Metal Artifact Reduction Sequences MRI: A Useful Reference for Preoperative Diagnosis and Debridement Planning of Periprosthetic Joint Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participant Selection and Clinical Data Collection
2.2. MARS Protocol
2.3. Definition of MARS Characteristic Performance on PJI
2.4. MRI Analysis
2.5. Definition of Suspicious Occult Infectious Lesions
2.6. Statistical Analysis
3. Results
3.1. Demographics of Cases
3.2. MARS MRI Features of PJI and its Diagnostic Efficiency
3.3. Diagnostic Efficacy of MARS MRI
3.4. Suspicious Occult Infectious Lesions Revealed by MARS MRI
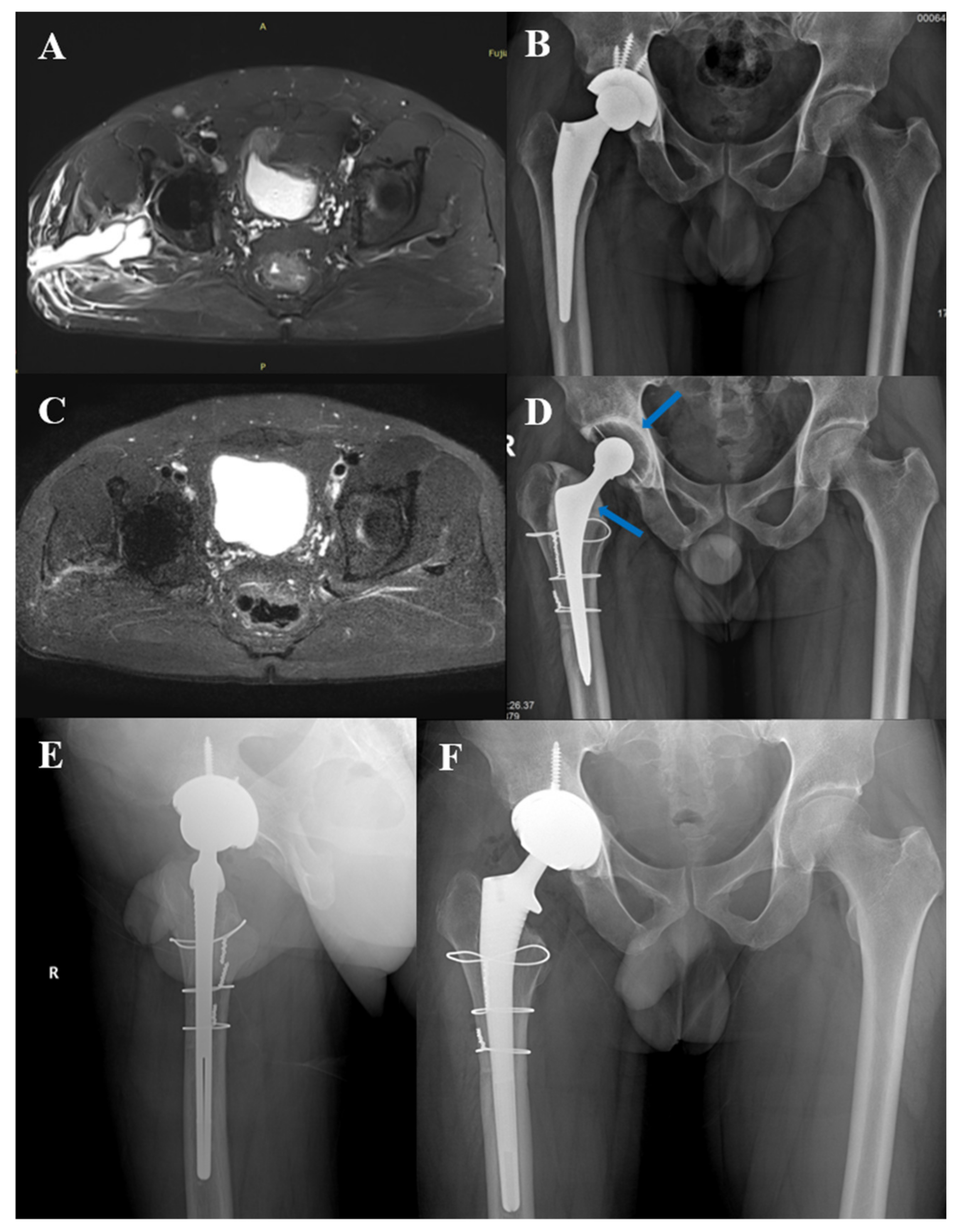
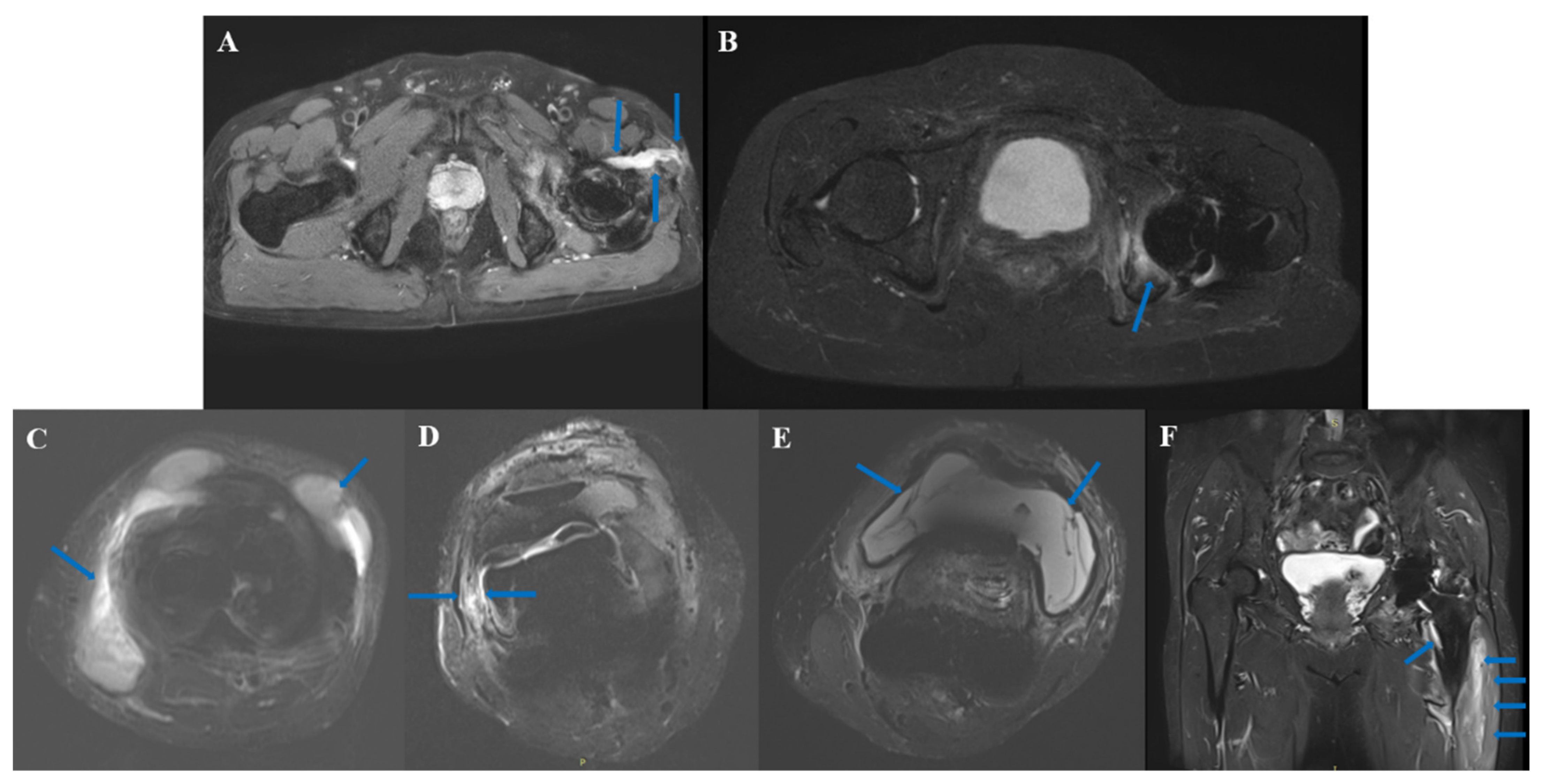
3.5. Typical Cases
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kapadia, B.H.; Berg, R.A.; Daley, J.A.; Fritz, J.; Bhave, A.; Mont, M.A. Periprosthetic joint infection. Lancet 2016, 387, 386–394. [Google Scholar] [CrossRef]
- Tande, A.J.; Gomez-Urena, E.O.; Berbari, E.F.; Osmon, D.R. Management of Prosthetic Joint Infection. Infect. Dis. Clin. N. Am. 2017, 31, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, J.; Gehrke, T.; Chen, A.F. Proceedings of the International Consensus on Periprosthetic Joint Infection. Bone Jt. J. 2013, 95, 1450–1452. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, J.; Tan, T.L.; Goswami, K.; Higuera, C.; Della Valle, C.; Chen, A.F.; Shohat, N. The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria. J. Arthroplast. 2018, 33, 1309–1314.e2. [Google Scholar] [CrossRef] [PubMed]
- Karim, M.A.; Andrawis, J.; Bengoa, F.; Bracho, C.; Compagnoni, R.; Cross, M.; Danoff, J.; Valle, C.J.D.; Foguet, P.; Fraguas, T.; et al. Hip and Knee Section, Diagnosis, Algorithm: Proceedings of International Consensus on Orthopedic Infections. J. Arthroplast. 2019, 34, S339–S350. [Google Scholar] [CrossRef] [PubMed]
- Tubb, C.C.; Polkowksi, G.G.; Krause, B. Diagnosis and Prevention of Periprosthetic Joint Infections. J. Am. Acad. Orthop. Surg. 2020, 28, e340–e348. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.A.; Chen, W.; Goodman, S.B.; Hargreaves, B.A.; Koch, K.M.; Lu, W.; Brau, A.C.; Draper, C.E.; Delp, S.L.; Gold, G.E. New MR imaging methods for metallic implants in the knee: Artifact correction and clinical impact. J. Magn. Reson Imaging 2011, 33, 1121–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jungmann, P.M.; Agten, C.A.; Pfirrmann, C.W.; Sutter, R. Advances in MRI around metal: MRI Around Metal. J. Magn. Reson Imaging 2017, 46, 972–991. [Google Scholar] [CrossRef] [PubMed]
- Khodarahmi, I.; Nittka, M.; Fritz, J. Leaps in Technology: Advanced MR Imaging after Total Hip Arthroplasty. Semin. Musculoskelet Radiol. 2017, 21, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Galley, J.; Sutter, R.; Stern, C.; Filli, L.; Rahm, S.; Pfirrmann, C.W.A. Diagnosis of Periprosthetic Hip Joint Infection Using MRI with Metal Artifact Reduction at 1.5 T. Radiology 2020, 296, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Fang, X.; Huang, C.; Li, Z.; Huang, Z.; Zhang, C.; Li, W.; Zhang, Z.; Guan, Z.; Zhang, W. Destination Joint Spacers: A Similar Infection-Relief Rate But Higher Complication Rate Compared with Two-Stage Revision. Orthop Surg. 2021, 13, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Fritz, J.; Lurie, B.; Miller, T.T.; Potter, H.G. MR Imaging of Hip Arthroplasty Implants. RadioGraphics 2014, 34, E106–E132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fritz, J.; Lurie, B.; Potter, H.G. MR Imaging of Knee Arthroplasty Implants. RadioGraphics 2015, 35, 1483–1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plodkowski, A.J.; Hayter, C.L.; Miller, T.T.; Nguyen, J.T.; Potter, H.G. Lamellated Hyperintense Synovitis: Potential MR Imaging Sign of an Infected Knee Arthroplasty. Radiology 2013, 266, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Zhang, L.; Cai, Y.; Huang, Z.; Li, W.; Zhang, C.; Yang, B.; Lin, J.; Wahl, P.; Zhang, W. Effects of different tissue specimen pretreatment methods on microbial culture results in the diagnosis of periprosthetic joint infection. Bone Jt. Res. 2021, 10, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Li, W.; Lee, G.-C.; Fang, X.; Xing, L.; Yang, B.; Lin, J.; Zhang, W. Metagenomic next-generation sequencing of synovial fluid demonstrates high accuracy in prosthetic joint infection diagnostics: mNGS for diagnosing PJI. Bone Jt. Res. 2020, 9, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Sdao, S.; Orlandi, D.; Aliprandi, A.; Lacelli, F.; Sconfienza, L.M.; Randelli, F.; Sardanelli, F.; Serafini, G. The role of ultrasonography in the assessment of peri-prosthetic hip complications. J. Ultrasound. 2015, 18, 245–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pozzuoli, A.; Berizzi, A.; Crimì, A.; Belluzzi, E.; Frigo, A.C.; Conti, G.D.; Nicolli, A.; Trevisan, A.; Biz, C.; Ruggieri, P. Metal Ion Release, Clinical and Radiological Outcomes in Large Diameter Metal-on-Metal Total Hip Arthroplasty at Long-Term Follow-Up. Diagnostics 2020, 10, 941. [Google Scholar] [CrossRef] [PubMed]


| TE (ms) | TR (ms) | TI (ms) | FOV (mm) | NEX | RBW (kHZ) | ST (mm) | Matrix | VAT | Flip Angle (Degrees) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hip | T1w tse Tra | 7.7 | 620 | 360 × 360 | 1 | 625 | 4 | 320 × 192 | On | 144 | |
| PD tirm Cor | 36 | 4160 | 220 | 340 × 340 | 2 | 601 | 4 | 320 × 256 | On | 138 | |
| PD tirm Tra | 36 | 3500 | 220 | 360 × 270 | 2 | 601 | 4 | 320 × 256 | On | 137 | |
| Knee | T1w tse Sag | 8.7 | 420 | 180 × 180 | 1 | 625 | 4 | 320 × 224 | On | 137 | |
| PD tirm Sag | 33 | 5380 | 220 | 180 × 180 | 2 | 601 | 4 | 320 × 192 | On | 138 | |
| PD tirm Cor | 33 | 5380 | 220 | 180 × 180 | 2 | 601 | 4 | 320 × 192 | On | 138 | |
| PD tirm Tra | 33 | 3500 | 220 | 200 × 162 | 2 | 601 | 4 | 320 × 256 | On | 137 |
| PJI, n = 31 | Non-PJI, n = 20 | |||
|---|---|---|---|---|
| Mono-Pathogen, n = 21 | Multi-Pathogen, n = 2 | CN PJI, n = 8 | Aseptic Loosening, n = 6 | Stage II Reimplantation Surgery, n = 14 |
| Methicillin sensitive staphylococcus aureus, n = 3 | Stenotrophomonas maltophilia, Pseudomonas monteilii, Enterococcus faecalis, n = 1 | Staphylococcus hominis, n = 1 | ||
| Klebsiella peneumoniae, n = 1 | Ralstonia pickettii, Staphylococcus epidermidis, n = 1 | Staphylococcus haemolyticus, n = 1 | ||
| Escherichia coli, n = 1 | ||||
| Methicillin-resistant staphylococcus aureus, n = 5 | ||||
| Aggregatibacter aphrophilus, n = 1 | ||||
| Methicillin sensitive staphylococcus epidermidis, n = 2 | ||||
| Enterobacter cloacae, n = 1 | ||||
| Staphylococcus warneri, n = 1 | ||||
| Staphylococcus epidermidis, n = 2 | ||||
| Pseudomonas aeruginosa, n = 1 | ||||
| Streptococcus hemolyticus, n = 1 | ||||
| Candida albicans, n = 2 | ||||
| Characteristic | All Cases | PJI | Non-PJI | p-Value (PJI vs. Non-PJI) | |
|---|---|---|---|---|---|
| Female (n) | 34 | 18 | 16 | 0.105 a | |
| Age (yrs) | 60.569 ± 12.400 | 60.774 ± 13.266 | 60.250 ± 11.252 | 0.885 b | |
| Joint involved (n) | 0.891 a | ||||
| hip | 30 | 18 | 12 | ||
| knee | 21 | 13 | 8 | ||
| CRP (mg/L), median, IQR | 11.020 (4.210, 27.260) | 19.29 (11.100, 69.300) | 4.380 (2.120, 5.823) | <0.001 c | |
| ESR (mm/h), ± S | 47.800 ± 33.829 | 64.650 ± 33.602 | 28.700 ± 17.147 | <0.001 b | |
| SF-WBC (×106/L), median, IQR | 2623.000 (795.000, 8024.000) | 6185.000 (2623.000, 35,883.000) | 672.000 (278.250, 1110.750) | <0.001 c | |
| SF-PMN% (%), median, IQR | 66.600 (45.200, 83.900) | 81.900 (66.900, 88.100) | 45.200 (39.025, 51.225) | <0.001 c | |
| Variable | PJI | Non-PJI | p- Value a | Sensitivity | Specificity | Youden’s Index | PPV | NPV | LR+ | LR− |
|---|---|---|---|---|---|---|---|---|---|---|
| Sinus | 4/31 | 0/20 | 0.145 | 12.90% | 100% | 0.129 | 100% | 42.55% | N/A | 0.871 |
| Bone destruction | 17/31 | 5/20 | 0.046 | 54.84% | 75% | 0.298 | 77.27% | 51.72% | 2.194 | 0.602 |
| Bone edema | 23/31 | 10/20 | 0.132 | 74.19% | 50% | 0.242 | 69.70% | 55.56% | 1.484 | 0.516 |
| Lamellated synovitis | 20/31 | 5/20 | 0.009 | 64.52% | 75% | 0.395 | 80.00% | 57.69% | 2.581 | 0.473 |
| Extracapsular edema | 28/31 | 7/20 | <0.001 | 90.32% | 65% | 0.7 | 80.00% | 81.25% | 2.581 | 0.149 |
| Intracapsular collections | 24/31 | 14/20 | 0.743 | 77.42% | 30% | 0.074 | 63.16% | 46.15% | 1.106 | 0.753 |
| Extracapsular collections | 11/31 | 5/20 | 0.543 | 35.48% | 75% | 0.105 | 68.75% | 42.86% | 1.419 | 0.860 |
| Variable | PJI | Non-PJI | Sensitivity | p-Value a (VS. MARS) | Specificity | p-Value a (VS. MARS) | Youden’s Index | PPV | NPV | LR+ | LR− |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MARS MRI | 25/31 | 5/20 | 80.65% | N/A | 75% | N/A | 0.556 | 83.33% | 71.43% | 3.226 | 0.258 |
| Culture | 23/31 | 5/20 | 74.19% | 0.727 | 75% | 1.000 | 0.642 | 92% | 69.23% | 7.419 | 0.287 |
| Pre-OP culture | 10/31 | 3/20 | 32.26% | 0.001 | 85% | 0.625 | 0.173 | 76.92% | 44.74% | 2.151 | 0.797 |
| Intra-OP culture | 21/31 | 2/20 | 67.74% | 0.344 | 90% | 0.453 | 0.577 | 91.30% | 64.29% | 6.774 | 0.358 |
| CRP (>10 mg/L) | 26/31 | 2/20 | 83.87% | 1.000 | 90% | 0.375 | 0.739 | 92.86% | 78.26% | 8.387 | 0.179 |
| ESR (>30 mm/h) | 25/31 | 8/20 | 80.65% | 1.000 | 60% | 0.180 | 0.406 | 75.76% | 66.67% | 2.016 | 0.323 |
| SF-WBC (>3000 × 106/L) | 23/31 | 1/20 | 74.19% | 0.687 | 95% | 0.125 | 0.692 | 95.83% | 70.37% | 14.84 | 0.272 |
| SF-PMN% (>80%) | 19/31 | 0/20 | 61.30% | 0.109 | 100% | N/A | 0.613 | 100% | 62.50% | N/A | 0.387 |
| Bone destruction+ Lamellated synovitis | 26/31 | 7/20 | 83.87% | - | 65% | - | 0.489 | 78.79% | 72.22% | 2.396 | 0.248 |
| Bone destruction+ Extracapsular edema | 28/31 | 9/20 | 90.32% | - | 55% | - | 0.453 | 75.68% | 78.57% | 2.007 | 0.176 |
| Lamellated synovitis+ Extracapsular edema | 29/31 | 10/20 | 93.55% | - | 50% | - | 0.435 | 74.36% | 83.33% | 1.871 | 0.129 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Chen, Y.; Ding, H.; Huang, Z.; Zhang, C.; Li, W.; Liu, X.; Tu, Z.; Zhang, W.; Fang, X. Metal Artifact Reduction Sequences MRI: A Useful Reference for Preoperative Diagnosis and Debridement Planning of Periprosthetic Joint Infection. J. Clin. Med. 2022, 11, 4371. https://doi.org/10.3390/jcm11154371
Huang C, Chen Y, Ding H, Huang Z, Zhang C, Li W, Liu X, Tu Z, Zhang W, Fang X. Metal Artifact Reduction Sequences MRI: A Useful Reference for Preoperative Diagnosis and Debridement Planning of Periprosthetic Joint Infection. Journal of Clinical Medicine. 2022; 11(15):4371. https://doi.org/10.3390/jcm11154371
Chicago/Turabian StyleHuang, Changyu, Yang Chen, Haiqi Ding, Zida Huang, Chaofan Zhang, Wenbo Li, Xi Liu, Zhanhai Tu, Wenming Zhang, and Xinyu Fang. 2022. "Metal Artifact Reduction Sequences MRI: A Useful Reference for Preoperative Diagnosis and Debridement Planning of Periprosthetic Joint Infection" Journal of Clinical Medicine 11, no. 15: 4371. https://doi.org/10.3390/jcm11154371
APA StyleHuang, C., Chen, Y., Ding, H., Huang, Z., Zhang, C., Li, W., Liu, X., Tu, Z., Zhang, W., & Fang, X. (2022). Metal Artifact Reduction Sequences MRI: A Useful Reference for Preoperative Diagnosis and Debridement Planning of Periprosthetic Joint Infection. Journal of Clinical Medicine, 11(15), 4371. https://doi.org/10.3390/jcm11154371






