Motor Coordination and Global Development in Subjects with Down Syndrome: The Influence of Physical Activity
Abstract
:1. Introduction
1.1. Motor Development and Down Syndrome
1.2. Benefits of Physical Activity
1.3. Aim of the Study
2. Materials and Methods
2.1. Study Procedure
2.1.1. Participants Recruitment
2.1.2. Participants Characteristics
2.1.3. Participants Allocation
2.1.4. Motor Coordination Test Selection
2.1.5. Motor Coordination Evaluation
2.1.6. Global Development Evaluation
2.2. Statistical Analysis
3. Results
4. Discussion
Strength and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, H.I.; Kim, S.W.; Kim, J.; Jeon, H.R.; Jung, D.W. Motor and Cognitive Developmental Profiles in Children With Down Syndrome. Ann. Rehabil. Med. 2017, 41, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Simons, J.; Daly, D.; Theodorou, F.; Caron, C.; Simons, J.; Andoniadou, E. Validity and reliability of the TGMD-2 in 7-10-year-old Flemish children with intellectual disability. Adapt. Phys. Act. Q. 2008, 25, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Smits-Engelsman, B.; Hill, E.L. The relationship between motor coordination and intelligence across the IQ range. Pediatrics 2012, 130, e950–e956. [Google Scholar] [CrossRef] [PubMed]
- Wuang, Y.P.; Wang, C.C.; Huang, M.H.; Su, C.Y. Profiles and cognitive predictors of motor functions among early school-age children with mild intellectual disabilities. J. Intellect. Disabil. Res. 2008, 52, 1048–1060. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, G.; Giustino, V.; Tabacchi, G.; Lanza, M.; Schena, F.; Biino, V.; Giuriato, M.; Gallotta, M.C.; Guidetti, L.; Baldari, C.; et al. Interrelationship Between Age, Gender, and Weight Status on Motor Coordination in Italian Children and Early Adolescents Aged 6–13 Years Old. Front. Pediatr. 2021, 9, 738294. [Google Scholar] [CrossRef]
- World Health Organization. International Classification of Functioning, Disability and Health: ICF; World Health Organization: Geneva, Switzerland, 2001.
- Jankowicz-Szymanska, A.; Mikolajczyk, E.; Wojtanowski, W. The effect of physical training on static balance in young people with intellectual disability. Res. Dev. Disabil. 2012, 33, 675–681. [Google Scholar] [CrossRef]
- Holzapfel, S.D.; Ringenbach, S.D.; Mulvey, G.M.; Sandoval-Menendez, A.M.; Cook, M.R.; Ganger, R.O.; Bennett, K. Improvements in manual dexterity relate to improvements in cognitive planning after assisted cycling therapy (ACT) in adolescents with down syndrome. Res. Dev. Disabil. 2015, 45–46, 261–270. [Google Scholar] [CrossRef]
- Sollerhed, A.C.; Hedov, G. Active Parents-Active Children-A Study among Families with Children and Adolescents with Down Syndrome. Int. J. Environ. Res. Public Health 2021, 18, 660. [Google Scholar] [CrossRef]
- Dipasquale, S.; Canter, B.; Roberts, M. Integrative Dance for Adults with Down Syndrome: Effects on Postural Stability. Int. J. Exerc. Sci. 2020, 13, 1317–1325. [Google Scholar]
- Chen, C.C.; Ringenbach, S.D.; Albert, A.R. Assisted cycling exercise improves fine manual dexterity in persons with Down’s syndrome. J. Appl. Res. Intellect. Disabil. 2014, 27, 264–272. [Google Scholar] [CrossRef]
- Terblanche, E.; Boer, P.H. The functional fitness capacity of adults with Down syndrome in South Africa. J. Intellect. Disabil. Res. 2013, 57, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Alesi, M.; Battaglia, G. Chapter Six—Motor development and Down syndrome. In International Review of Research in Developmental Disabilities; Lanfranchi, S., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 56, pp. 169–211. [Google Scholar]
- Frank, K.; Esbensen, A.J. Fine motor and self-care milestones for individuals with Down syndrome using a Retrospective Chart Review. J. Intellect. Disabil. Res. 2015, 59, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Pereira, K.; Basso, R.P.; Lindquist, A.R.; da Silva, L.G.; Tudella, E. Infants with Down syndrome: Percentage and age for acquisition of gross motor skills. Res. Dev. Disabil. 2013, 34, 894–901. [Google Scholar] [CrossRef]
- Palisano, R.J.; Walter, S.D.; Russell, D.J.; Rosenbaum, P.L.; Gemus, M.; Galuppi, B.E.; Cunningham, L. Gross motor function of children with down syndrome: Creation of motor growth curves. Arch. Phys. Med. Rehabil. 2001, 82, 494–500. [Google Scholar] [CrossRef]
- Winders, P.; Wolter-Warmerdam, K.; Hickey, F. A schedule of gross motor development for children with Down syndrome. J. Intellect. Disabil. Res. 2019, 63, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Abd, G.; El, M. Fine motor skill proficiency in children with and without down syndrome. J. Phys. Ther. Health Promot. 2016, 4, 43–50. [Google Scholar] [CrossRef]
- Alesi, M.; Battaglia, G.; Pepi, A.; Bianco, A.; Palma, A. Gross motor proficiency and intellectual functioning: A comparison among children with Down syndrome, children with borderline intellectual functioning, and typically developing children. Medicine 2018, 97, e12737. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, G.P.; Reilly, N.E.; Mottram, T.M.; Khan, M.A.; Elliott, D. Sequential aiming movements and the one-target advantage in individuals with Down syndrome. Res. Dev. Disabil. 2013, 34, 3858–3866. [Google Scholar] [CrossRef]
- Vuijk, P.J.; Hartman, E.; Scherder, E.; Visscher, C. Motor performance of children with mild intellectual disability and borderline intellectual functioning. J. Intellect. Disabil. Res. 2010, 54, 955–965. [Google Scholar] [CrossRef]
- Westendorp, M.; Hartman, E.; Houwen, S.; Smith, J.; Visscher, C. The relationship between gross motor skills and academic achievement in children with learning disabilities. Res. Dev. Disabil. 2011, 32, 2773–2779. [Google Scholar] [CrossRef]
- Galli, M.; Rigoldi, C.; Mainardi, L.; Tenore, N.; Onorati, P.; Albertini, G. Postural control in patients with Down syndrome. Disabil. Rehabil. 2008, 30, 1274–1278. [Google Scholar] [CrossRef] [PubMed]
- Malak, R.; Kostiukow, A.; Krawczyk-Wasielewska, A.; Mojs, E.; Samborski, W. Delays in Motor Development in Children with Down Syndrome. Med. Sci. Monit. 2015, 21, 1904–1910. [Google Scholar] [CrossRef] [PubMed]
- Pinter, J.D.; Eliez, S.; Schmitt, J.E.; Capone, G.T.; Reiss, A.L. Neuroanatomy of Down’s syndrome: A high-resolution MRI study. Am. J. Psychiatry 2001, 158, 1659–1665. [Google Scholar] [CrossRef] [PubMed]
- Rondal, J.A.; Perera, J. Down Syndrome: Neurobehavioural Specificity; John Wiley: Chichester, UK, 2006. [Google Scholar]
- Saavedra, S.; Joshi, A.; Woollacott, M.; van Donkelaar, P. Eye hand coordination in children with cerebral palsy. Exp. Brain Res. 2009, 192, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Sveljo, O.; Culic, M.; Koprivsek, K.; Lucic, M. The functional neuroimaging evidence of cerebellar involvement in the simple cognitive task. Brain Imaging Behav. 2014, 8, 480–486. [Google Scholar] [CrossRef]
- Teipel, S.J.; Alexander, G.E.; Schapiro, M.B.; Moller, H.J.; Rapoport, S.I.; Hampel, H. Age-related cortical grey matter reductions in non-demented Down’s syndrome adults determined by MRI with voxel-based morphometry. Brain 2004, 127, 811–824. [Google Scholar] [CrossRef]
- Maiano, C.; Hue, O.; Tracey, D.; Lepage, G.; Morin, A.J.S.; Moullec, G. Static postural control among school-aged youth with Down syndrome: A systematic review. Gait Posture 2018, 62, 426–433. [Google Scholar] [CrossRef]
- Giustino, V.; Messina, G.; Alesi, M.; La Mantia, L.; Palma, A.; Battaglia, G. Study of postural control and body balance in subjects with Down syndrome. Hum. Mov. 2021, 22, 66–71. [Google Scholar] [CrossRef]
- Rigoldi, C.; Galli, M.; Celletti, C.; Blow, D.; Camerota, F.; Albertini, G. Does neuromuscular taping influence hand kinesiology? A pilot study on Down’s Syndrome. Clin. Ter. 2015, 166, e188–e194. [Google Scholar] [CrossRef]
- Aoki, S.; Yamauchi, Y.; Hashimoto, K. Developmental trend of children with Down’s syndrome—How do sex and neonatal conditions influence their developmental patterns? Brain Dev. 2018, 40, 181–187. [Google Scholar] [CrossRef]
- Patterson, T.; Rapsey, C.M.; Glue, P. Systematic review of cognitive development across childhood in Down syndrome: Implications for treatment interventions. J. Intellect. Disabil. Res. 2013, 57, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Biec, E.; Zima, J.; Wojtowicz, D.; Wojciechowska-Maszkowska, B.; Krecisz, K.; Kuczynski, M. Postural stability in young adults with Down syndrome in challenging conditions. PLoS ONE 2014, 9, e94247. [Google Scholar] [CrossRef]
- Frey, G.C.; Chow, B. Relationship between BMI, physical fitness, and motor skills in youth with mild intellectual disabilities. Int. J. Obes. 2006, 30, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias, M.; Gatti, E.; Bordegoni, M.; Cugini, U.; Mansutti, A. Improving manual skills in persons with disabilities (PWD) through a multimodal assistance system. Disabil. Rehabil. Assist. Technol. 2014, 9, 335–343. [Google Scholar] [CrossRef]
- Vianello, R. Sindrome di Down. Sviluppo Psicologico e Integrazione Dalla Nascita All’età Adulta; Edizioni Junior: Bergamo, Italy, 2006. [Google Scholar]
- Gonzalez-Aguero, A.; Vicente-Rodriguez, G.; Moreno, L.A.; Guerra-Balic, M.; Ara, I.; Casajus, J.A. Health-related physical fitness in children and adolescents with Down syndrome and response to training. Scand. J. Med. Sci. Sports 2010, 20, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Scifo, L.; Chicau Borrego, C.; Monteiro, D.; Matosic, D.; Feka, K.; Bianco, A.; Alesi, M. Sport Intervention Programs (SIPs) to Improve Health and Social Inclusion in People with Intellectual Disabilities: A Systematic Review. J. Funct. Morphol. Kinesiol. 2019, 4, 57. [Google Scholar] [CrossRef] [PubMed]
- Alsakhawi, R.S.; Elshafey, M.A. Effect of Core Stability Exercises and Treadmill Training on Balance in Children with Down Syndrome: Randomized Controlled Trial. Adv. Ther. 2019, 36, 2364–2373. [Google Scholar] [CrossRef] [PubMed]
- Ptomey, L.T.; Szabo, A.N.; Willis, E.A.; Gorczyca, A.M.; Greene, J.L.; Danon, J.C.; Donnelly, J.E. Changes in cognitive function after a 12-week exercise intervention in adults with Down syndrome. Disabil. Health J. 2018, 11, 486–490. [Google Scholar] [CrossRef]
- Fairclough, S.J.; McGrane, B.; Sanders, G.; Taylor, S.; Owen, M.; Curry, W. A non-equivalent group pilot trial of a school-based physical activity and fitness intervention for 10–11 year old english children: Born to move. BMC Public Health 2016, 16, 861. [Google Scholar] [CrossRef]
- Mastebroek, M.; Naaldenberg, J.; Lagro-Janssen, A.L.; van Schrojenstein Lantman de Valk, H. Health information exchange in general practice care for people with intellectual disabilities—A qualitative review of the literature. Res. Dev. Disabil. 2014, 35, 1978–1987. [Google Scholar] [CrossRef]
- McGarty, A.M.; Downs, S.J.; Melville, C.A.; Harris, L. A systematic review and meta-analysis of interventions to increase physical activity in children and adolescents with intellectual disabilities. J. Intellect. Disabil. Res. 2018, 62, 312–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogg-Groenendaal, M.; Hermans, H.; Claessens, B. A systematic review on the effect of exercise interventions on challenging behavior for people with intellectual disabilities. Res. Dev. Disabil. 2014, 35, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Alesi, M.; Battaglia, G.; Roccella, M.; Testa, D.; Palma, A.; Pepi, A. Improvement of gross motor and cognitive abilities by an exercise training program: Three case reports. Neuropsychiatr. Dis. Treat. 2014, 10, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Grondhuis, S.N.; Aman, M.G. Overweight and obesity in youth with developmental disabilities: A call to action. J. Intellect. Disabil. Res. 2014, 58, 787–799. [Google Scholar] [CrossRef]
- Ara, I.; Moreno, L.A.; Leiva, M.T.; Gutin, B.; Casajús, J.A. Adiposity, physical activity, and physical fitness among children from Aragon, Spain. Obesity 2007, 15, 1918–1924. [Google Scholar] [CrossRef]
- Zemel, B.S.; Pipan, M.; Stallings, V.A.; Hall, W.; Schadt, K.; Freedman, D.S.; Thorpe, P. Growth Charts for Children With Down Syndrome in the United States. Pediatrics 2015, 136, e1204–e1211. [Google Scholar] [CrossRef]
- Patel, D.R.; Cabral, M.D.; Ho, A.; Merrick, J. A clinical primer on intellectual disability. Transl. Pediatr. 2020, 9, S23–S35. [Google Scholar] [CrossRef]
- Henderson, S.E.; Sugden, D.A.; Barnett, A. Movement Assessment Battery for Children—Second Edition (Movement ABC-2); The Psychological Corporation: London, UK, 2007. [Google Scholar]
- Alpern, G.D.; Lanfranchi, S.; Vianello, R. DP-3—Developmental Profile-3; Hogrefe Editore: Firenze, Italia, 2015. [Google Scholar]
- Alpern, G.D. Developmental Profile 3: DP-3; Western Psychological Services (WPS): Torrance, CA, USA, 2007. [Google Scholar]
- Daunhauer, L.A.; Fidler, D.J. The down syndrome behavioral phenotype: Implications for practice and research in occupational therapy. Occup. Ther. Health Care 2011, 25, 7–25. [Google Scholar] [CrossRef]
- Holzapfel, G.A.; Niestrawska, J.A.; Ogden, R.W.; Reinisch, A.J.; Schriefl, A.J. Modelling non-symmetric collagen fibre dispersion in arterial walls. J. R. Soc. Interface 2015, 12, 20150188. [Google Scholar] [CrossRef]
- Memisevic, H.; Macak, A. Fine motor skills in children with Down syndrome. Spec. Edukac. Rehabil. 2014, 13, 365–377. [Google Scholar] [CrossRef] [Green Version]
- Cowley, P.M.; Ploutz-Snyder, L.L.; Baynard, T.; Heffernan, K.; Jae, S.Y.; Hsu, S.; Lee, M.; Pitetti, K.H.; Reiman, M.P.; Fernhall, B. Physical fitness predicts functional tasks in individuals with Down syndrome. Med. Sci. Sports Exerc. 2010, 42, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Boncoddo, R.; Dixon, J.A.; Kelley, E. The emergence of a novel representation from action: Evidence from preschoolers. Dev. Sci. 2010, 13, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, G.; Giustino, V.; Messina, G.; Faraone, M.; Brusa, J.; Bordonali, A.; Barbagallo, M.; Palma, A.; Dominguez, L.J. Walking in Natural Environments as Geriatrician’s Recommendation for Fall Prevention: Preliminary Outcomes from the “Passiata Day” Model. Sustainability 2020, 12, 2684. [Google Scholar] [CrossRef]
- Buchner, D.M.; Rillamas-Sun, E.; Di, C.; LaMonte, M.J.; Marshall, S.W.; Hunt, J.; Zhang, Y.; Rosenberg, D.E.; Lee, I.M.; Evenson, K.R.; et al. Accelerometer-Measured Moderate to Vigorous Physical Activity and Incidence Rates of Falls in Older Women. J. Am. Geriatr. Soc. 2017, 65, 2480–2487. [Google Scholar] [CrossRef]
- Warburton, D.E.R.; Bredin, S.S.D. Health benefits of physical activity: A systematic review of current systematic reviews. Curr. Opin. Cardiol. 2017, 32, 541–556. [Google Scholar] [CrossRef]
- Shin, C.N.; Lee, Y.S.; Belyea, M. Physical activity, benefits, and barriers across the aging continuum. Appl. Nurs. Res. 2018, 44, 107–112. [Google Scholar] [CrossRef]
- Missiuna, C.; Cairney, J.; Pollock, N.; Campbell, W.; Russell, D.J.; Macdonald, K.; Schmidt, L.; Heath, N.; Veldhuizen, S.; Cousins, M. Psychological distress in children with developmental coordination disorder and attention-deficit hyperactivity disorder. Res. Dev. Disabil. 2014, 35, 1198–1207. [Google Scholar] [CrossRef]
- Andriolo, R.B.; El Dib, R.P.; Ramos, L.; Atallah, A.N.; da Silva, E.M. Aerobic exercise training programmes for improving physical and psychosocial health in adults with Down syndrome. Cochrane Database Syst. Rev. 2010, 5, CD005176. [Google Scholar] [CrossRef]
- Carter, K.; Sunderman, S.; Wooten Burnett, S. The Effect of Vestibular Stimulation Exercises on Balance, Coordination, and Agility in Children with Down Syndrome. Am. J. Psychiatry Neurosci. 2018, 6, 28–32. [Google Scholar] [CrossRef]
- Fragala-Pinkham, M.; O’Neil, M.E.; Haley, S.M. Summative evaluation of a pilot aquatic exercise program for children with disabilities. Disabil. Health J. 2010, 3, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Heller, T.; Hsieh, K.; Rimmer, J.H. Attitudinal and psychosocial outcomes of a fitness and health education program on adults with down syndrome. Am. J. Ment. Retard. 2004, 109, 175–185. [Google Scholar] [CrossRef]
- Jobling, A.; Virji-Babul, N.; Nichols, D. Children with Down Syndrome. J. Phys. Educ. Recreat. Danc. 2006, 77, 34–54. [Google Scholar] [CrossRef]






| Variable | Min | 25th p | Median | Mean | SD | 75th p | Max |
|---|---|---|---|---|---|---|---|
| M-ABC | |||||||
| Manual dexterity | 3.0 | 3.0 | 3.0 | 3.72 | 2.39 | 3.0 | 14.0 |
| Aiming and catching | 2.0 | 6.0 | 9.0 | 9.16 | 3.85 | 12.0 | 16.0 |
| Static and dynamic balance | 5.0 | 5.50 | 8.0 | 9.17 | 5.06 | 10.0 | 26.0 |
| Global motor coordination | 14.0 | 15.50 | 21.0 | 22.25 | 8.21 | 25.0 | 41.0 |
| DP-3 | |||||||
| Physical area | 0.11 | 2.36 | 4.11 | 4.24 | 2.21 | 7.0 | 7.70 |
| Adaptive behavior area | 1.80 | 4.50 | 6.50 | 6.66 | 2.76 | 8.65 | 12.60 |
| Social-emotional area | 1.80 | 3.05 | 4.10 | 5.14 | 2.84 | 7.16 | 10.80 |
| Cognitive area | 1.40 | 4.0 | 4.80 | 4.93 | 1.68 | 5.86 | 8.20 |
| Communication area | 1.10 | 2.80 | 4.10 | 4.49 | 2.47 | 5.31 | 10.20 |
| Global development | 25.0 | 25.0 | 25.0 | 38.40 | 21.46 | 51.0 | 101.0 |
| Variable | PA-G Means ± SD | PI-G Means ± SD |
|---|---|---|
| M-ABC | ||
| Manual dexterity | 3.73 ± 2.84 | 3.70 ± 1.64 |
| Aiming and catching | 10.53 ± 4.09 | 7.10 ± 2.38 |
| Static and dynamic balance | 10.57 ± 6.07 | 7.20 ± 2.20 |
| Global motor coordination | 25.50 ± 8.57 | 17.70 ± 5.17 |
| DP-3 | ||
| Physical area | 5.00 ± 2.05 | 3.18 ± 2.07 |
| Adaptive behavior area | 7.21 ± 2.73 | 5.88 ± 2.74 |
| Social-emotional area | 5.93 ± 3.21 | 4.02 ± 1.86 |
| Cognitive area | 5.02 ± 1.65 | 4.81 ± 1.81 |
| Communication area | 4.77 ± 2.50 | 4.10 ± 2.50 |
| Global development | 42.07 ± 23.81 | 32.90 ± 17.03 |
| M-ABC | |||||
|---|---|---|---|---|---|
| Manual Dexterity | Aiming and Catching | Static and Dynamic Balance | Global Motor Coordination | ||
| DP-3 | Physical area | ρ = 0.23, p = 0.138 | ρ = 0.32, p = 0.06 | ρ = 0.10, p = 0.320 | ρ = 0.31, p = 0.078 |
| Adaptive behavior area | ρ = 0.20, p = 0.176 | ρ = 0.41, p = 0.023 | ρ = 0.24, p = 0.135 | ρ = 0.43, p = 0.021 | |
| Social-emotional area | ρ = 0.15, p = 0.243 | ρ = 0.34, p = 0.05 | ρ = 0.06, p = 0.391 | ρ = 0.24, p = 0.131 | |
| Cognitive area | ρ = 0.17, p = 0.213 | ρ = 0.10, p = 0.327 | ρ = 0.04, p = 0.422 | ρ = 0.09, p = 0.340 | |
| Communication area | ρ = 0.23, p = 0.136 | ρ = 0.34, p = 0.05 | ρ = −0.02, p = 0.467 | ρ = 0.25, p = 0.122 | |
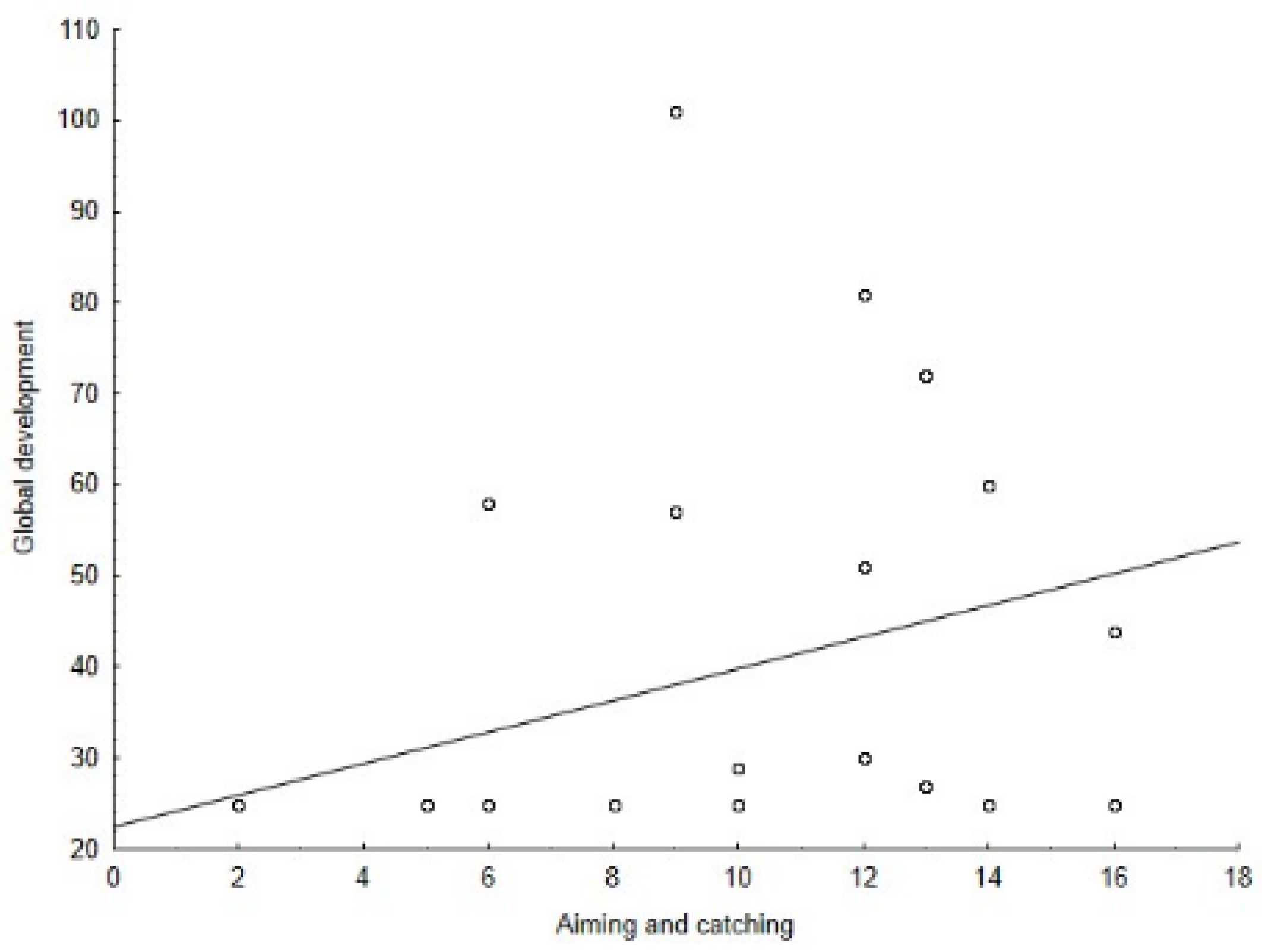
| Global development | ρ = 0.34, p = 0.05 | ρ = 0.50, p = 0.006 | ρ = 0.29, p = 0.081 | ρ = 0.56, p = 0.002 | |
| Variable | Rank Sum PA-G | Rank Sum PI-G | Z | p |
|---|---|---|---|---|
| M-ABC | ||||
| Manual dexterity | 186.0 | 139.0 | −0.835 | 0.404 |
| Aiming and catching | 235.0 | 90.0 | 2.248 | 0.025 |
| Static and dynamic balance | 197.50 | 102.50 | 1.304 | 0.192 |
| Global motor coordination | 218.0 | 82.0 | 2.504 | 0.012 |
| DP-3 | ||||
| Physical area | 209.0 | 91.0 | 1.980 | 0.048 |
| Adaptive behavior area | 196.0 | 104.0 | 1.204 | 0.223 |
| Social-emotional area | 199.50 | 100.50 | 1.409 | 0.159 |
| Cognitive area | 175.50 | 124.50 | 0.000 | 0.775 |
| Communication area | 187.0 | 113.0 | 0.676 | 0.527 |
| Global development | 222.0 | 103.0 | 1.618 | 0.106 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alesi, M.; Giustino, V.; Gentile, A.; Gómez-López, M.; Battaglia, G. Motor Coordination and Global Development in Subjects with Down Syndrome: The Influence of Physical Activity. J. Clin. Med. 2022, 11, 5031. https://doi.org/10.3390/jcm11175031
Alesi M, Giustino V, Gentile A, Gómez-López M, Battaglia G. Motor Coordination and Global Development in Subjects with Down Syndrome: The Influence of Physical Activity. Journal of Clinical Medicine. 2022; 11(17):5031. https://doi.org/10.3390/jcm11175031
Chicago/Turabian StyleAlesi, Marianna, Valerio Giustino, Ambra Gentile, Manuel Gómez-López, and Giuseppe Battaglia. 2022. "Motor Coordination and Global Development in Subjects with Down Syndrome: The Influence of Physical Activity" Journal of Clinical Medicine 11, no. 17: 5031. https://doi.org/10.3390/jcm11175031
APA StyleAlesi, M., Giustino, V., Gentile, A., Gómez-López, M., & Battaglia, G. (2022). Motor Coordination and Global Development in Subjects with Down Syndrome: The Influence of Physical Activity. Journal of Clinical Medicine, 11(17), 5031. https://doi.org/10.3390/jcm11175031










