Ultrasound Imaging in Predicting the Autograft Size in Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Protocol Registration
2.2. Data Sources and Search Strategy
2.3. Inclusion and Exclusion Criteria
2.4. Data Extraction
2.5. Outcomes
2.6. Study Quality Assessment
2.7. Statistical Analysis
3. Results
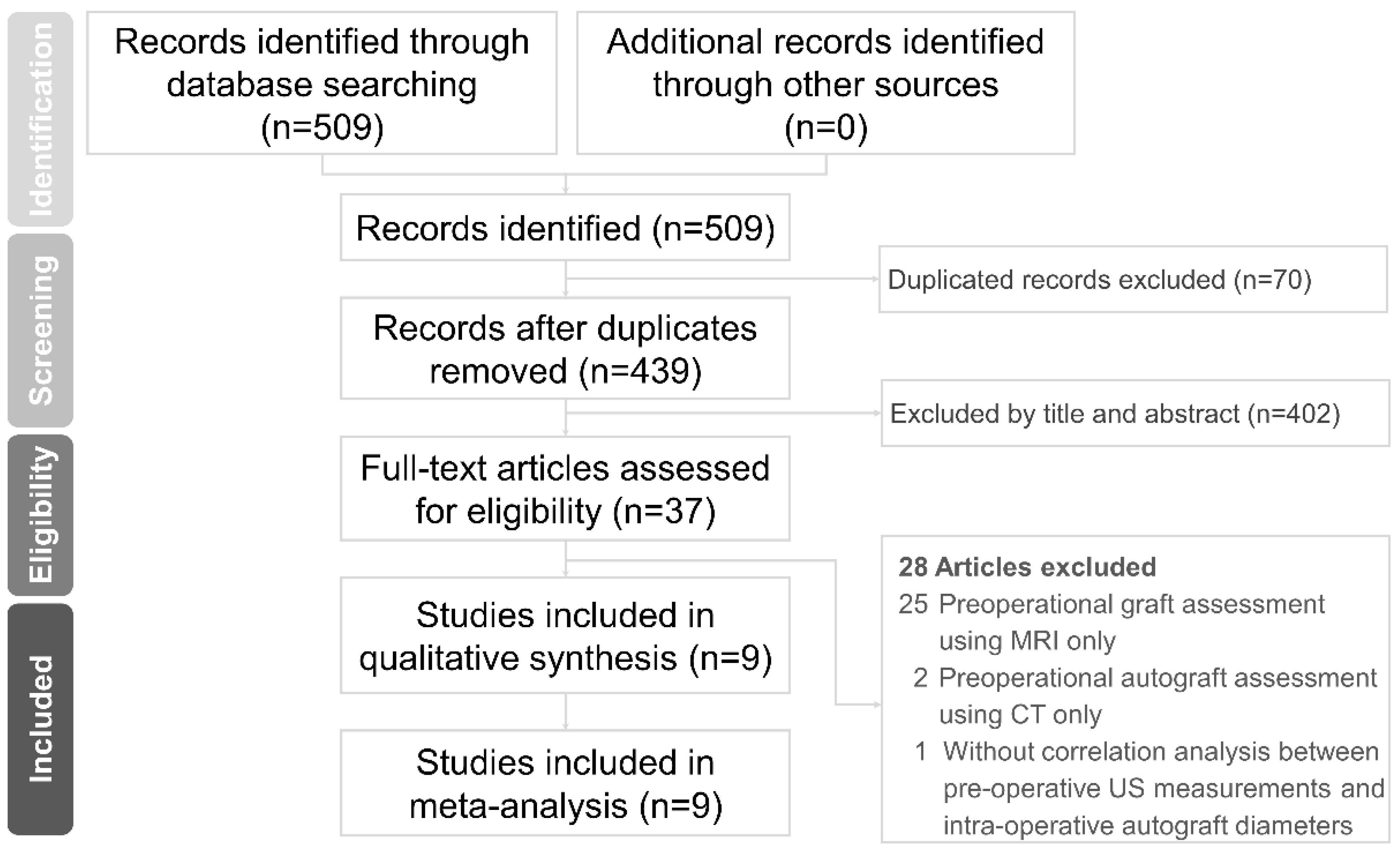
3.1. Literature Search
3.2. Study Characteristics
3.3. Quality Assessment
3.4. Outcome
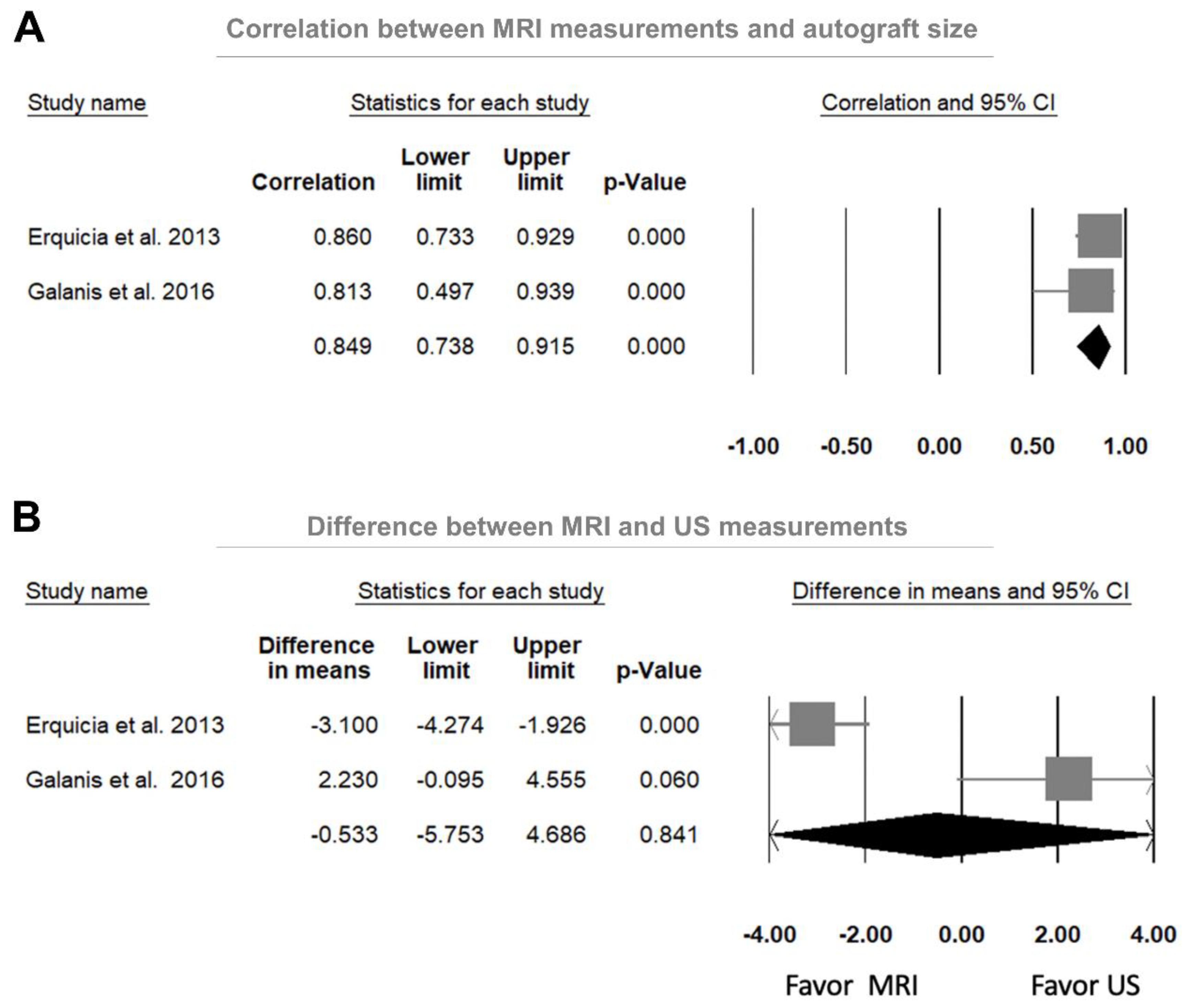
3.4.1. Correlations between Preoperative US and Intraoperative Autograft Measurements
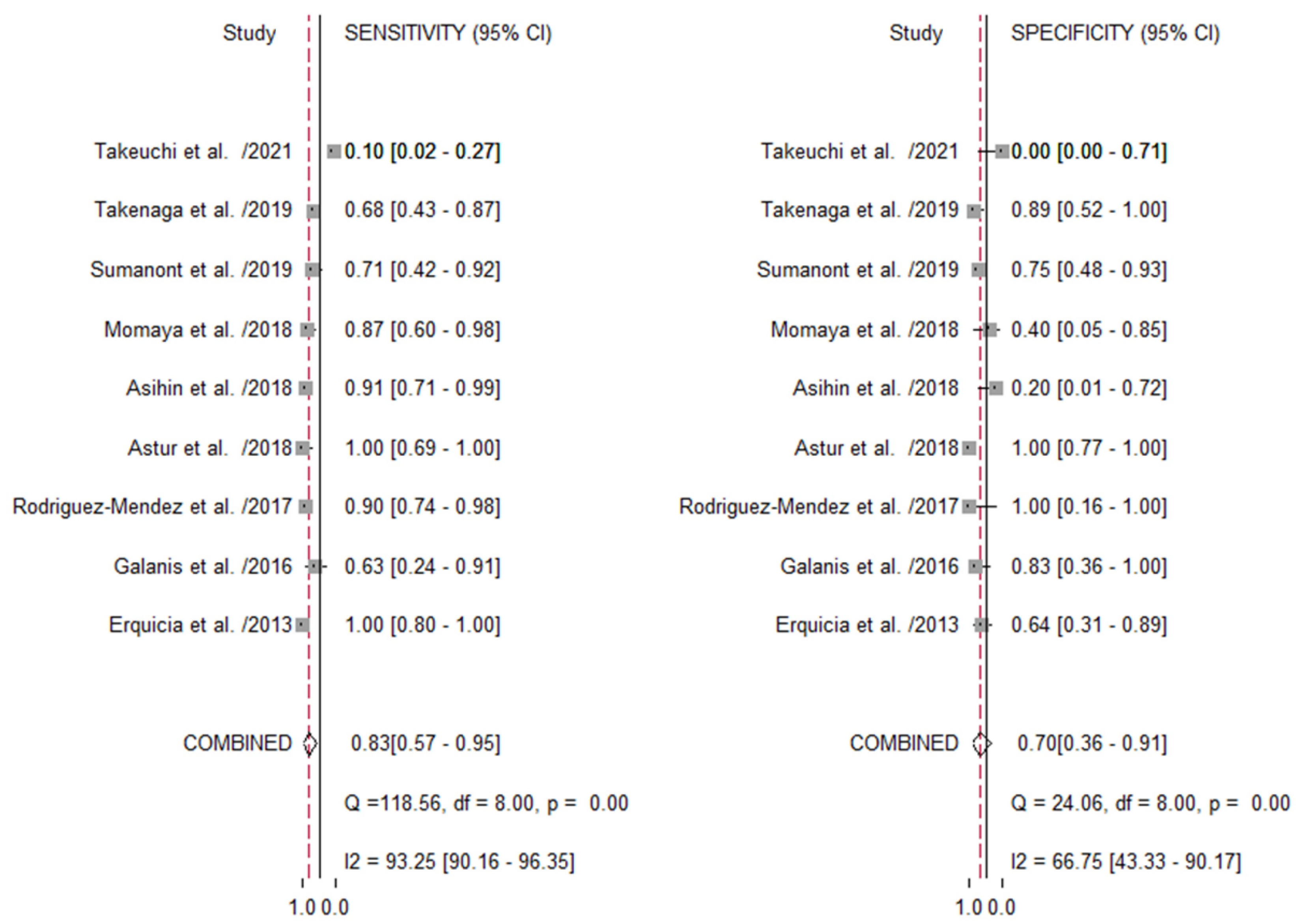
3.4.2. US Imaging in Predicting the Size Adequacy of the Autograft
3.4.3. Comparison between US and MRI Measurements in Predicting the Autograft Size
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gianotti, S.M.; Marshall, S.W.; Hume, P.A.; Bunt, L. Incidence of anterior cruciate ligament injury and other knee ligament injuries: A national population-based study. J. Sci. Med. Sport 2009, 12, 622–627. [Google Scholar] [CrossRef]
- Parkkari, J.; Pasanen, K.; Mattila, V.M.; Kannus, P.; Rimpelä, A. The risk for a cruciate ligament injury of the knee in adolescents and young adults: A population-based cohort study of 46 500 people with a 9 year follow-up. Br. J. Sports Med. 2008, 42, 422–426. [Google Scholar] [CrossRef]
- Boden, B.P.; Sheehan, F.T. Mechanism of Non-Contact ACL Injury. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2021, 41, i47–i51. [Google Scholar] [CrossRef]
- Alazzawi, S.; Sukeik, M.; Ibrahim, M.; Haddad, F.S. Management of anterior cruciate ligament injury: Pathophysiology and treatment. Br. J. Hosp. Med. 2016, 77, 222–225. [Google Scholar] [CrossRef]
- Samitier, G.; Marcano, A.I.; Alentorn-Geli, E.; Cugat, R.; Farmer, K.W.; Moser, M.W. Failure of Anterior Cruciate Ligament Reconstruction. Arch. Bone Jt. Surg. 2015, 3, 220–240. [Google Scholar]
- Moksnes, H.; Risberg, M.A. Performance-based functional evaluation of non-operative and operative treatment after anterior cruciate ligament injury. Scand. J. Med. Sci. Sports 2009, 19, 345–355. [Google Scholar] [CrossRef]
- Hettrich, C.M.; Dunn, W.R.; Reinke, E.K.; Group, M.; Spindler, K.P. The rate of subsequent surgery and predictors after anterior cruciate ligament reconstruction: Two- and 6-year follow-up results from a multicenter cohort. Am. J. Sports Med. 2013, 41, 1534–1540. [Google Scholar] [CrossRef] [PubMed]
- Grassi, A.; Ardern, C.L.; Marcheggiani Muccioli, G.M.; Neri, M.P.; Marcacci, M.; Zaffagnini, S. Does revision ACL reconstruction measure up to primary surgery? A meta-analysis comparing patient-reported and clinician-reported outcomes, and radiographic results. Br. J. Sports Med. 2016, 50, 716–724. [Google Scholar] [CrossRef]
- Magnussen, R.A.; Lawrence, J.T.; West, R.L.; Toth, A.P.; Taylor, D.C.; Garrett, W.E. Graft size and patient age are predictors of early revision after anterior cruciate ligament reconstruction with hamstring autograft. Arthroscopy 2012, 28, 526–531. [Google Scholar] [CrossRef]
- Conte, E.J.; Hyatt, A.E.; Gatt, C.J., Jr.; Dhawan, A. Hamstring autograft size can be predicted and is a potential risk factor for anterior cruciate ligament reconstruction failure. Arthroscopy 2014, 30, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Kamien, P.M.; Hydrick, J.M.; Replogle, W.H.; Go, L.T.; Barrett, G.R. Age, graft size, and Tegner activity level as predictors of failure in anterior cruciate ligament reconstruction with hamstring autograft. Am. J. Sports Med. 2013, 41, 1808–1812. [Google Scholar] [CrossRef]
- Yasumoto, M.; Deie, M.; Sunagawa, T.; Adachi, N.; Kobayashi, K.; Ochi, M. Predictive value of preoperative 3-dimensional computer tomography measurement of semitendinosus tendon harvested for anterior cruciate ligament reconstruction. Arthroscopy 2006, 22, 259–264. [Google Scholar] [CrossRef]
- Bickel, B.A.; Fowler, T.T.; Mowbray, J.G.; Adler, B.; Klingele, K.; Phillips, G. Preoperative magnetic resonance imaging cross-sectional area for the measurement of hamstring autograft diameter for reconstruction of the adolescent anterior cruciate ligament. Arthroscopy 2008, 24, 1336–1341. [Google Scholar] [CrossRef]
- Galanis, N.; Savvidis, M.; Tsifountoudis, I.; Gkouvas, G.; Alafropatis, I.; Kirkos, J.; Kellis, E. Correlation between semitendinosus and gracilis tendon cross-sectional area determined using ultrasound, magnetic resonance imaging and intraoperative tendon measurements. J. Electromyogr. Kinesiol. 2016, 26, 44–51. [Google Scholar] [CrossRef]
- Erquicia, J.I.; Gelber, P.E.; Doreste, J.L.; Pelfort, X.; Abat, F.; Monllau, J.C. How to improve the prediction of quadrupled semitendinosus and gracilis autograft sizes with magnetic resonance imaging and ultrasonography. Am. J. Sports Med. 2013, 41, 1857–1863. [Google Scholar] [CrossRef]
- Rodriguez-Mendez, L.M.; Martinez-Ruiz, J.J.; Perez-Manzo, R.; Corona-Hernandez, J.L.; Alcala-Zermeno, J.L.; Sanchez-Enriquez, S. Preoperative Ultrasonographic Prediction of Hamstring Tendon Diameter for Anterior Cruciate Ligament Repair. J. Knee Surg. 2017, 30, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Mohd Asihin, M.A.; Bajuri, M.Y.; Ahmad, J.; Syed Kamaruddin, S.F. Pre-operative ultrasonographic prediction of hamstring autograft size for anterior cruciate ligament reconstruction surgery. Ceylon Med. J. 2018, 63, 11–16. [Google Scholar] [CrossRef]
- Sumanont, S.; Mahaweerawat, C.; Boonrod, A.; Thammaroj, P.; Boonrod, A. Preoperative Ultrasound Evaluation of the Semitendinosus Tendon for Anterior Cruciate Ligament Reconstruction. Orthop. J. Sports Med. 2019, 7, 2325967118822318. [Google Scholar] [CrossRef] [Green Version]
- Takenaga, T.; Yoshida, M.; Albers, M.; Nagai, K.; Nakamura, T.; Fu, F.H.; Onishi, K. Preoperative sonographic measurement can accurately predict quadrupled hamstring tendon graft diameter for ACL reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, S.; Rothrauff, B.B.; Taguchi, M.; Onishi, K.; Fu, F.H. Preoperative ultrasound predicts the intraoperative diameter of the quadriceps tendon autograft more accurately than preoperative magnetic resonance imaging for anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol. Arthrosc. 2021, 30, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Astur, D.D.C.; Novaretti, J.V.; Liggieri, A.C.; Janovsky, C.; Nicolini, A.P.; Cohen, M. Ultrasonography for evaluation of hamstring tendon diameter: Is it possible to predict the size of the graft? Rev. Bras. Ortop. 2018, 53, 404–409. [Google Scholar] [CrossRef]
- Momaya, A.M.; Beicker, C.; Siffri, P.; Kissenberth, M.J.; Backes, J.; Bailey, L.; Rulewicz, G.J.; Mercuri, J.M.; Shealy, E.C.; Tokish, J.M.; et al. Preoperative Ultrasonography Is Unreliable in Predicting Hamstring Tendon Graft Diameter for ACL Reconstruction. Orthop. J. Sports Med. 2018, 6, 2325967117746146. [Google Scholar] [CrossRef]
- Wu, W.T.; Lee, T.M.; Mezian, K.; Nanka, O.; Chang, K.V.; Ozcakar, L. Ultrasound Imaging of the Anterior Cruciate Ligament: A Pictorial Essay and Narrative Review. Ultrasound Med. Biol. 2021, 30, 52–60. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 339, b2535. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Chen, K.C.; Lee, T.M.; Wu, W.T.; Wang, T.G.; Han, D.S.; Chang, K.V. Assessment of Tongue Strength in Sarcopenia and Sarcopenic Dysphagia: A Systematic Review and Meta-Analysis. Front. Nutr. 2021, 8, 684840. [Google Scholar] [CrossRef]
- Bland, J.M.; Kerry, S.M. Statistics notes. Weighted comparison of means. BMJ 1998, 316, 129. [Google Scholar] [CrossRef] [Green Version]
- Chang, K.V.; Wu, W.T.; Han, D.S.; Özçakar, L. Ulnar Nerve Cross-Sectional Area for the Diagnosis of Cubital Tunnel Syndrome: A Meta-Analysis of Ultrasonographic Measurements. Arch. Phys. Med. Rehabil. 2018, 99, 743–757. [Google Scholar] [CrossRef]
- Chen, I.J.; Chang, K.V.; Wu, W.T.; Özçakar, L. Ultrasound Parameters Other Than the Direct Measurement of Ulnar Nerve Size for Diagnosing Cubital Tunnel Syndrome: A Systemic Review and Meta-analysis. Arch. Phys. Med. Rehabil. 2019, 100, 1114–1130. [Google Scholar] [CrossRef]
- Walter, S.D. Properties of the summary receiver operating characteristic (SROC) curve for diagnostic test data. Stat. Med. 2002, 21, 1237–1256. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Chu, H. Quantifying publication bias in meta-analysis. Biometrics 2018, 74, 785–794. [Google Scholar] [CrossRef]
- Deeks, J.J.; Macaskill, P.; Irwig, L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J. Clin. Epidemiol. 2005, 58, 882–893. [Google Scholar] [CrossRef]
- Mukaka, M.M. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Treme, G.; Diduch, D.R.; Billante, M.J.; Miller, M.D.; Hart, J.M. Hamstring graft size prediction: A prospective clinical evaluation. Am. J. Sports Med. 2008, 36, 2204–2209. [Google Scholar] [CrossRef]
- Mariscalco, M.W.; Flanigan, D.C.; Mitchell, J.; Pedroza, A.D.; Jones, M.H.; Andrish, J.T.; Parker, R.D.; Kaeding, C.C.; Magnussen, R.A. The influence of hamstring autograft size on patient-reported outcomes and risk of revision after anterior cruciate ligament reconstruction: A Multicenter Orthopaedic Outcomes Network (MOON) Cohort Study. Arthroscopy 2013, 29, 1948–1953. [Google Scholar] [CrossRef] [Green Version]
- Alkhalaf, F.N.A.; Hanna, S.; Alkhaldi, M.S.H.; Alenezi, F.; Khaja, A. Autograft diameter in ACL reconstruction: Size does matter. SICOT J. 2021, 7, 16. [Google Scholar] [CrossRef]
- Grawe, B.M.; Williams, P.N.; Burge, A.; Voigt, M.; Altchek, D.W.; Hannafin, J.A.; Allen, A.A. Anterior Cruciate Ligament Reconstruction With Autologous Hamstring: Can Preoperative Magnetic Resonance Imaging Accurately Predict Graft Diameter? Orthop. J. Sports Med. 2016, 4, 2325967116646360. [Google Scholar] [CrossRef] [Green Version]
- Leiter, J.; Elkurbo, M.; McRae, S.; Chiu, J.; Froese, W.; MacDonald, P. Using pre-operative MRI to predict intraoperative hamstring graft size for anterior cruciate ligament reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 229–235. [Google Scholar] [CrossRef]
- Grassi, C.A.; Fruheling, V.M.; Abdo, J.C.; de Moura, M.F.A.; Namba, M.; da Silva, J.L.V.; da Cunha, L.A.M.; de Oliveira Franco, A.P.G.; Costa, I.Z.; Filho, E.S. Hamstring tendons insertion—An anatomical study. Rev. Bras. Ortop. 2013, 48, 417–420. [Google Scholar] [CrossRef] [Green Version]
- Kositsky, A.; Gonçalves, B.A.M.; Stenroth, L.; Barrett, R.S.; Diamond, L.E.; Saxby, D.J. Reliability and Validity of Ultrasonography for Measurement of Hamstring Muscle and Tendon Cross-Sectional Area. Ultrasound Med. Biol. 2020, 46, 55–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamada, M.; Shino, K.; Mitsuoka, T.; Abe, N.; Horibe, S. Cross-sectional area measurement of the semitendinosus tendon for anterior cruciate ligament reconstruction. Arthroscopy 1998, 14, 696–701. [Google Scholar] [CrossRef]
- Wernecke, G.; Harris, I.A.; Houang, M.T.; Seeto, B.G.; Chen, D.B.; MacDessi, S.J. Using magnetic resonance imaging to predict adequate graft diameters for autologous hamstring double-bundle anterior cruciate ligament reconstruction. Arthroscopy 2011, 27, 1055–1059. [Google Scholar] [CrossRef] [PubMed]
- Beyzadeoglu, T.; Akgun, U.; Tasdelen, N.; Karahan, M. Prediction of semitendinosus and gracilis autograft sizes for ACL reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 1293–1297. [Google Scholar] [CrossRef]
- Cobanoglu, M.; Ozgezmez, F.T.; Omurlu, I.K.; Ozkan, I.; Savk, S.O.; Cullu, E. Preoperative magnetic resonance imaging evaluation of semitendinosus tendon in anterior cruciate ligament reconstruction: Does this have an effect on graft choice? Indian J. Orthop. 2016, 50, 499–504. [Google Scholar] [CrossRef]
- Serino, J.; Murray, R.; Argintar, E.H. Use of Magnetic Resonance Imaging to Predict Quadrupled Semitendinosus Graft Diameter in All-Inside Anterior Cruciate Ligament Reconstruction. Orthopedics 2017, 40, e617–e622. [Google Scholar] [CrossRef] [Green Version]
- Zakko, P.; van Eck, C.F.; Guenther, D.; Irrgang, J.J.; Fu, F.H. Can we predict the size of frequently used autografts in ACL reconstruction? Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 3704–3710. [Google Scholar] [CrossRef]
- Ashford, W.B.; Kelly, T.H.; Chapin, R.W.; Xerogeanes, J.W.; Slone, H.S. Predicted quadriceps vs. quadrupled hamstring tendon graft size using 3-dimensional MRI. Knee 2018, 25, 1100–1106. [Google Scholar] [CrossRef]
- Corey, S.; Mueller, T.; Hartness, C.; Prasad, B.M. Correlation of intra-operative hamstring autograft size with pre-operative anthropometric and MRI measurements. J. Orthop. 2018, 15, 988–991. [Google Scholar] [CrossRef]
- Ilahi, O.A.; Staewen, R.S.; Stautberg, E.F., 3rd; Qadeer, A.A. Estimating Lengths of Semitendinosus and Gracilis Tendons by Magnetic Resonance Imaging. Arthroscopy 2018, 34, 2457–2462. [Google Scholar] [CrossRef]
- Hodges, C.T.; Shelton, T.J.; Bateni, C.P.; Henrichon, S.S.; Skaggs, A.W.; Boutin, R.D.; Lee, C.A.; Haus, B.M.; Marder, R.A. The medial epicondyle of the distal femur is the optimal location for MRI measurement of semitendinosus and gracilis tendon cross-sectional area. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 3498–3504. [Google Scholar] [CrossRef] [PubMed]
- Hollnagel, K.; Johnson, B.M.; Whitmer, K.K.; Hanna, A.; Miller, T.K. Prediction of Autograft Hamstring Size for Anterior Cruciate Ligament Reconstruction Using MRI. Clin. Orthop. Relat. Res. 2019, 477, 2677–2684. [Google Scholar] [CrossRef] [PubMed]
- Vardiabasis, N.; Mosier, B.; Walters, J.; Burgess, A.; Altman, G.; Akhavan, S. Can We Accurately Predict the Quadruple Hamstring Graft Diameter From Preoperative Magnetic Resonance Imaging? Orthop. J. Sports Med. 2019, 7, 2325967119834504. [Google Scholar] [CrossRef] [PubMed]
- Oliva Moya, F.; Sotelo Sevillano, B.; Vilches Fernández, J.M.; Mantic Lugo, M.; Orta Chincoa, J.; Andrés García, J.A. Can we predict the graft diameter for autologous hamstring in anterior cruciate ligament reconstruction? Rev. Esp. Cir. Ortop. Traumatol. 2020, 64, 145–150. [Google Scholar] [CrossRef]
- Pérez-Mozas, M.; Payo-Ollero, J.; Montiel-Terrón, V.; Valentí-Nin, J.R.; Valentí-Azcárate, A. Preoperative prediction of autologous hamstring graft diameter in anterior cruciate ligament reconstruction. Rev. Esp. Cir. Ortop. Traumatol. 2020, 64, 310–317. [Google Scholar] [CrossRef]
- Thwin, L.; Ho, S.W.; Tan, T.J.L.; Lim, W.Y.; Lee, K.T. Pre-operative MRI measurements versus anthropometric data: Which is more accurate in predicting 4-stranded hamstring graft size in anterior cruciate ligament reconstruction? Asia-Pac. J. Sports Med. Arthrosc. Rehabil. Technol. 2020, 22, 5–9. [Google Scholar] [CrossRef]
- Heijboer, W.M.P.; Suijkerbuijk, M.A.M.; van Meer, B.L.; Bakker, E.W.P.; Meuffels, D.E. Predictive Factors for Hamstring Autograft Diameter in Anterior Cruciate Ligament Reconstruction. J. Knee Surg. 2021, 34, 605–611. [Google Scholar] [CrossRef]
- Partan, M.J.; Stapleton, E.J.; Atlas, A.M.; DiMauro, J.P. Predicting Autologous Hamstring Graft Diameter in the Pediatric Population Using Preoperative Magnetic Resonance Imaging and Demographic Data. Am. J. Sports Med. 2021, 49, 1482–1491. [Google Scholar] [CrossRef]
- Sherman, B.; Kwan, K.; Schlechter, J. Magnetic Resonance Imaging Predictive Model Determines Hamstring Autograft Size for Anterior Cruciate Ligament Reconstruction in Patients Under 18 Years Old. Arthrosc. Sports Med. Rehabil. 2021, 3, e715–e720. [Google Scholar] [CrossRef]
- Chang, C.B.; Seong, S.C.; Kim, T.K. Preoperative magnetic resonance assessment of patellar tendon dimensions for graft selection in anterior cruciate ligament reconstruction. Am. J. Sports Med. 2009, 37, 376–382. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Verma, N.; McNickle, A.G.; Zelazny, A.; Ghodadra, N.; Bach, B.R., Jr. Avoiding mismatch in allograft anterior cruciate ligament reconstruction: Correlation between patient height and patellar tendon length. Arthroscopy 2010, 26, 643–650. [Google Scholar] [CrossRef]
- Baghdadi, S.; VanEenenaam, D.P., Jr.; Williams, B.A.; Lawrence, J.T.R.; Maguire, K.J.; Wells, L.; Ganley, T.J. Quadriceps Tendon Autograft in Pediatric ACL Reconstruction: Graft Dimensions and Prediction of Size on Preoperative MRI. Orthop. J. Sports Med. 2021, 9, 23259671211056678. [Google Scholar] [CrossRef]
- Gagliardi, A.G.; Howell, D.R.; Stein, J.M.; Monson, M.A.; Pearce, S.S.; Albright, J.C. Prediction of quadriceps tendon-patellar bone autograft diameter in adolescents with 2-dimensional magnetic resonance imaging and anthropometric measures. Skelet. Radiol. 2021, 51, 619–623. [Google Scholar] [CrossRef]
- Truong, P.N.; Toan, N.V.; Nam, V.H.; Fang, W.H.; Vangsness, C.T., Jr.; Han, B.; Hoang, B.X. Preoperative Determination of the Size of the Semitendinosus and Gracilis Tendon by Multidetector Row CT Scanner for Anterior Cruciate Ligament Reconstruction. J. Knee Surg. 2021. [Google Scholar] [CrossRef]
- Seijas, R.; Rius, M.; Barastegui, D.; Ares, O.; Rivera, E.; Alvarez-Diaz, P. Sonographic Measurement of the Patellar Tendon Should Predict Autograft Bone Patellar Tendon Bone (BPTB) Size: Comparison of Anatomical and Clinical Findings. J. Investig. Surg. 2020, 33, 621–626. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]

| Study, Year | Study Design | Autograft | Age | M/F | Ultrasound Setting | Surgical Procedure | Interval US—OP | Outcome | Reference Standard | |
|---|---|---|---|---|---|---|---|---|---|---|
| Manufacturer, Transducer Frequency, CSA Measurement | Probe Position, Examinee Posture, Site of US Measurements | |||||||||
| Erquicia, 2013 [15] | Prospective cohort | 4S-GST | 32 (16–59) † | 25/8 | LOGIQe, GE Healthcare Linear array probe, 7–12 MHz, ellipse tool | NA, prone, knee flexion 90°, proximal to the medial joint line | GT, ST harvested GT, ST paired, closed-hole sizing block | 15 days | CSA: GT (US, MRI), ST (US, MRI), GT + ST (US, MRI) Diameter: 4S-GST (OP) No inter-rater, intra-rater reliability | Autograft diameter |
| Galanis, 2016 [14] | Prospective cohort | 4S-GST | 31.14 ± 3.11 * | 14/0 | Siemens Acuson S2000 Linear array probe, 10 MHz, ellipse or dotted line tool | Perpendicular to the tendon, prone, knee flexion 30°, near the widest point of the medial femoral epicondyle | GT, ST tendons harvested GT, ST paired, closed-hole sizing block | NA | CSA: GT + ST (US, MRI), ST (US, MRI), GT (US, MRI) Diameter: 4S-GST (OP), ST (US, MRI), GT (US, MRI) Inter-rater and intra-rater reliability | Autograft diameter |
| Rodriguez-Mendez, 2017 [16] | Prospective cohort | 4S-GST | (16–43) † | 33/0 | Siemens Acuson S2000 Linear array probe, 14 MHz, NA | Perpendicular to the tendon, prone, knee flexion 0°, posterior medial of proximal tibia with widest zone | GT, ST tendons harvested GT, ST folded a quadruple tendon | NA | Diameter: GT + ST (US), GT (US, OP), ST (US, OP), 4S-GST (OP) Length: 4S-GST (OP), ST (OP), GT (OP) No inter-rater, intra-rater reliability | Autograft diameter |
| Astur, 2018 [21] | Cross-sectional | 4S-GST | 24.8 ± 8.4 * | 19/5 | Logic P6 device, 7–11 MHz, NA | NA, ventral recumbent, the articular line | GT, ST tendons harvested ST, GT folded in half to form a quadruple graft | 7 days | CSA: GT + ST (US) Diameter: GT (US), ST (US), 4S-GST (OP) No inter-rater, intra-rater reliability | Autograft diameter |
| Asihin, 2018 [17] | Prospective cohort | 4S-GST | 28.48 ± 6.0 * | 23/4 | Philips HD11 XE Linear array probe, 5–12 MHz, ellipse tool | NA, prone with knee flexion in 30°, the medial joint line | GT, ST harvested with a closed-end tendon harvester | 1 day | CSA: ST + GT (US) Diameter: 4S-GST (OP) No inter-rater, intra-rater reliability | Autograft diameter |
| Momaya, 2018 [22] | Prospective cohort | 4S-GST | 22.8 ± 6.6 * | 10/10 | Fujifilm SonoSite, NA, NA | NA, prone with knee flexion in 30° | GT, ST harvested with a closed-loop tendon stripper | 14 days | CSA: ST + GT (US) Diameter: 4S-GST (OP) Inter-rater, intra-rater reliability | Autograft diameter |
| Sumanont, 2019 [18] | Prospective cohort | 4S-ST | 29.3 ± 9.6 * | 37/3 | NA, NA, NA | NA, supine with knee flexion in 30°, the posterior medial aspect of the knee joint | ST harvested with a closed tendon stripper | NA | Diameter: ST (US, OP), 4S-ST (OP) Length: ST (US) CSA: ST (US) Inter-rater, intra-rater reliability | Autograft diameter |
| Takenaga, 2019 [19] | Prospective cohort | 4S-GST | 21.9 ± 8.6 * | 11/17 | Medicine RS80 Prestige linear-array probe, 4–18 MHz, freehand tracing | NA, supine with the hip in maximal ER and the knee in flexion 20°, the myotendinous junction of the sartorius muscle | GT, ST harvested with tendon stripper, suturing the distal end of tendon | 11.3 ± 9.9 days * | CSA: GT + ST (US), ST (US), GT (US) Thickness: GT (US), ST (US) Width: GT (US), ST (US) Diameter: 4S-GST (OP), 2GT (OP), 2ST (OP) Inter-rater, intra-rater reliability | Autograft diameter |
| Takeuchi, 2021 [20] | Prospective cohort | QT | 19.9 ± 5.0 * | 18/12 | Medicine RS80 Prestige linear-array probe, 4–18 MHz, NA | Perpendicular to the tendon, supine with the knee flexion in 20°, anterior knee proximal to the superior pole of the patella at a distance of 15 mm & 30 mm | QT harvested | 17.9 ± 22.1 days * | CSA: QT (US, MRI) Diameter: QT (OP), QT (US, MRI) Inter-rater, intra-rater reliability | Autograft diameter |
| Risk of Bias | Applicability Concerns | ||||||
|---|---|---|---|---|---|---|---|
| Study | Patient Selection | Index Test (US Measurement) | Reference Standard (Autograft Size) | Flow and Timing | Patient Selection | Index Test (US Measurement) | Reference Standard (Autograft Size) |
| Erquicia, 2013 [15] | Low | Low | Low | Low | Low | Low | Low |
| Galanis, 2016 [14] | Low | Low | Low | High | Low | Low | Low |
| Rodriguez-Mendez, 2017 [16] | Low | Low | Low | High | Low | Low | Low |
| Astur, 2018 [21] | Low | Low | Low | Low | Low | Low | Low |
| Asihin, 2018 [17] | Low | Low | Low | Low | Low | Low | Low |
| Momaya, 2018 [22] | Low | Low | Low | Low | Low | Low | Low |
| Sumanont, 2019 [18] | Low | Low | Low | High | Low | Low | Low |
| Takenaga, 2019 [19] | Low | Low | Low | Low | Low | Low | Low |
| Takeuchi, 2021 [20] | Low | Low | Low | Low | Low | Low | Low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, T.-M.; Wu, W.-T.; Chiu, Y.-H.; Chang, K.-V.; Özçakar, L. Ultrasound Imaging in Predicting the Autograft Size in Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 3876. https://doi.org/10.3390/jcm11133876
Lee T-M, Wu W-T, Chiu Y-H, Chang K-V, Özçakar L. Ultrasound Imaging in Predicting the Autograft Size in Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2022; 11(13):3876. https://doi.org/10.3390/jcm11133876
Chicago/Turabian StyleLee, Tsung-Min, Wei-Ting Wu, Yi-Hsiang Chiu, Ke-Vin Chang, and Levent Özçakar. 2022. "Ultrasound Imaging in Predicting the Autograft Size in Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 11, no. 13: 3876. https://doi.org/10.3390/jcm11133876
APA StyleLee, T.-M., Wu, W.-T., Chiu, Y.-H., Chang, K.-V., & Özçakar, L. (2022). Ultrasound Imaging in Predicting the Autograft Size in Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 11(13), 3876. https://doi.org/10.3390/jcm11133876








