Structural Changes in Trabecular Bone, Cortical Bone and Hyaline Cartilage as Well as Disturbances in Bone Metabolism and Mineralization in an Animal Model of Secondary Osteoporosis in Clostridium perfringens Infection
Abstract
:1. Introduction
2. Material and Methods
2.1. Animal Model
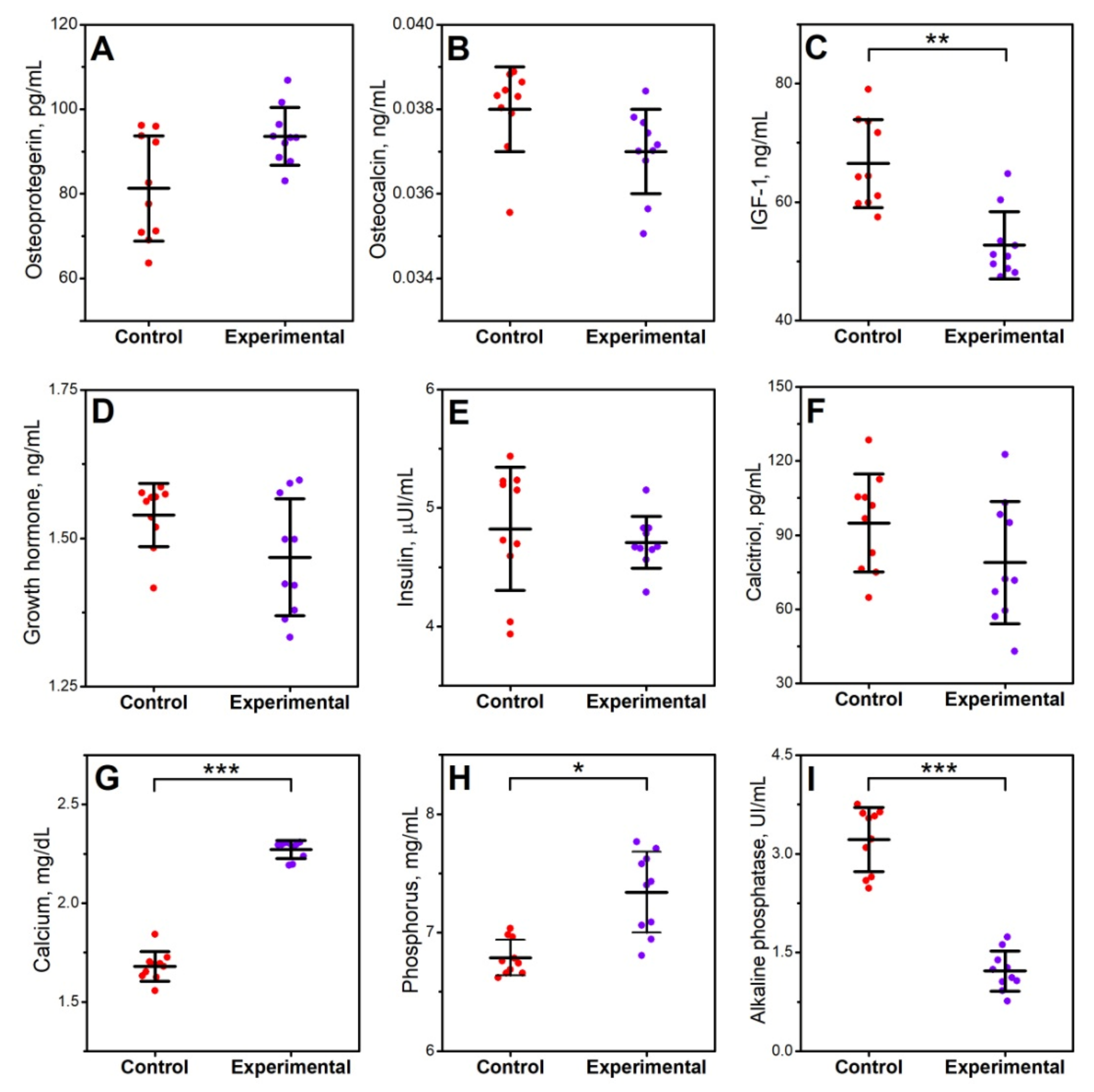
2.2. Blood Serum Analysis
2.3. Mechanical Properties of the Tibia
2.4. Geometrical Properties of the Tibia
2.5. Structural Properties of the Tibia
2.6. Densitometry Measurements of the Tibia
2.7. X-ray Diffraction
2.8. Composition of Bone Mineral Fraction
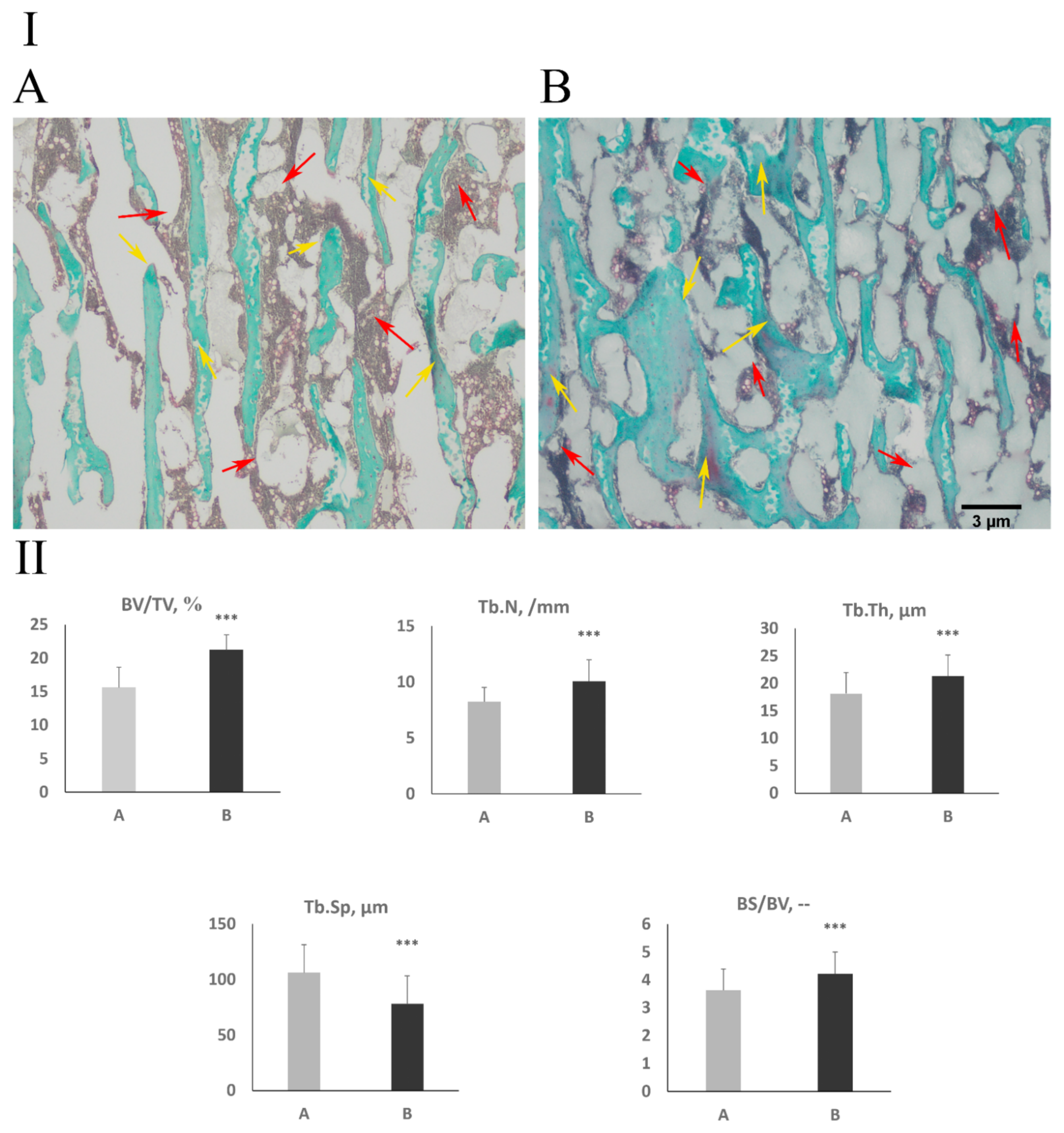
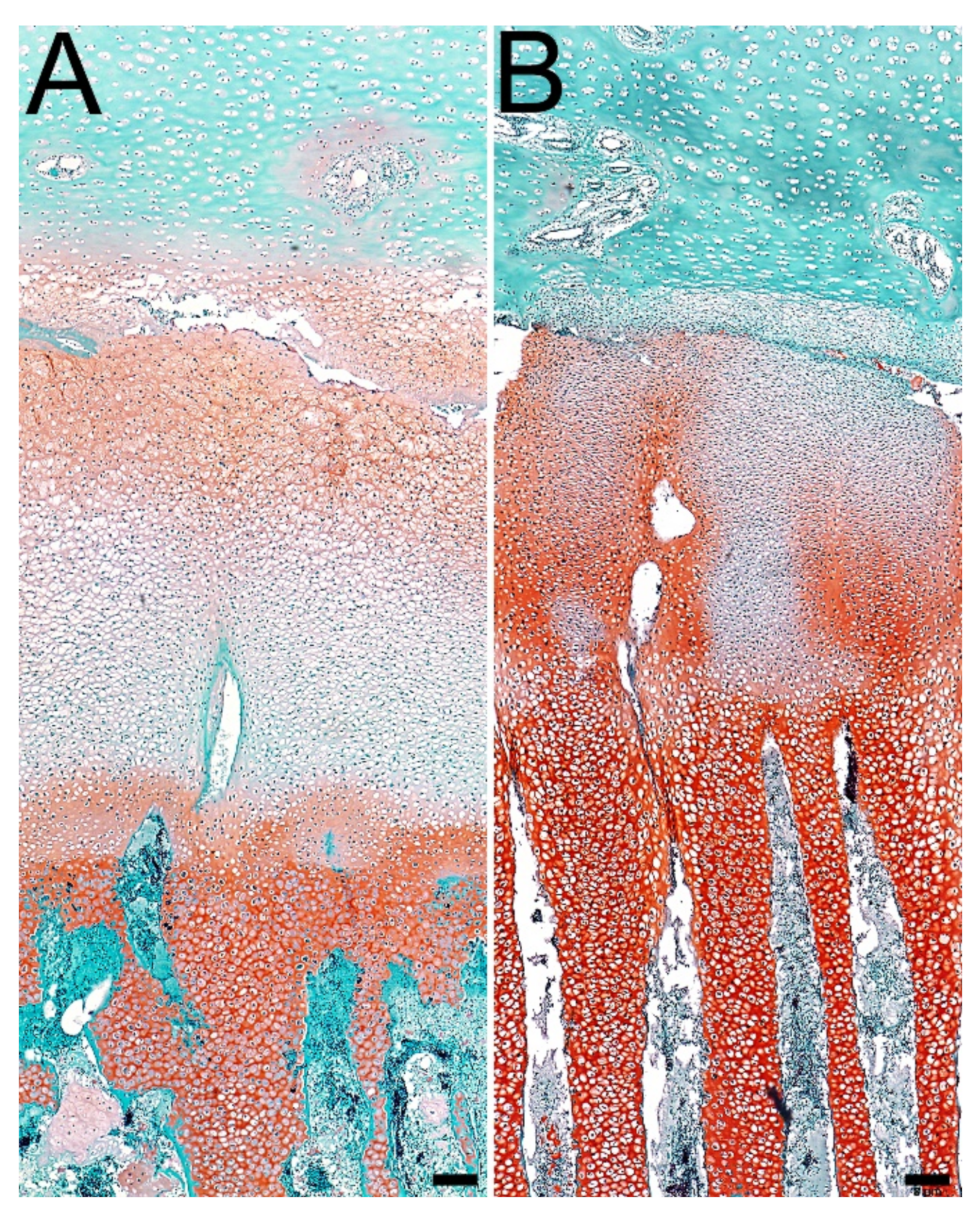
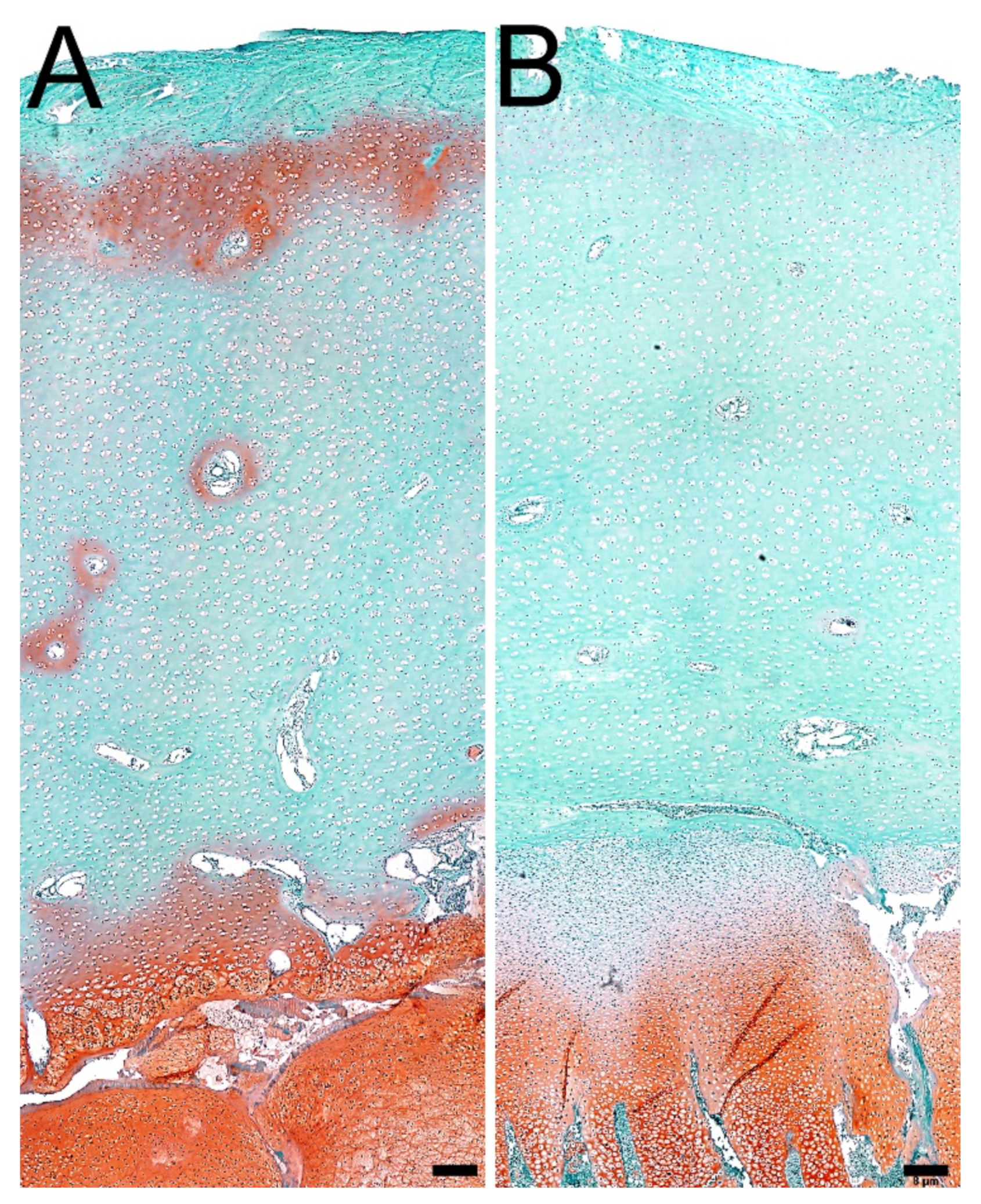
2.9. Bone, Articular Cartilage, and Growth Plate Histomorphometry
2.10. Statistical Analysis
3. Results
3.1. Blood Serum Analysis
3.2. Bone Osteometry, Density, Geometry, Mechanical, and Structural Properties
3.3. Cancellous Bone Histomorphometry and Bone Collagen Structure
3.4. Articular Cartilage and Growth Plate Cartilage Morphology
3.5. Proteoglycans in Articular Cartilage and Growth Plate Cartilage
3.6. Ash Content and Composition of the Mineral Fraction in Bone
3.7. Analysis of the Crystallinity of the Bone Mineral Phase
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benno, Y.; Mitsuoka, T. Development of intestinal microflora in humans and animals. Bifidobact. Microfi. 1986, 5, 13–25. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised estimated for the number of human and bacteria cells in the body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bäckhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-bacterial mutualism in the human intestine. Science 2005, 25, 1915–1920. [Google Scholar] [CrossRef] [Green Version]
- Guarner, F.; Malagelada, J.R. Gut flora in health and disease. Lancet 2003, 361, 512–519. [Google Scholar] [CrossRef]
- Zubadalashvili, N.G.; Makhviladze, M.A.; Diasamidze, M.T.; Abdulashvili, N.N. The comparative study of Linex and Lacto-G in treatment of adult patients with disbacteriosis. Georgian Med. News 2009, 170, 38–42. [Google Scholar]
- Yoo, H.S.; Lee, S.U.; Park, K.Y.; Park, Y.H. Molecular typing and epidemiological survey of prevalence of Clostridium perfringens types by.ultiplex PCR. Int. J. Clin. Microbiol. 1997, 35, 228–232. [Google Scholar] [CrossRef] [Green Version]
- Higgs, J.; Derbyshire, E.; Styles, K. Nutrition and osteoporosis prevention for the orthopaedic surgeon: A wholefoods approach. EFORT Open Rev. 2017, 2, 300–308. [Google Scholar] [CrossRef]
- Sarker, M.R.; Carman, R.J.; McClane, B.A. Inactivation of the gene (cpe) encoding Clostridium perfringens enterotoxin eliminates the ability of two cpe-positive C. perfringens type A human gastrointestinal disease isolates to affect rabbit ileal loops. Mol. Microbiol. 1999, 33, 946–958. [Google Scholar] [CrossRef]
- Sirous, M.; Namaki, S.; Mirshafiey, A. Clostridia. J. Chin. Clin. Med. 2009, 4, 35–47. [Google Scholar]
- Fisher, D.J.; Miyamoto, K.; Harrison, B.; Akimoto, S.; Sarker, M.R.; McClane, B.A. Association of beta2 toxin production with Clostridium perfringens type A human gastrointestinal disease isolates carrying a plasmid enterotoxin gene. Mol. Microbiol. 2005, 56, 747–762. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Rafii, F. Effects of bile acids and bisin on the production of enterotoxin by Clostridium perfringens in a nutrient-rich medium. Int. J. Med. Microbiol. 2018, 3, 1–9. [Google Scholar]
- Li, J.; McClane, B.A. A novel small acid soluble protein variant is important for spore resistance of most Clostridium perfringens food poisoning isolates. PLoS Pathog. 2008, 4, e1000056. [Google Scholar] [CrossRef] [Green Version]
- Van Immerseel, F.; De Buck, J.; Pasmans, F.; Huyghebaert, G.; Haesebrouck, F.; Ducatelle, R. Clostridium perfringens in poultry: An emerging threat for animal and public health. Avian Pathol. 2004, 33, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Borriello, S.P.; Barclay, F.E.; Welch, A.R.; Stringer, M.F.; Watson, G.N.; Williams, R.K.T.; Seal, D.V.; Sullens, K. Epidemiology of diarrhoea caused by enterotoxigenic Clostridium perfringens. J. Med. Microbiol. 1985, 20, 363–372. [Google Scholar] [CrossRef]
- Asha, N.J.; Tompkins, D.; Wilcox, M.H. Comparative analysis of prevalence, risk factors, and molecular epidemiology of antibiotic-associated diarrhea due to Clostridium difficile, Clostridium perfringens, and Staphylococcus aureu. J. Clin. Microbiol. 2006, 44, 2785–2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Fabala, F.; Sanza, N.; Ruiz-Bastiana, M.; Barrosb, C.; Gomez-Garces, J.L. Clostridium perfringens bacteraemia, an analysis of 28 cases over 10 years in a university hospital of Madrid. Enferm. Infecc. Microbiol. Clin. 2018, 36, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Hsu, P.C.; Chang, H.J.; Cheng, C.W.; Lee, M.H. Clinical significance and outcomes of Clostridium perfringens bacteremia—A 10-year experience at a tertiary care hospital. Int. J. Infect. Dis. 2013, 17, 955–960. [Google Scholar] [CrossRef] [Green Version]
- Asha, N.J.; Wilcox, M.H. Laboratory diagnosis of Clostridium perfringens antibiotic-associated diarrhoea. J. Med. Microbiol. 2002, 51, 891–894. [Google Scholar] [CrossRef] [PubMed]
- Baxter, M.; Latorre, J.; Koltes, D.; Dridi, S.; Greene, S.E.; Bickler, S.; Kim, J.H.; Merino-Guzman, R.; Hernandez-Valasco, X.; Anthony, N.B.; et al. Assessment of a nutritional rehabilitation model in two modern broilers and their jungle fowl ancestor: A model for better understanding childhood undernutrition. Front. Nutr. 2018, 5, 18. [Google Scholar] [CrossRef] [Green Version]
- Aguado, E.; Pascaretti-Grizon, F.; Goyenvalle, E.; Audran, M.; Chappard, D. Bone mass and bone quality are altered by hypoactivity in the chicken. PLoS ONE 2015, 10, e0116763. [Google Scholar]
- Aerssens, J.; Boonen, S.; Lowet, G.; Dequeker, J. Interspecies differences in bone composition, density, and quality: Potential implications for in vivo bone research. Endocrinology 1998, 139, 663–670. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Dobrowolski, P.; Winiarska-Mieczan, A.; Kwiecień, M.; Muszyński, S.; Tomczyk, A. The effect of tannic acid on bone mechanical and geometric properties, bone density, and trabecular histomorphometry as well as the morphology of articular and growth cartilages in rats co-exposed to cadmium and lead is dose dependent. Toxicol. Ind. Health 2017, 33, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.A.; Lyra, A.C.; Mendes, C.M.C.; Lopes, M.B.; Coqueiro, F.G.; Rocha, R.; Santana, G.O. Bone mineral density and inflammatory bowel disease severity. Braz. J. Med. Biol. Res. 2017, 50, e6374. [Google Scholar] [CrossRef]
- Muszyński, S.; Kwiecień, M.; Tomaszewska, E.; Świetlicka, I.; Dobrowolski, P.; Kasperek, K.; Jeżewska-Witkowska, G. Effect of caponization on performance and quality characteristics of long bones in Polbar chickens. Poult. Sci. 2017, 96, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Rudyk, H.; Tomaszewska, E.; Kotsyumbas, I.; Muszyński, S.; Tomczyk-Warunek, A.; Szymańczyk, S.; Dobrowolski, P.; Wiącek, D.; Kamiński, D.; Brezvyn, O. Bone homeostasis in experimental fumonisins intoxication of rats. Ann. Anim. Sci. 2019, 19, 403–419. [Google Scholar] [CrossRef] [Green Version]
- Tomaszewska, E.; Dobrowolski, P.; Muszyński, S.; Kostro, K.; Taszkun, I.; Żmuda, A.; Blicharski, T.; Hułas-Stasiak, M. DON-induced changes in bone homeostasis in mink dams. J. Vet. Res. 2017, 61, 357–362. [Google Scholar] [CrossRef] [Green Version]
- Muszyński, S.; Tomaszewska, E.; Dobrowolski, P.; Kwiecień, M.; Wiącek, D.; Świetlicka, I.; Skibińska, M.; Szymańska-Chargot, M.; Orzeł, J.; Świetlicki, M.; et al. Analysis of bone osteometry, mineralization, mechanical and histomorphometrical properties of tibiotarsus in broiler chickens demonstrates a influence of dietary chickpea seeds (Cicer arietinum L.) inclusion as a primary protein source. PLoS ONE 2018, 13, e0208921. [Google Scholar] [CrossRef]
- Muszyński, S.; Tomaszewska, E.; Kwiecień, M.; Dobrowolski, P.; Tomczyk-Warunek, A. Subsequent somatic axis and bone tissue metabolism responses to a low-zinc diet with or without phytase inclusion in broiler chickens. PLoS ONE 2018, 13, e0191964. [Google Scholar] [CrossRef] [Green Version]
- Tomaszewska, E.; Dobrowolski, P.; Puzio, I.; Donaldson, J.; Muszyński, S. Acrylamide-induced prenatal programming of bone structure in mammal model. Ann. Anim. Sci. 2020, 20, 1257–1287. [Google Scholar] [CrossRef]
- Miyazawa, E.; Iwabuchi, A.; Yoshida, T. Phytate breakdown and apparent absorption of phosphorus, calcium and magnesium in germfree and conventionalized rats. Nutr. Res. 1996, 16, 603–613. [Google Scholar] [CrossRef]
- Younes, H.; Coudray, C.; Bellanger, J.; Demigne, C.; Rayssiguierm, Y.; Remesy, C. Effects of two fermentable carbohydrates (inulin and resistant starch) and their combination on calcium and magnesium balance in rats. Br. J. Nutr. 2001, 86, 479–485. [Google Scholar] [CrossRef]
- Ibáneza, L.; Rouleaua, M.; Wakkacha, A.; Blin-Wakkach, C. Gut microbiome and bone. Jt. Bone Spine 2019, 86, 43–47. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, Y.; Wang, Y.; Ren, X.; Han, J. The impact of the intestinal microbiome on bone health. Intractable Rare Dis. Res. 2018, 7, 148–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut microbiota and IBD: Causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 573–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, I.; Ullah, N.; Zha, L.; Bai, Y.; Khan, A.; Zhao, T.; Che, T.; Zhang, C. Alteration of gut microbiota in inflammatory bowel disease (IBD): Cause or consequence? IBD treatment targeting the gut microbiome. Pathogens 2019, 8, 126. [Google Scholar] [CrossRef] [Green Version]
- Banaszkiewicz, A.; Kądzielska, J.; Gawrońska, A.; Pituch, H.; Obuch-Woszczatyński, P.; Albrecht, P.; Młynarczyk, G.; Radzikowski, A. Enterotoxigenic Clostridium perfringens infection and pediatric patients with inflammatory bowel disease. J. Crohns Colitis. 2014, 8, 276–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miheller, P.; Gesztes, W.; Lakatos, P.L. Manipulating bone disease in inflammatory bowel disease patients. Ann. Gastroenterol. 2013, 3, 148–155. [Google Scholar]
- Pappa, H.; Thayu, M.; Sylvester, F.; Leonard, M.; Zemel, B.; Gordon, C. Skeletal health of children and adolescents with inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 11–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, S.; Li, C.; Liu, D.; Guo, Y. Inflammatory responses to a Clostridium perfringens type A strain and α-toxin in primary intestinal epithelial cells of chicken embryos. Avian Pathol. 2015, 44, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Lillehoj, H.S.; Allen, P.C.; Park, D.W.; Fitz Coy, S.; Bautista, D.A.; Lillehoj, E.P. Immunopathology and cytokine responses in broiler chickens coinfected with Eimeria maxima and Clostridium perfringens with the use of an animal model of necrotic enteritis. Avian Dis. 2008, 52, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Collier, C.T.; Hofacre, C.L.; Payne, A.M.; Anderson, D.B.; Kaiser, P.; Mackie, R.I.; Gaskins, H.R. Coccidia-induced mucogenesis promotes the onset of necrotic enteritis by supporting Clostridium perfringens growth. Vet. Immunol. Immunopathol. 2008, 122, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Bryant, A.E.; Stevens, D.L. Phospholipase C and perfringolysin O from Clostridium perfringens upregulate endothelial cell-leukocyte adherence molecule 1 and intercellular leukocyte adherence molecule 1 expression and induce interleukin-8 synthesis in cultured human umbilical vein endothelial cells. Infect. Immun. 1996, 64, 358–362. [Google Scholar] [PubMed]
- Kilby, K.; Mathias, H.; Boisvenue, L.; Heisler, C.; Jones, J.L. Micronutrient absorption and related outcomes in people with inflammatory bowel disease: A review. Nutrients 2019, 11, 1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilg, H.; Moschen, A.R.; Kaser, A.; Pines, A.; Dotan, I. Gut, inflammation and osteoporosis: Basic and clinical concepts. Gut 2008, 57, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Schulte, C.M.; Dignass, A.U.; Goebell, H.; Röher, H.D.; Schulte, K.M. Genetic factors determine extent of bone loss in inflammatory bowel disease. Gastroenterology 2000, 119, 909–920. [Google Scholar] [CrossRef]
- Beaugerie, L.; Petit, J.C. Antibiotic-associated diarrhoea. BestPract. Res. Clin. Gastroenterol. 2004, 18, 337–352. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Tellez, G.; Richards, J.D.; Escobar, J. Identification of potential biomarkers for gut barrier failure in broiler chickens. Front. Vet. Sci. 2015, 2, 14. [Google Scholar] [CrossRef] [Green Version]
- Gharib-Naseri, K.; Kheravii, S.; Keerqin, C.; Swick, R.A.; Choct, M.; Wu, S.B. Differential expression of intestinal genes in necrotic enteritis challenged broiler chickens with 2 different Clostridium perfringens strains. Poult. Sci. 2021, 100, 100886. [Google Scholar] [CrossRef] [PubMed]
- Criado-Mesas, L.; Abdelli, N.; Noce, A.; Farré, M.; Pérez, J.F.; Solà-Oriol, D.; Martin-Venegas, R.; Forouzandeh, A.; González-Solé, F.; Folch, J.M. Transversal gene expression panel to evaluate intestinal health in broiler chickens in different challenging conditions. Sci. Rep. 2021, 11, 6315. [Google Scholar] [CrossRef]
- Collier, C.T.; van der Klis, J.D.; Deplancke, B.; Anderson, D.B.; Gaskins, H.R. Effects of tylosin on bacterial mucolysis, Clostridium perfringens colonization, and intestinal barrier function in a chick model of necrotic enteritis. Antimicrob. Agents Chemother. 2003, 47, 3311–3317. [Google Scholar] [CrossRef] [Green Version]
- Tomaszewska, E.; Muszyński, S.; Dobrowolski, P.; Kwiecień, M.; Winiarska-Mieczan, A.; Świetlicka, I.; Wawrzyniak, A. Effect of zinc level and source (zinc oxide vs. zinc glycine) on bone mechanical and geometric parameters, and histomorphology in male Ross 308 broiler chicken. Braz. J. Poult. Sci. 2017, 19, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Weaver, C.M.; Gordon, C.M.; Janz, K.F.; Kalkwarf, H.J.; Lappe, J.M.; Lewis, R.; O’Karma, M.; Wallace, T.C.; Zemel, B.S. The national osteoporosis foundation’s position statement on peak bone mass development and lifestyle factors: A systematic review and implementation recommendations. Osteoporos. Int. 2016, 27, 1281–1386. [Google Scholar] [CrossRef] [Green Version]
- Sjögren, K.; Engdahl, C.; Henning, P.; Lerner, U.H.; Tremaroli, V.; Lagerquist, M.K.; Bäckhed, F.; Ohlsson, C. The gut microbiota regulates bone mass in mice. J. Bone Miner. Res. 2012, 27, 1357–1367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarzer, M.; Makki, K.; Storelli, G.; Machuca-Gayet, I.; Srutkova, D.; Hermanova, P.; Martino, E.; Balmand, S.; Hudovic, T.; Heddi, A.; et al. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science 2016, 351, 854–857. [Google Scholar] [CrossRef]
- Irwin, R.; Lee, T.; Young, V.B.; Parameswaran, N.; McCabe, L.R. Colitis induced bone loss is gender dependent and associated with increased inflammation. Inflamm. Bowel Dis. 2013, 19, 1586–1597. [Google Scholar] [CrossRef] [PubMed]
- Skrovanek, S.; DiGuilio, K.; Bailey, R.; Huntington, W.; Urbas, R.; Mayilvaganan, B.; Mercogliano, G.; Mullin, J.M. Zinc and gastrointestinal disease. World J. Gastrointest. Pathophysiol. 2014, 5, 496–513. [Google Scholar] [CrossRef]
- Brodziak-Doperała, B.; Kwapuliński, J.; Paukszto, A.; Kowol, J.; Bogunia, M.; Ahnert, B. Interactions of copper and iron with other elements in the osseous tissue of the femur head. Fresen Enaviron. Bull. 2009, 18, 1963–1966. [Google Scholar]
- Thomas, M.L.; Ramp, W.K. Effects of parathyroid hormone on alkaline phosphatase activity and mineralization of cultured chick embryo tibiae. Calcif. Tissue Int. 1979, 27, 137–142. [Google Scholar] [CrossRef]
- Guss, J.D.; Horsfield, M.W.; Fontenele, F.F.; Sandoval, T.N.; Luna, M.; Apoorva, F.; Lima, S.F.; Bicalho, R.C.; Singh, A.; Ley, R.E.; et al. Alterations to the gut gicrobiome impair bone strength and tissue material properties. J. Bone Miner. Res. 2017, 32, 1343–1353. [Google Scholar] [CrossRef] [Green Version]
- Haschka, J.; Hirschmann, S.; Kleyer, A.; Englbrecht, M.; Faustini, F.; Simon, D.; Figueiredo, C.P.; Schuster, L.; Muschitz, C.; Kocijan, R.; et al. High-resolution quantitative computed tomography demonstrates structural defects in Cortical and trabecular bone in IBD patients. J. Crohns Colitis. 2016, 10, 532–540. [Google Scholar] [CrossRef] [Green Version]
- Oostlander, A.E.; Bravenboer, N.; Sohl, E.; Holzmann, P.J.; van der Woude, C.J.; Dijkstra, G.; Stokkers, P.C.F.; Oldenburg, B.; Netelenbos, J.C.; Hommes, D.W.; et al. Dutch Initiative on Crohn and Colitis (ICC). Histomorphometric analysis reveals reduced bone mass and bone formation in patients with quiescent Crohn’s disease. Gastroenterology 2011, 140, 116–123. [Google Scholar] [CrossRef] [Green Version]
- Fox, S.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar]
- Poole, A.R.; Kojima, T.; Yasuda, T.; Mwale, F.; Kobayashi, M.; Laverty, S. Composition and structure of articular cartilage: A template for tissue repair. Clin. Orthop. Relat. Res. 2001, 391, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Szychlińska, M.A.; Di Rosa, M.; Castorina, A.; Mobasheri, A.; Musumeci, G. A correlation between intestinal microbiota dysbiosis and osteoarthritis. Heliyon 2019, 12, e01134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunziker, E.B. Mechanism of longitudinal bone growth and its regulation by growth plate chondrocytes. Microsci. Res. Tech. 1994, 28, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Boer, C.G.; Radjabzadeh, D.; Medina-Gomez, C.; Garmaeva, S.; Schipof, D.; Arp, P.; Koet, T.; Kurilshikov, A.; Fu, J.; Ikram, M.A.; et al. Intestinal microbiome composition and its relation to joint pain and inflammation. Nat. Commun. 2019, 10, 4881. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Lv, J.; Jia, Y.; Wang, R.; Zhang, Z.; Liu, J.; Jia, C. Effect of moxibustion on the intestinal flora of rats with knee osteoarthritis induced by monosodium iodoacetate. Evid. Based Complement. Alternat. Med. 2020, 2020, 3196427. [Google Scholar] [CrossRef]
- Yan, J.; Herzog, J.W.; Tsang, K.; Brennan, C.A.; Bower, M.A.; Garrett, W.S.; Sartor, B.R.; Aliprantis, A.O.; Charles, J.F. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc. Natl. Acad. Sci. USA 2016, 113, 7554–7563. [Google Scholar] [CrossRef] [Green Version]
- Abad, V.; Meyers, J.L.; Weise, M.; Gafni, R.I.; Barnes, K.M.; Nilsson, O.; Bacher, J.D.; Baron, J. The role of the resting zone in growth plate chondrogenesis. Endocrinology 2002, 143, 1851–1857. [Google Scholar] [CrossRef]

| Control | Experimental | p-Value | |
|---|---|---|---|
| Bone osteometric properties | |||
| Bone weight, g | 20.8 ± 1.55 | 21.7 ± 1.82 | NS |
| Bone length, mm | 1131 ± 36.0 | 1152 ± 29.7 | NS |
| Length/weight bone ration | 18.4 ± 1.17 | 18.8 ± 1.33 | NS |
| Bone densitometry | |||
| BMD, g/cm2 | 0.267 ± 0.015 | 0.248 ± 0.012 | * |
| BMC, g | 3.52 ± 0.326 | 3.51 ± 0.228 | NS |
| BTD cortical bone, g/cm3 | 1.87 ± 0.047 | 1.91 ± 0.086 | NS |
| Bone geometrical properties | |||
| Horizontal external diameter, mm | 9.76 ± 0.399 | 9.94 ± 0.658 | NS |
| Horizontal internal diameter, mm | 5.81 ± 0.506 | 6.44 ± 0.592 | * |
| Vertical external diameter, mm | 7.73 ± 0.460 | 7.98 ± 0.522 | NS |
| Vertical internal diameter, mm | 5.09 ± 0.451 | 5.44 ± 0.499 | NS |
| Cortical cross-sectional area, mm2 | 36.6 ± 2.606 | 33.4 ± 3.24 | * |
| MRTW | 0.61 ± 0.082 | 0.51 ± 0.083 | * |
| Cortical index, % | 37.7 ± 2.78 | 33.0 ± 2.64 | ** |
| Secondary moment of inertia, mm4 | 192 ± 28.54 | 197 ± 29.7 | NS |
| Radius of gyration, mm | 2.24 ± 0.056 | 2.40 ± 0.090 | *** |
| Control | Experimental | p-Value | |
|---|---|---|---|
| Mechanical properties | |||
| Max. elastic strength, N | 211 ± 23.7 | 215 ± 20.2 | NS |
| Elastic energy, MJ | 64.5 ± 10.6 | 77.8 ± 11.4 | NS |
| Ultimate strength, N | 378 ± 35.6 | 389 ± 50.6 | NS |
| Work to fracture, MJ | 509 ± 59.0 | 520 ± 32.1 | NS |
| Stiffness, N/mm | 375 ± 8.07 | 310 ± 7.86 | *** |
| Structural properties | |||
| Young’s modulus of elasticity, GPa | 3867 ± 160 | 3037 ± 224 | *** |
| Bending moment, Nm | 23.4 ± 1.91 | 23.6 ± 1.66 | NS |
| Toughness Modulus, mJ/mm3 | 0.268 ± 0.057 | 0.279 ± 0.063 | NS |
| Yield strain | 0.013 ± 0.001 | 0.018 ± 0.003 | ** |
| Yield stress, MPa | 520 ± 63.0 | 471 ± 53.4 | NS |
| Ultimate strain | 0.045 ± 0.002 | 0.047 ± 0.006 | NS |
| Ultimate stress, MPa | 836 ± 97.1 | 892 ± 66.3 | NS |
| Control | Experimental | p-Value | |
|---|---|---|---|
| Growth plate cartilage | |||
| Resting zone, µm | 311 ± 71.0 | 379 ± 89.2 | ** |
| Proliferation zone, µm | 1001 ± 176 | 864 ± 213 | *** |
| Hypertrophy zone, µm | 812 ± 132 | 1297 ± 135 | *** |
| Calcification zone, µm | 1295 ± 101 | 1207 ± 199 | ** |
| Articular cartilage | |||
| Surperficial zone, µm | 85.1 ± 10.3 | 65.8 ± 11.0 | *** |
| Transition zone, µm | 613 ± 52.0 | 699 ± 20.8 | *** |
| Deep zone, µm | 177 ± 19.9 | 181 ± 15.2 | NS |
| Control | Experimental | p-Value | |
|---|---|---|---|
| Ash, % | 58.8 ± 2.18 | 56.9 ± 3.18 | NS |
| Ca, g/kg | 494 ± 3.68 | 487 ± 10.0 | * |
| P, g/kg | 258 ± 3.16 | 259 ± 2.98 | NS |
| Mg, g/kg | 10.5 ± 0.175 | 9.91 ± 0.225 | NS |
| S, g/kg | 11.4 ± 0.239 | 11.5 ± 0.135 | NS |
| Na, g/kg | 3.18 ± 0.156 | 3.16 ± 0.140 | NS |
| Co, mg/kg | 0.351 ± 0.039 | 0.347 ± 0.033 | NS |
| Cu, mg/kg | 27.4 ± 0.702 | 29.1 ± 1.18 | ** |
| Fe, mg/kg | 294 ± 17.3 | 286 ± 21.58 | NS |
| Se, mg/kg | 1.21 ± 0.109 | 1.09 ± 0.168 | NS |
| Si, mg/kg | 4.05 ± 0.744 | 3.14 ± 1.46 | NS |
| Zn, mg/kg | 445 ± 14.8 | 423 ± 13.5 | ** |
| Control | Experimental | p-Value | |
|---|---|---|---|
| a-b plane, µm | 69.0 ± 2.23 | 68.0 ± 2.32 | NS |
| c axis, µm | 94.5 ± 1.73 | 95.8 ± 1.19 | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomczyk-Warunek, A.; Blicharski, T.; Muszyński, S.; Tomaszewska, E.; Dobrowolski, P.; Blicharski, R.; Jarecki, J.; Arczewska-Włosek, A.; Świątkiewicz, S.; Józefiak, D. Structural Changes in Trabecular Bone, Cortical Bone and Hyaline Cartilage as Well as Disturbances in Bone Metabolism and Mineralization in an Animal Model of Secondary Osteoporosis in Clostridium perfringens Infection. J. Clin. Med. 2022, 11, 205. https://doi.org/10.3390/jcm11010205
Tomczyk-Warunek A, Blicharski T, Muszyński S, Tomaszewska E, Dobrowolski P, Blicharski R, Jarecki J, Arczewska-Włosek A, Świątkiewicz S, Józefiak D. Structural Changes in Trabecular Bone, Cortical Bone and Hyaline Cartilage as Well as Disturbances in Bone Metabolism and Mineralization in an Animal Model of Secondary Osteoporosis in Clostridium perfringens Infection. Journal of Clinical Medicine. 2022; 11(1):205. https://doi.org/10.3390/jcm11010205
Chicago/Turabian StyleTomczyk-Warunek, Agnieszka, Tomasz Blicharski, Siemowit Muszyński, Ewa Tomaszewska, Piotr Dobrowolski, Rudolf Blicharski, Jaromir Jarecki, Anna Arczewska-Włosek, Sylwester Świątkiewicz, and Damian Józefiak. 2022. "Structural Changes in Trabecular Bone, Cortical Bone and Hyaline Cartilage as Well as Disturbances in Bone Metabolism and Mineralization in an Animal Model of Secondary Osteoporosis in Clostridium perfringens Infection" Journal of Clinical Medicine 11, no. 1: 205. https://doi.org/10.3390/jcm11010205








