- García-Miguel, F.; Serrano-Aguilar, P.; López-Bastida, J. Preoperative assessment. Lancet 2003, 362, 1749–1757. [Google Scholar] [CrossRef]
- Djulbegovic, B.; Guyatt, G.H. Progress in evidence-based medicine: A quarter century on. Lancet 2017, 390, 415–423. [Google Scholar] [CrossRef]
- Bowyer, A.J.; Royse, C.F. Postoperative recovery and outcomes—What are we measuring and for whom? Anaesthesia 2015, 71, 72–77. [Google Scholar] [CrossRef] [Green Version]
- Institute of Medicine (IOM). Crossing the Quality Chasm: A New Health System for the 21st Century; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Harrington, L. Quality measurement. In Medical Quality Management: Theory and Practice; Giardino, A.P., Riesenberg, L.A., Varkey, P., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 33–51. [Google Scholar]
- Gittell, J.H.; Fairfield, K.M.; Bierbaum, B.; Head, W.; Jackson, R.; Kelly, M.; Laskin, R.; Lipson, S.; Siliski, J.; Thornhill, T.; et al. Impact of relational coordination on quality of care, postoperative pain and functioning, and length of stay: A nine-hospital study of surgical patients. Med. Care 2000, 38, 807–819. [Google Scholar] [CrossRef]
- Salmon, P.; Hall, G.M.; Peerbhoy, D.; Shenkin, A.; Parker, C. Recovery from hip and knee arthroplasty: Patients’ perspective on pain, function, quality of life, and well-being up to 6 months postoperatively. Arch. Phys. Med. Rehabil. 2001, 82, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Donovan, M.; Dillon, P.; McGuire, L. Incidence and characteristics of pain in a sample of medical-surgical inpatients. Pain 1987, 30, 69–78. [Google Scholar] [CrossRef]
- Marks, R.M.; Sachar, E.J. Undertreatment of Medical Inpatients with Narcotic Analgesics. Surv. Anesthesiol. 1973, 17, 515–516. [Google Scholar] [CrossRef]
- Sommer, M.; De Rijke, J.M.; Van Kleef, M.; Kessels, A.G.H.; Peters, M.L.; Geurts, J.W.J.M.; Gramke, H.-F.; Marcus, M.A.E. The prevalence of postoperative pain in a sample of 1490 surgical inpatients. Eur. J. Anaesthesiol. 2008, 25, 267–274. [Google Scholar] [CrossRef]
- Apfelbaum, J.L.; Chen, C.; Mehta, S.S.; Gan, A.T.J. Postoperative Pain Experience: Results from a National Survey Suggest Postoperative Pain Continues to Be Undermanaged. Anesth. Analg. 2003, 97, 534–540. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.L.; Raja, S.N. Treatment of acute postoperative pain. Lancet 2011, 377, 2215–2225. [Google Scholar] [CrossRef]
- Kehlet, H.; Wilmore, D.W. Evidence-Based Surgical Care and the Evolution of Fast-Track Surgery. Ann. Surg. 2008, 248, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Kehlet, H.; Wilmore, D.W. Multimodal strategies to improve surgical outcome. Am. J. Surg. 2002, 183, 630–641. [Google Scholar] [CrossRef]
- Goldberg, D.S.; McGee, S.J. Pain as a global public health priority. BMC Public Heal. 2011, 11, 770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fishbain, D.A.; Cutler, R.; Rosomoff, H.L.; Rosomoff, R.S. Chronic Pain-Associated Depression: Antecedent or Consequence of Chronic Pain? A Review. Clin. J. Pain 1997, 13, 116–137. [Google Scholar] [CrossRef]
- McWilliams, L.A.; Cox, B.J.; Enns, M.W. Mood and anxiety disorders associated with chronic pain: An examination in a nationally representative sample. Pain 2003, 106, 127–133. [Google Scholar] [CrossRef]
- Michaelides, A.; Zis, P. Depression, anxiety and acute pain: Links and management challenges. Postgrad. Med. 2019, 131, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Bruce, J.; on behalf of the Recovery Study Group; Thornton, A.J.; Scott, N.W.; Marfizo, S.; Powell, R.; Johnston, M.; Wells, M.; Heys, S.D.; Thompson, A.M. Chronic preoperative pain and psychological robustness predict acute postoperative pain outcomes after surgery for breast cancer. Br. J. Cancer 2012, 107, 937–946. [Google Scholar] [CrossRef] [Green Version]
- Pinto, P.R.; McIntyre, T.; Almeida, A.; Araújo-Soares, V. The mediating role of pain catastrophizing in the relationship between presurgical anxiety and acute postsurgical pain after hysterectomy. Pain 2012, 153, 218–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theunissen, M.; Peters, M.L.; Bruce, J.; Gramke, H.F.; Marcus, M.A. Preoperative anxiety and catastrophizing: A systematic review and meta-analysis of the association with chronic postsurgical pain. Clin. J. Pain 2012, 28, 819–841. [Google Scholar] [CrossRef]
- Khan, R.S.; Ahmed, K.; Blakeway, E.; Skapinakis, P.; Nihoyannopoulos, L.; Macleod, K.; Sevdalis, N.; Ashrafian, H.; Platt, M.; Darzi, A.; et al. Catastrophizing: A predictive factor for postoperative pain. Am. J. Surg. 2011, 201, 122–131. [Google Scholar] [CrossRef]
- Sjöling, M.; Nordahl, G.; Olofsson, N.; Asplund, K. The impact of preoperative information on state anxiety, postoperative pain and satisfaction with pain management. Patient Educ. Couns. 2003, 51, 169–176. [Google Scholar] [CrossRef]
- Trivedi, M.H. The link between depression and physical symptoms. Prim. Care Companion J. Clin. Psychiatry 2004, 6 (Suppl. 1), 12. [Google Scholar]
- Klinger, R.; Krug, F.; Goßmann, M.; Damzog, U.; Dahme, B.; Bruch, H.-P. Das perioperative schmerz- und befindlichkeitsinventar: Ein fragebogen zur erfassung der perioperativen lebensqualität. Der. Schmerz. 1999, 13, 86. [Google Scholar]
- Chapman, C.R.; Casey, K.L.; Dubner, R.; Foley, K.M.; Gracely, R.H.; Reading, A.E. Pain measurement: An overview. Pain 1985, 22, 1–31. [Google Scholar] [CrossRef]
- Thong, I.S.K.; Jensen, M.P.; Miró, J.; Tan, G. The validity of pain intensity measures: What do the NRS, VAS, VRS, and FPS-R measure? Scand. J. Pain 2018, 18, 99–107. [Google Scholar] [CrossRef]
- Jensen, M.P.; Karoly, P. Self-report scales and procedures for assessing pain in adults. In Handbook of Pain Assessment, 3rd ed.; The Guilford Press: New York, NY, USA, 2011; pp. 19–44. [Google Scholar]
- Hjermstad, M.J.; Fayers, P.M.; Haugen, D.F.; Caraceni, A.; Hanks, G.W.; Loge, J.H.; Fainsinger, R.; Aass, N.; Kaasa, S. Studies Comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for Assessment of Pain Intensity in Adults: A Systematic Literature Review. J. Pain Symptom Manag. 2011, 41, 1073–1093. [Google Scholar] [CrossRef] [PubMed]
- Karcioglu, O.; Topacoglu, H.; Dikme, O.; Dikme, O. A systematic review of the pain scales in adults: Which to use? Am. J. Emerg. Med. 2018, 36, 707–714. [Google Scholar] [CrossRef]
- Karoly, P.; Jensen, M.P. Multimethod Assessment of Chronic Pain: Psychology Practitioner Guidebooks; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Wulf, H. Die Behandlung Akuter Perioperativer Und Posttraumatischer Schmerzen: Empfehlungen Einer Interdisziplinären Expertenkommission; Thieme: Stuttgart, Germany, 1997. [Google Scholar]
- Schott, G.D. The cartography of pain: The evolving contribution of pain maps. Eur. J. Pain 2010, 14, 784–791. [Google Scholar] [CrossRef]
- Shaballout, N.; Neubert, T.-A.; Boudreau, S.; Beissner, F. From Paper to Digital Applications of the Pain Drawing: Systematic Review of Methodological Milestones. JMIR mHealth uHealth 2019, 7, e14569. [Google Scholar] [CrossRef] [Green Version]
- Hamill, J.K.; Lyndon, M.; Liley, A.; Hill, A.G. Where it hurts: A systematic review of pain-location tools for children. Pain 2014, 155, 851–858. [Google Scholar] [CrossRef]
- Haefeli, M.; Elfering, A. Pain assessment. Eur. Spine J. 2006, 15 (Suppl. 1), S17–S24. [Google Scholar] [CrossRef] [PubMed]
- Aluoja, A.; Shlik, J.; Vasar, V.; Luuk, K.; Leinsalu, M. Development and psychometric properties of the Emotional State Questionnaire, a self-report questionnaire for depression and anxiety. Nord. J. Psychiatry 1999, 53, 443–449. [Google Scholar]
- Lopez-Delgado, J.C.; Rio, G.M.-D.; Flordelís-Lasierra, J.L.; Putzu, A. Nutrition in Adult Cardiac Surgery: Preoperative Evaluation, Management in the Postoperative Period, and Clinical Implications for Outcomes. J. Cardiothorac. Vasc. Anesth. 2019, 33, 3143–3162. [Google Scholar] [CrossRef]
- Klein, J.D.; Hey, L.A.; Yu, C.S.; Klein, B.B.; Coufal, F.J.; Young, E.P.; Marshall, L.F.; Garfin, S.R. Perioperative Nutrition and Postoperative Complications in Patients Undergoing Spinal Surgery. Spine 1996, 21, 2676–2682. [Google Scholar] [CrossRef] [PubMed]
- Zafiropoulos, B.; A Alison, J.; McCarren, B. Physiological responses to the early mobilisation of the intubated, ventilated abdominal surgery patient. Aust. J. Physiother. 2004, 50, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.S.; Alazzawi, S.; Nizam, I.; Haddad, F.S. An evidence-based review of enhanced recovery interventions in knee replacement surgery. Ann. R. Coll. Surg. Engl. 2013, 95, 386–389. [Google Scholar] [CrossRef]
- Sagar, S.; Harland, P.; Shields, R. Early postoperative feeding with elemental diet. BMJ 1979, 1, 293–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hubner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.; et al. Espen guideline: Clinical nutrition in surgery. Clin. Nutr. 2017, 36, 623–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petermann, F. Hospital Anxiety and Depression Scale, Deutsche Version (HADS-D). Z. Psychiatr. Psychol. Psychother. 2011, 59, 251–253. [Google Scholar] [CrossRef]
- von Zerssen, D. Die befindlichkeitsskala. In Manual; Beltz Test: Weinheim, Germany, 1976. [Google Scholar]
- Janke, W.; Debus, G. Die Eigenschaftswörterliste: Ewl; Verlag für Psychologie CJ Hogrefe: Göttingen, Germany, 1978. [Google Scholar]
- Morfeld, M.; Petersen, C.; Krüger-Bödeker, A.; Von Mackensen, S.; Bullinger, M. The Assessment of Mood at Workplace—Psychometric Analyses of the Revised Profile of Mood States (POMS) Questionnaire. Psychosoc. Med. 2007, 4, 1–9. [Google Scholar]
- Guy, W. ECDEU Assessment Manual for Psychopharmacology; US Department of Health, Education, and Welfare, Public Health Service: Washington, DC, USA, 1976.
- Von Korff, M.; Ormel, J.; Keefe, F.J.; Dworkin, S.F. Grading the severity of chronic pain. Pain 1992, 50, 133–149. [Google Scholar] [CrossRef]
- Flor, H.; Turk, D. Chronic back pain and rheumatoid arthritis: Relationship of pain-related cognitions, pain severity, and pain behaviors. J. Behav. Med. 1988, 11, 251–265. [Google Scholar] [CrossRef]
- Löwe, B.; Wahl, I.; Rose, M.; Spitzer, C.; Glaesmer, H.; Wingenfeld, K.; Schneider, A.; Brähler, E. A 4-item measure of depression and anxiety: Validation and standardization of the Patient Health Questionnaire-4 (PHQ-4) in the general population. J. Affect. Disord. 2010, 122, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Cattell, R.B. The Scree Test For The Number Of Factors. Multivar. Behav. Res. 1966, 1, 245–276. [Google Scholar] [CrossRef]
- Kaiser, H.F. The Application of Electronic Computers to Factor Analysis. Educ. Psychol. Meas. 1960, 20, 141–151. [Google Scholar] [CrossRef]
- Möhring, W.; Schlütz, D. Standardisierte Befragung: Grundprinzipien, Einsatz und Anwendung. In Handbuch Standardisierte Erhebungsverfahren in der Kommunikationswissenschaft; Metzler, J.B., Ed.; Springer: Wiesbaden, Germany, 2013; pp. 183–200. [Google Scholar]
- Tabachnick, B.G.; Fidell, L.S. Using Multivariate Statistics; Pearson Education: Boston, MA, USA, 2013. [Google Scholar]
- Cronbach, L.J. Coefficient alpha and the internal structure of tests. Psychometrika 1951, 16, 297–334. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1988. [Google Scholar]
- Guttman, L. Some necessary conditions for common-factor analysis. Psychometrika 1954, 19, 149–161. [Google Scholar] [CrossRef]
- Sinatra, R. Causes and Consequences of Inadequate Management of Acute Pain. Pain Med. 2010, 11, 1859–1871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ip, H.Y.; Abrishami, A.; Peng, P.W.; Wong, J.; Chung, F. Predictors of postoperative pain and analgesic consumption: A qualitative systematic review. Anesthesiology 2009, 111, 657–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taenzer, P.; Melzack, R.; Jeans, M.E. Influence of psychological factors on postoperative pain, mood and analgesic requirements. Pain 1986, 24, 331–342. [Google Scholar] [CrossRef]
- White, P.F.; Kehlet, H. Improving postoperative pain management: What are the unresolved issues? Anesthesiology 2010, 112, 220–225. [Google Scholar] [CrossRef] [Green Version]
- Langer, E.J.; Janis, I.L.; Wolfer, J.A. Reduction of psychological stress in surgical patients. J. Exp. Soc. Psychol. 1975, 11, 155–165. [Google Scholar] [CrossRef]
- Wetsch, W.A.; Pircher, I.; Lederer, W.; Kinzl, J.F.; Traweger, C.; Heinz-Erian, P.; Benzer, A. Preoperative stress and anxiety in day-care patients and inpatients undergoing fast-track surgery. Br. J. Anaesth. 2009, 103, 199–205. [Google Scholar] [CrossRef] [Green Version]
- López-Jornet, P.; Camacho-Alonso, F.; Sanchez-Siles, M. Assessment of general pre and postoperative anxiety in patients undergoing tooth extraction: A prospective study. Br. J. Oral Maxillofac. Surg. 2014, 52, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, D.A.; Brodie, F.L.; Rose-Nussbaumer, J.; Ramanathan, S. Anxiety in patients undergoing cataract surgery: A pre- and postoperative comparison. Clin. Ophthalmol. 2017, 11, 1979–1986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Item | Sample (n = 132) |
|---|---|
| Mean age, years (SD) | 68 (9.4) |
| Male/female | 58/74 |
| Marital status | |
| Single | 10 |
| Married | 88 |
| Widowed | 18 |
| Divorced | 11 |
| Serious relationship | 3 |
| Living separately | 1 |
| Missing | 1 |
| Employment | |
| Incapacitated to work | 7 |
| Unemployed | 4 |
| Employed | 32 |
| Retired due to illness | 10 |
| Retired due to age | 75 |
| Item | Component | |
|---|---|---|
| 1 | 2 | |
| Postoperatively | ||
| General mood | 0.862 | |
| Sad | 0.798 | |
| Anxious | 0.779 | |
| Weak | 0.733 | |
| Irritated | 0.720 | |
| Numb/dizzy | 0.684 | |
| Tired | 0.672 | |
| Preoperatively | ||
| General mood | 0.840 | |
| Weak | 0.811 | |
| Tired | 0.758 | |
| Numb/dizzy | 0.711 | |
| Sad | 0.697 | |
| Anxious | 0.664 | |
| Irritated | 0.654 | |
| Item | F | p | Partial η2 | MeanPre (SD) | MeanPost (SD) |
|---|---|---|---|---|---|
| Pain | |||||
| Pain | 42.33 | <0.001 | 0.28 | 3.95 (2.58) | 2.09 (1.91) |
| Emotional state | |||||
| Sad | 29.03 | <0.001 | 0.21 | 2.00 (2.25) | 1.01 (1.47) |
| Anxious | 50.45 | <0.001 | 0.32 | 2.23 (2.43) | 0.71 (1.20) |
| Tired | 1.10 | 0.30 | 0.01 | 2.30 (2.12) | 2.16 (2.03) |
| Weak | 0.97 | 0.33 | 0.01 | 1.95 (2.24) | 1.74 (1.94) |
| Irritated | 21.18 | <0.001 | 0.17 | 1.50 (1.85) | 0.67 (1.20) |
| General mood | 42.17 | <0.001 | 0.17 | 2.05 (2.00) | 1.16 (1.51) |
| State of health | |||||
| State of health | 20.22 | <0.001 | 0.15 | 2.11 (1.04) | 1.61 (0.94) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stuhlreyer, J.; Klinger, R. Development and Validation of the Pain and State of Health Inventory (PHI): Application for the Perioperative Setting. J. Clin. Med. 2021, 10, 1965. https://doi.org/10.3390/jcm10091965
Stuhlreyer J, Klinger R. Development and Validation of the Pain and State of Health Inventory (PHI): Application for the Perioperative Setting. Journal of Clinical Medicine. 2021; 10(9):1965. https://doi.org/10.3390/jcm10091965
Chicago/Turabian StyleStuhlreyer, Julia, and Regine Klinger. 2021. "Development and Validation of the Pain and State of Health Inventory (PHI): Application for the Perioperative Setting" Journal of Clinical Medicine 10, no. 9: 1965. https://doi.org/10.3390/jcm10091965





