ATOMS (Adjustable Transobturator Male System) Is an Effective and Safe Second-Line Treatment Option for Recurrent Urinary Incontinence after Implantation of an AdVance/AdVance XP Fixed Male Sling? A Multicenter Cohort Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Endpoints
2.3. Variables Evaluated
2.4. Statistical Analysis
3. Results
3.1. Effectiveness of Secondary ATOMS Implant
3.2. Safety of Secondary ATOMS Implant
3.3. Patient Reported Outcome Measures (PROMs) of Secondary ATOMS Implant
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Averbeck, M.A.; Woodhouse, C.; Comiter, C.; Bruschini, H.; Hanus, T.; Herschorn, S.; Goldman, H.B. Surgical treatment of post-prostatectomy stress urinary incontinence in adult men: Report from the 6th international consultation on incontinence. Neurourol. Urodyn. 2019, 38, 398–406. [Google Scholar] [CrossRef]
- Rehder, P.; Staudacher, N.M.; Schachtner, J.; Berger, M.E.; Schillfahrt, F.; Hauser, V.; Mueller, R.; Skradski, V.; Horninger, W.; Glodny, B. Hypothesis that urethral bulb (corpus spongiosum) plays an active role in male urinary continence. Adv. Urol. 2016, 2016, 6054730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mumm, J.N.; Klehr, B.; Rodler, S.; Kretschmer, A.; Vilsmaier, T.; Westhofen, T.; Chaloupka, M.; Schulz, G.B.; Gozzi, C.; Rehder, P.; et al. Five-year results of a prospective multicenter trial: AdVance XP for postprostatectomy-incontinence in patients with favorable prognostic factors. Urol. Int. 2021, 105, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Meisterhofer, K.; Herzog, S.; Strini, K.A.; Sebastianelli, L.; Bauer, R.; Dalpiaz, O. Male slings for postprostatectomy incontinence: A systematic review and meta-analysis. Eur. Urol. Focus 2020, 6, 575–592. [Google Scholar] [CrossRef] [PubMed]
- Abrams, P.; Constable, L.D.; Cooper, D.; MacLennan, G.; Drake, M.J.; Harding, C.; Mundy, A.; McCormack, K.; McDonald, A.; Norrie, J.; et al. Outcomes of a noninferiority randomised controlled trial of surgery for men with urodynamic stress incontinence after prostate surgery (MASTER). Eur. Urol. 2021, 79, 812–823. [Google Scholar] [CrossRef] [PubMed]
- Ajay, D.; Zhang, H.; Gupta, S.; Selph, J.P.; Belsante, M.J.; Lentz, A.C.; Webster, G.D.; Peterson, A.C. The artificial urinary sphincter is superior to a secondary transobturator male sling in cases of a primary sling failure. J. Urol. 2015, 194, 1038–1042. [Google Scholar] [CrossRef] [PubMed]
- Rehder, P.; Haab, F.; Cornu, J.N.; Gozzi, C.; Bauer, R.M. Treatment of postprostatectomy male urinary incontinence with the transobturator retroluminal repositioning sling suspension: 3-year follow-up. Eur. Urol. 2012, 62, 140–145. [Google Scholar] [CrossRef]
- Soljanik, I.; Becker, A.J.; Stief, C.G.; Gozzi, C.; Bauer, R.M. Repeat retrourethral transobturator sling in the management of recurrent postprostatectomy stress urinary incontinence after failed first male sling. Eur. Urol. 2010, 58, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Munier, P.; Nicolas, M.; Tricard, T.; Droupy, S.; Costa, P.; Saussine, C. What if artificial urinary sphincter is not possible? Feasibility and effectiveness of ProACT for patients with persistent stress urinary incontinence after radical prostatectomy treated by sling. Neurourol. Urodyn. 2020, 39, 1417–1422. [Google Scholar] [CrossRef] [PubMed]
- Baron, M.G.; Delcourt, C.; Nouhaud, F.X.; Gillibert, A.; Pfister, C.; Grise, P.; Cornu, J.N. Sequential treatment with ProACT device implantation after male sling failure for male urinary incontinence. Prog. Urol. 2017, 27, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Lentz, A.C.; Peterson, A.C.; Webster, G.D. Outcomes following artificial sphincter implantation after prior unsuccessful male sling. J. Urol. 2012, 187, 2149–2153. [Google Scholar] [CrossRef] [PubMed]
- Grabbert, M.; Husch, T.; Kretschmer, A.; Kirschner-Hermanns, R.; Anding, R.; Brehmer, B.; Naumann, C.M.; Queissert, F.; Loertzer, H.; Khoder, W.; et al. Secondary sling implantation after failure of primary surgical treatment for male stress urinary incontinence: A retrospective study. Urol. Int. 2020, 104, 625–630. [Google Scholar] [CrossRef]
- Van der Aa, F.; Drake, M.J.; Kasyan, G.R.; Petrolekas, A.; Cornu, J.N. The artificial urinary sphincter after a quarter of a century: A critical systematic review of its use in male non-neurogenic incontinence. Eur. Urol. 2013, 63, 681–689. [Google Scholar] [CrossRef]
- Kumar, A.; Litt, E.R.; Ballert, K.N.; Nitti, V.W. Artificial urinary sphincter versus male sling for post-prostatectomy incontinence-what do patients choose? J. Urol. 2009, 181, 1231–1235. [Google Scholar] [CrossRef] [PubMed]
- Seweryn, J.; Bauer, W.; Ponholzer, A.; Schramek, P. Initial experience and results with a new adjustable transobturator male system for the treatment of stress urinary incontinence. J. Urol. 2012, 187, 956–961. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, S.; Virseda-Chamorro, M.; Queissert, F.; López, A.; Arance, I.; Angulo, J.C. The mode of action of adjustable transobturator male system (atoms): Intraoperative urethral pressure measurements. Uro 2021, 1, 45–53. [Google Scholar] [CrossRef]
- Angulo, J.C.; Virseda-Chamorro, M.; Arance, I.; Ruiz, S.; Ojea, A.; Carballo, M.; Rodriguez, A.; Pereira, J.; Teyrouz, A.; Rebassa, M.; et al. Long-term outcome of adjustable transobturator male system for stress urinary incontinence in the Iberian multicentre study. Neurourol. Urodyn. 2020, 39, 1737–1745. [Google Scholar] [CrossRef] [PubMed]
- Esquinas, C.; Angulo, J.C. Effectiveness of adjustable transobturator male system (ATOMS) to treat male stress incontinence: A systematic review and meta-analysis. Adv. Ther. 2019, 36, 426–441. [Google Scholar] [CrossRef] [Green Version]
- Angulo, J.C.; Esquinas, C.; Arance, I.; Rodriguez, A.; Pereira, J.; Rabassa, M.; Teyrouz, A.; Teba, F.; Celada, G.; Marcelino, J.P.; et al. Adjustable transobturator male system after failed surgical devices for male stress urinary incontinence: A feasibility study. Urol. Int. 2018, 101, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Friedl, A.; Muhlstadt, S.; Zachoval, R.; Giammò, A.; Kivaranovic, D.; Rom, M.; Fornara, P.; Brossner, C. Long-term outcome of the adjustable transobturator male system (ATOMS): Results of a European multicentre study. BJU Int. 2017, 119, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Angulo, J.C.; Arance, I.; Esquinas, C.; Dorado, J.F.; Marcelino, J.P.; Martins, F.E. Outcome measures of adjustable transobturator male system with pre-attached scrotal port for male stress urinary incontinence after radical prostatectomy: A prospective study. Adv. Ther. 2017, 34, 1173–1183. [Google Scholar] [CrossRef]
- Ziegelmann, M.J.; Linder, B.J.; Rivera, M.E.; Viers, B.R.; Elliott, D.S. The impact of prior urethral sling on artificial urinary sphincter outcomes. Can. Urol. Assoc. J. 2016, 10, 405–409. [Google Scholar] [CrossRef]
- Husch, T.; Kretschmer, A.; Thomsen, F.; Kronlachner, D.; Kurosch, M.; Obaje, A.; Anding, R.; Pottek, T.; Rose, A.; Olianas, R.; et al. Risk factors for failure of male slings and artificial urinary sphincters: Results from a large middle European cohort study. Urol. Int. 2017, 99, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Queissert, F.; Husch, T.; Kretschmer, A.; Anding, R.; Kirschner-Hermanns, R.; Pottek, T.; Olianas, R.; Friedl, A.; Homberg, R.; Pfitzenmaier, J.; et al. High/low-volume center experience predicts outcome of AMS 800 in male stress incontinence: Results of a large middle European multicenter case series. Neurourol. Urodyn. 2020, 39, 1856–1861. [Google Scholar] [CrossRef]
- Machioka, K.; Kadono, Y.; Naito, R.; Nakashima, K.; Iijima, M.; Kawaguchi, S.; Shigehara, K.; Nohara, T.; Izumi, K.; Mizokami, A. Evaluating urinary incontinence before and after radical prostatectomy using the international consultation on incontinence questionnaire-short form. Neurourol. Urodyn. 2019, 38, 726–733. [Google Scholar] [CrossRef]
- Husch, T.; Kretschmer, A.; Thomsen, F.; Kronlachner, D.; Kurosch, M.; Obaje, A.; Anding, R.; Kirschner-Hermanns, R.; Pottek, T.; Olianas, R.; et al. The AdVance and AdVanceXP male sling in urinary incontinence: Is there a difference? World J. Urol. 2018, 36, 1657–1662. [Google Scholar] [CrossRef] [PubMed]



| Variable | n (%) |
|---|---|
| Data before Advance implant | |
| Type of retrourethral sling, n (%) | |
| Advance | 18 (23) |
| Advance XP | 24 (30.8) |
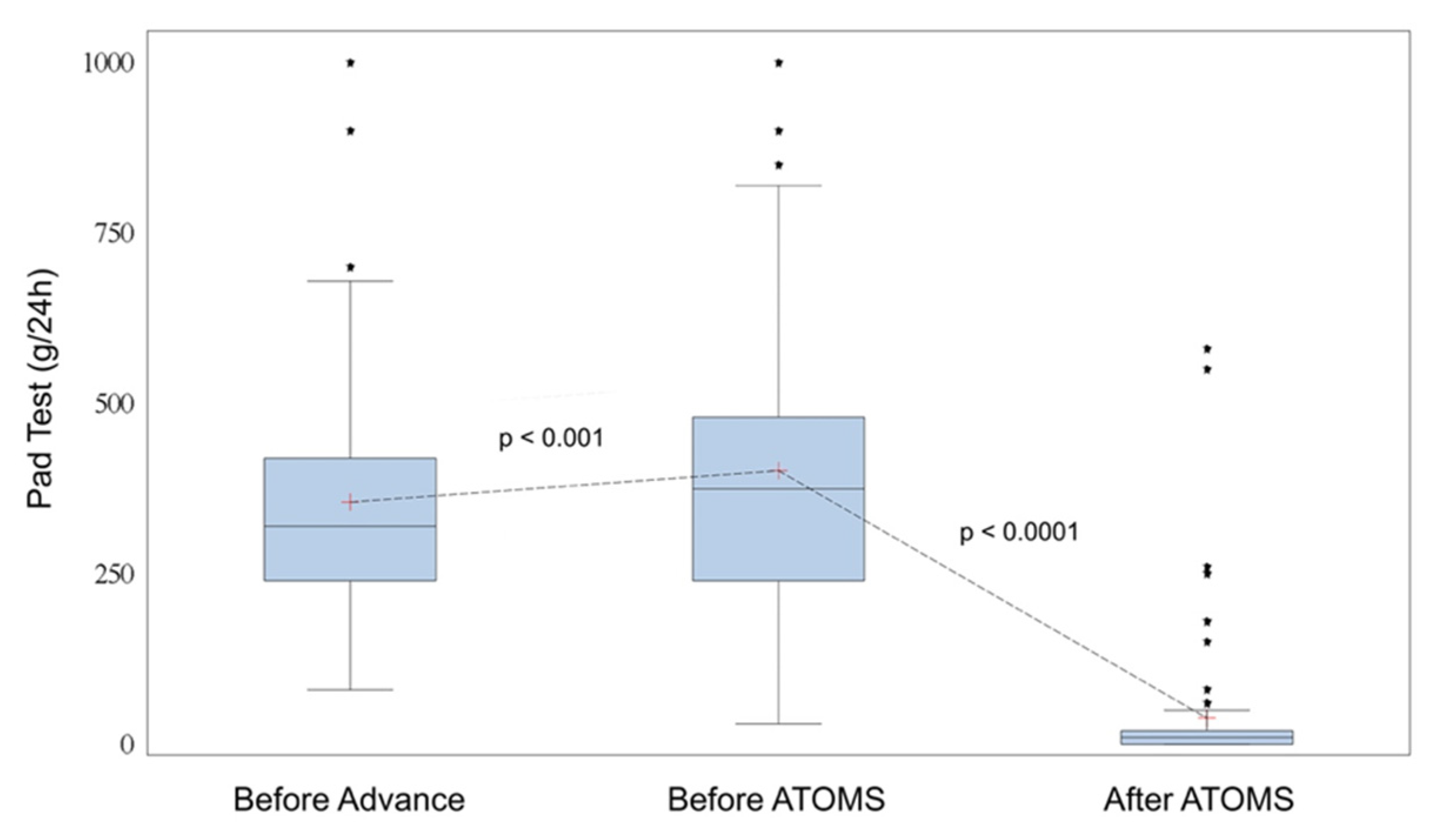
| 24-h pad test, mL, mean ± SD (range) (*) | 355 ± 194 (80–1200) |
| 24-h pad count, n, mean ± SD (range) (*) | 3.4 ± 1.3 (1–6) |
| Night pad count, n, mean ± SD (range) (*) | 0.4 ± 0.7 (0–2) |
| ICIQ-SF total (*) | 15.1 ± 3.8 (10–32) |
| ICIQ-SF Question 1 (*) | 3.4 ± 0.8 (2–5) |
| ICIQ-SF Question 2 (*) | 4.5 ± 2.3 (2–18) |
| ICIQ-SF Question 3 (*) | 7.1 ± 1.9 (4–10) |
| VAS for pain (0–10), mean ± SD (range) (*, #) | 0.9 ± 1.3 (0–8) |
| Data before ATOMS implant | |
| Age, years, mean ± SD (range) | 71.3 ± 5.9 (53–85) |
| Previous radiation, n (%) | 18 (20.5) |
| Previous urethral/bladder neck strictures, n (%) | 14 (15.9) |
| Time since sling surgery, months, mean ± SD (range) | 40.3 ± 34.1 (2–216) |
| 24-h pad test, mL, mean ± SD (range) | 422 + 280 (30–2000) |
| 24-h pad count, n, mean ± SD (range) | 3.9 ± 1.5 (2–8) |
| Night pad-count, n, mean ± SD (range) | 0.7 ± 0.8 (0–3) |
| ICIQ-SF total | 16.25 ± 2.5 (10–21) |
| ICIQ-SF Question 1 | 3.9 ± 0.7 (2–5) |
| ICIQ-SF Question 2 | 4.7 ± 1.1 (2–6) |
| ICIQ-SF Question 3 | 7.6 ± 1.5 (4–10) |
| Perioperative complication, n (%) | 0 (0) |
| Postoperative complications, n (%) | 7 (7.95) |
| VAS for pain (0–10), mean ± SD (range) (#) | 0.7 ± 1.4 (0–6) |
| Total filling volume, mL, mean ± SD (range) | 15.4 ± 5.4 (6–27) |
| Number of fillings, n, mean ± SD (range) | 2.8 ± 2.1 (0–8) |
| Data after ATOMS adjustment | |
| Follow-up after ATOMS, months, mean ± SD (range) | 42.5 ± 20 (7–108) |
| Patients with pad test < 20 mL, n (%) | 67 (76.1) |
| Patients with zero pad test, n (%) | 50 (56.8) |
| 24-h pad test, mL, mean ± SD (range) | 38 ± 97 (0–580) |
| 24-h pad count, n, mean ± SD (range) | 0.6 ± 0.9 (0–4) |
| Night pad count, n, mean ± SD (range) | 0.1 ± 0.25 (0–1) |
| ICIQ-SF total | 5.3 ± 3.3 (1–15) |
| ICIQ-SF Question 1 | 1.4 ± 1.2 (0–4) |
| ICIQ-SF Question 2 | 1.2 ± 1.2 (0–4) |
| ICIQ-SF Question 3 | 2.65 ± 1.5 (1–7) |
| PGI-I scale, n (%) | |
| Very much better | 56 (63.6) |
| Much better | 23 (26.2) |
| Better | 8 (9.1) |
| Same as before | 1 (1.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Queissert, F.; Rourke, K.; Schönburg, S.; Giammò, A.; Gonsior, A.; González-Enguita, C.; Romero, A.; Schrader, A.J.; Cruz, F.; Martins, F.E.; et al. ATOMS (Adjustable Transobturator Male System) Is an Effective and Safe Second-Line Treatment Option for Recurrent Urinary Incontinence after Implantation of an AdVance/AdVance XP Fixed Male Sling? A Multicenter Cohort Analysis. J. Clin. Med. 2022, 11, 81. https://doi.org/10.3390/jcm11010081
Queissert F, Rourke K, Schönburg S, Giammò A, Gonsior A, González-Enguita C, Romero A, Schrader AJ, Cruz F, Martins FE, et al. ATOMS (Adjustable Transobturator Male System) Is an Effective and Safe Second-Line Treatment Option for Recurrent Urinary Incontinence after Implantation of an AdVance/AdVance XP Fixed Male Sling? A Multicenter Cohort Analysis. Journal of Clinical Medicine. 2022; 11(1):81. https://doi.org/10.3390/jcm11010081
Chicago/Turabian StyleQueissert, Fabian, Keith Rourke, Sandra Schönburg, Alessandro Giammò, Andreas Gonsior, Carmen González-Enguita, Antonio Romero, Andres J. Schrader, Francisco Cruz, Francisco E. Martins, and et al. 2022. "ATOMS (Adjustable Transobturator Male System) Is an Effective and Safe Second-Line Treatment Option for Recurrent Urinary Incontinence after Implantation of an AdVance/AdVance XP Fixed Male Sling? A Multicenter Cohort Analysis" Journal of Clinical Medicine 11, no. 1: 81. https://doi.org/10.3390/jcm11010081
APA StyleQueissert, F., Rourke, K., Schönburg, S., Giammò, A., Gonsior, A., González-Enguita, C., Romero, A., Schrader, A. J., Cruz, F., Martins, F. E., Dorado, J. F., & Angulo, J. C. (2022). ATOMS (Adjustable Transobturator Male System) Is an Effective and Safe Second-Line Treatment Option for Recurrent Urinary Incontinence after Implantation of an AdVance/AdVance XP Fixed Male Sling? A Multicenter Cohort Analysis. Journal of Clinical Medicine, 11(1), 81. https://doi.org/10.3390/jcm11010081







