Air-Pressure-Supported Application of Cultured Human Keratinocytes in a Fibrin Sealant Suspension as a Potential Clinical Tool for Large-Scale Wounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Isolation and Cultivation of Human Keratinocytes
2.3. Preparation of Keratinocyte–Fibrin–Sealant–Suspension
2.4. CellTracker™ CM-Di-I Staining
2.5. Seeding Procedure
2.6. Keratinocyte Vitality in Fibrin
2.7. Microscopic Examination
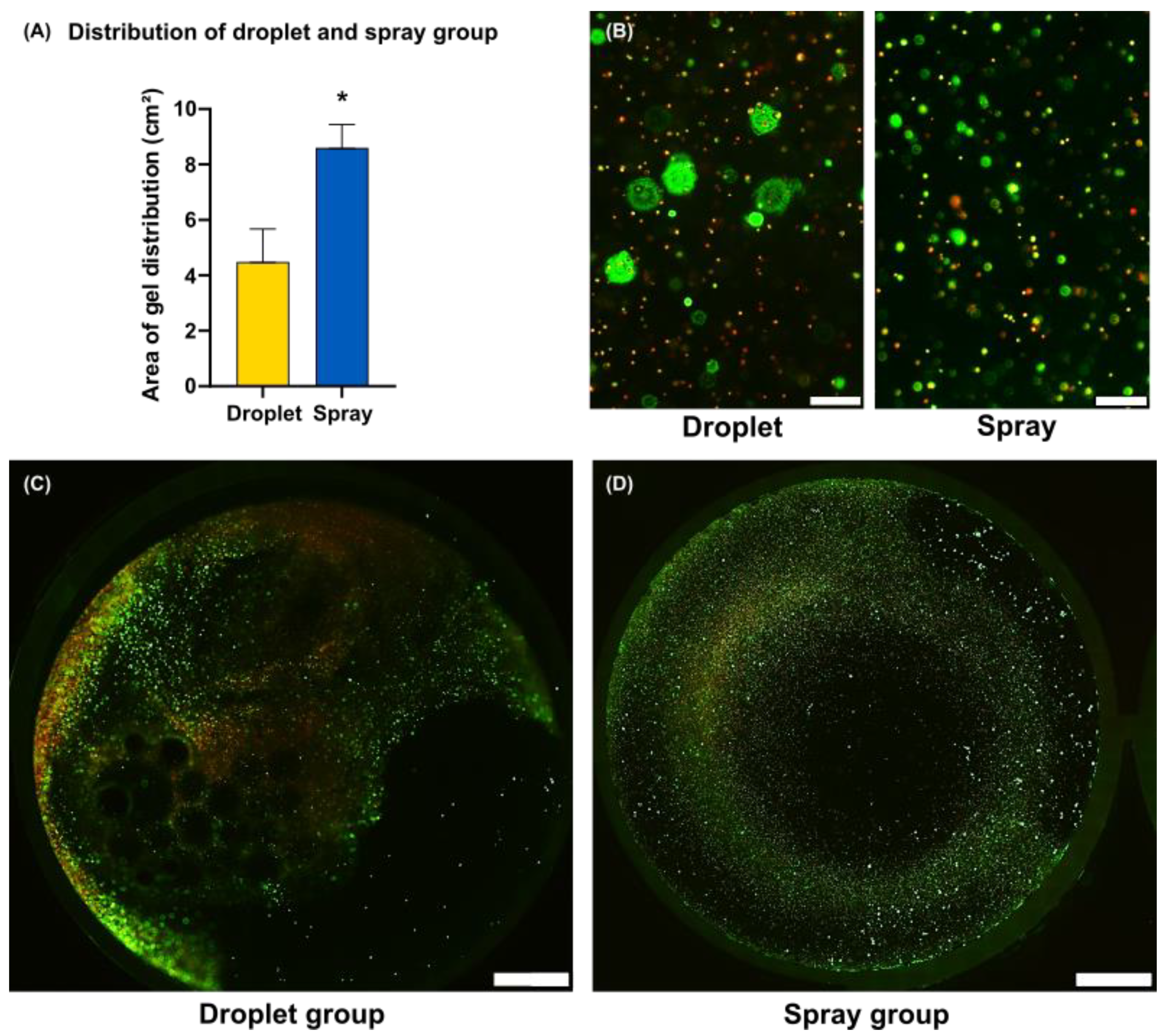
2.8. Area of Gel Distribution
2.9. Statistical Analysis
3. Results
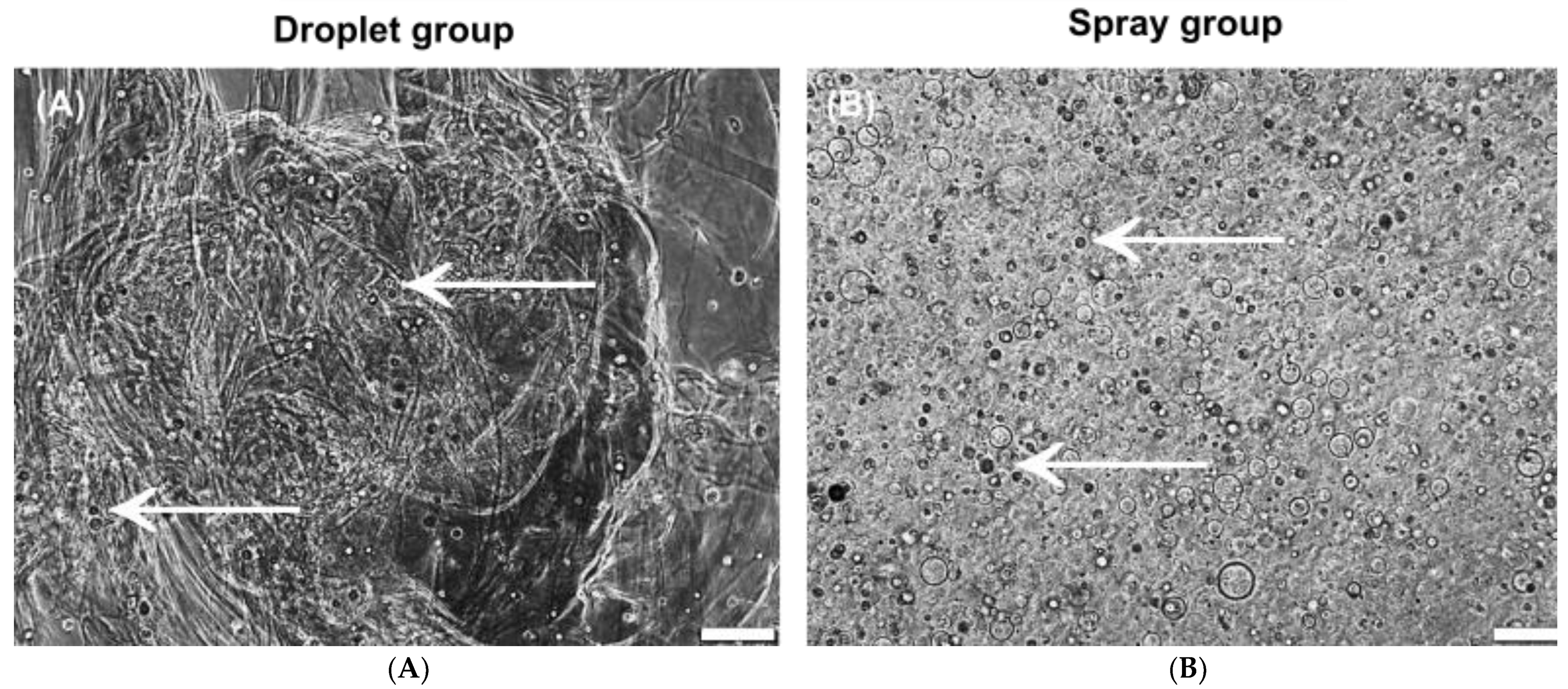
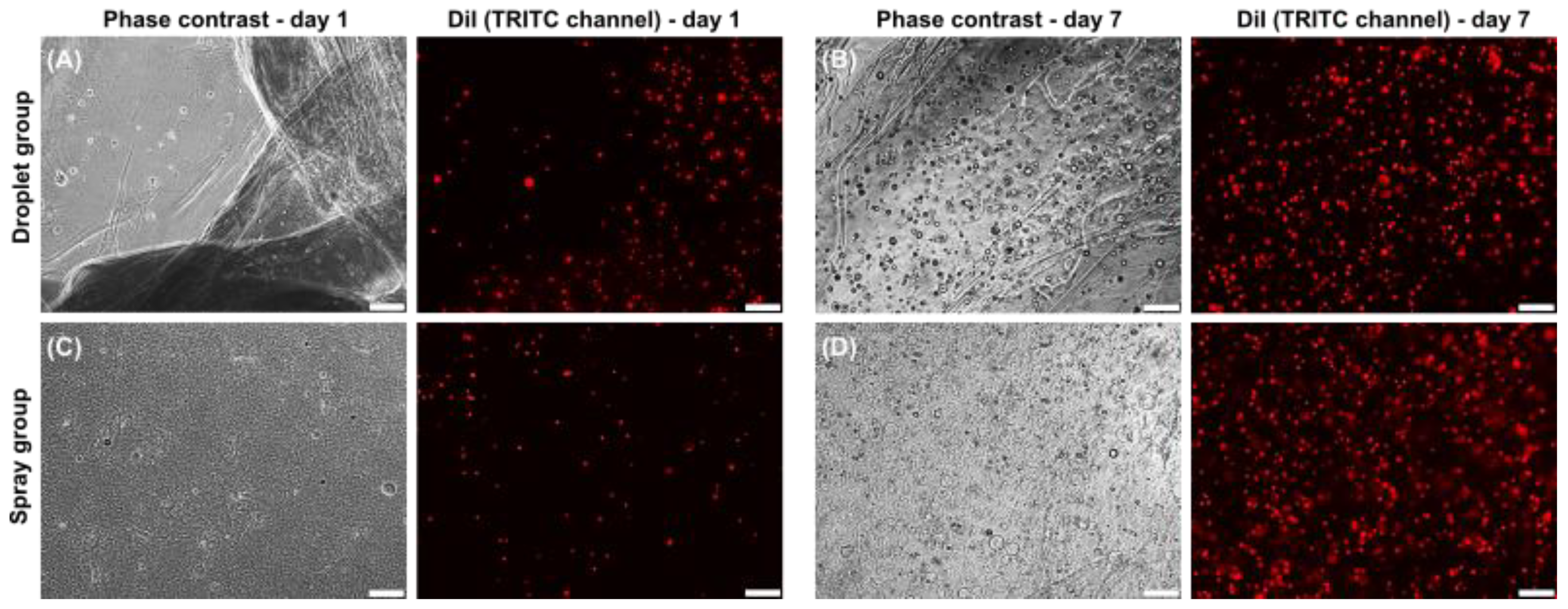
3.1. Cell Vitality
3.2. Morphology and Growth
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Pleguezuelos-Beltrán, P.; Gálvez-Martín, P.; Nieto-García, D.; Marchal, J.A.; López-Ruiz, E. Advances in spray products for skin regeneration. Bioact. Mater. 2022, 16, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Clugston, P.A.; Snelling, C.F.; Macdonald, I.B.; Maledy, H.L.; Boyle, J.C.; Germann, E.; Courtemanche, A.D.; Wirtz, P.; Fitzpatrick, D.J.; Kester, D.A.; et al. Cultured epithelial autografia: Three years of clinical experience with eighteen patients. J. Burn Care Rehabil. 1991, 12, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Atiyeh, B.S.; Costagliola, M. Cultured epithelial autograft (CEA) in burn treatment: Three decades later. Burns 2007, 33, 405–413. [Google Scholar] [CrossRef]
- Sörgel, C.A.; Schmid, R.; Stadelmann, N.; Weisbach, V.; Distel, L.; Horch, R.E.; Kengelbach-Weigand, A. IGF-I and Hyaluronic Acid Mitigate the Negative Effect of Irradiation on Human Skin Keratinocytes. Cancers 2022, 14, 588. [Google Scholar] [CrossRef]
- Horch, R.E.; Kopp, J.; Kneser, U.; Beier, J.; Bach, A.D. Tissue engineering of cultured skin substitutes. J. Cell. Mol. Med. 2005, 9, 592–608. [Google Scholar] [CrossRef]
- Horch, R.E.; Wagner, G.; Bannasch, H.; Kengelbach-Weigand, A.; Arkudas, A.; Schmitz, M. Keratinocyte Monolayers on Hyaluronic Acid Membranes as “Upside-Down” Grafts Reconstitute Full-Thickness Wounds. Med. Sci. Monit. 2019, 25, 6702–6710. [Google Scholar] [CrossRef]
- Horch, R.E.; Debus, M.; Wagner, G.; Stark, G.B. Cultured Human Keratinocytes on Type I Collagen Membranes to Reconstitute the Epidermis. Tissue Eng. 2000, 6, 53–67. [Google Scholar] [CrossRef]
- Vogt, P.M.; Thompson, S.; Andree, C.; Liu, P.; Breuing, K.; Hatzis, D.; Brown, H.; Mulligan, R.C.; Eriksson, E. Genetically modified keratinocytes transplanted to wounds reconstitute the epidermis. Proc. Natl. Acad. Sci. USA 1994, 91, 9307–9311. [Google Scholar] [CrossRef]
- Moustafa, M.; Bullock, A.J.; Creagh, F.M.; Heller, S.; Jeffcoate, W.; Game, F.; Amery, C.; Tesfaye, S.; Ince, Z.; Haddow, D.B.; et al. Randomized, controlled, single-blind study on use of autologous keratinocytes on a transfer dressing to treat nonhealing diabetic ulcers. Regen. Med. 2007, 2, 887–902. [Google Scholar] [CrossRef]
- Sood, R.; Roggy, D.; Zieger, M.; Balledux, J.; Chaudhari, S.; Koumanis, D.J.; Mir, H.S.; Cohen, A.; Knipe, C.; Gabehart, K.; et al. Cultured Epithelial Autografts for Coverage of Large Burn Wounds in Eighty-Eight Patients: The Indiana University Experience. J. Burn Care Res. 2010, 31, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.; Ali, M.N.; Barakullah, A.; Gulzar, A.; Arshad, M.; Fatima, S.; Asad, M. Synthetic polymeric biomaterials for wound healing: A review. Prog. Biomater. 2018, 7, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Dai, N.-T.; Williamson, M.; Khammo, N.; Adams, E.; Coombes, A. Composite cell support membranes based on collagen and polycaprolactone for tissue engineering of skin. Biomaterials 2004, 25, 4263–4271. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Shih, S.; Khachemoune, A. Skin substitutes for acute and chronic wound healing: An updated review. J. Dermatol. Treat. 2020, 31, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Bannasch, H.; Unterberg, T.; Föhn, M.; Weyand, B.; Horch, R.E.; Stark, G.B. Cultured keratinocytes in fibrin with decellularised dermis close porcine full-thickness wounds in a single step. Burns 2008, 34, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Horch, R.E.; Bannasch, H.; Kopp, J.; Andree, C.; Stark, G.B. Single-cell suspensions of cultured human keratinocytes in fibrin-glue reconstitute the epidermis. Cell Transpl. 1998, 7, 309–317. [Google Scholar] [CrossRef]
- Kopp, J.; Jeschke, M.G.; Bach, A.D.; Kneser, U.; Horch, R.E. Applied tissue engineering in the closure of severe burns and chronic wounds using cultured human autologous keratinocytes in a natural fibrin matrix. Cell Tissue Bank. 2004, 5, 81–87. [Google Scholar] [CrossRef]
- Johnsen, S.; Ermuth, T.; Tanczos, E.; Bannasch, H.; Horch, R.E.; Zschocke, I.; Peschen, M.; Schöpf, E.; Vanscheidt, W.; Augustin, M. Treatment of therapy-refractive ulcera cruris of various origins with autologous keratinocytes in fibrin sealant. Vasa 2005, 34, 25–29. [Google Scholar] [CrossRef]
- Losi, P.; Briganti, E.; Errico, C.; Lisella, A.; Sanguinetti, E.; Chiellini, F.; Soldani, G. Fibrin-based scaffold incorporating VEGF- and bFGF-loaded nanoparticles stimulates wound healing in diabetic mice. Acta Biomater. 2013, 9, 7814–7821. [Google Scholar] [CrossRef]
- Miron, R.J.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Hernandez, M.; Choukroun, J. Platelet-Rich Fibrin and Soft Tissue Wound Healing: A Systematic Review. Tissue Eng. Part B Rev. 2017, 23, 83–99. [Google Scholar] [CrossRef] [Green Version]
- Byrne, D.J.; Hardy, J.; Wood, R.A.B.; McIntosh, R.; Cuschieri, A. Effect of fibrin glues on the mechanical properties of healing wounds. Br. J. Surg. 1991, 78, 841–843. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.D.; Poventud-Fuentes, I.; Sampietro, S.; Diamond, S.L.; Stalker, T.J.; Brass, L.F. Hierarchical organization of the hemostatic response to penetrating injuries in the mouse macrovasculature. J. Thromb. Haemost. 2016, 15, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Hadjipanayi, E.; Kuhn, P.-H.; Moog, P.; Bauer, A.-T.; Kuekrek, H.; Mirzoyan, L.; Hummel, A.; Kirchhoff, K.; Salgin, B.; Isenburg, S.; et al. The Fibrin Matrix Regulates Angiogenic Responses within the Hemostatic Microenvironment through Biochemical Control. PLoS ONE 2015, 10, e0135618. [Google Scholar] [CrossRef]
- Litvinov, R.I.; Weisel, J.W. What Is the Biological and Clinical Relevance of Fibrin? Semin. Thromb. Hemost. 2016, 42, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Currie, L.; Martin, R.; Sharpe, J.R.; James, S. A comparison of keratinocyte cell sprays with and without fibrin glue. Burns 2003, 29, 677–685. [Google Scholar] [CrossRef]
- Chester, D.L.; Balderson, D.S.; Papini, R.P.G. A Review of Keratinocyte Delivery to the Wound Bed. J. Burn Care Rehabil. 2004, 25, 266–275. [Google Scholar] [CrossRef]
- Muhamed, I.; Sproul, E.P.; Ligler, F.S.; Brown, A.C. Fibrin Nanoparticles Coupled with Keratinocyte Growth Factor Enhance the Dermal Wound-Healing Rate. ACS Appl. Mater. Interfaces 2019, 11, 3771–3780. [Google Scholar] [CrossRef]
- Liu, J.; Bian, Z.; Kuijpers-Jagtman, A.; Hoff, J.V.D. Skin and oral mucosa equivalents: Construction and performance. Orthod. Craniofac. Res. 2010, 13, 11–20. [Google Scholar] [CrossRef]
- Xie, Y.; Rizzi, S.C.; Dawson, R.; Lynam, E.; Richards, S.; Leavesley, D.I.; Upton, Z. Development of a Three-Dimensional Human Skin Equivalent Wound Model for Investigating Novel Wound Healing Therapies. Tissue Eng. Part C Methods 2010, 16, 1111–1123. [Google Scholar] [CrossRef]
- Horch, R.E.; Ludolph, I.; Arkudas, A.; Cai, A. Personalized Reconstruction of Genital Defects in Complicated Wounds with Vertical Rectus Abdominis Myocutaneous Flaps including Urethral Neo-Orifice. J. Pers. Med. 2021, 11, 1076. [Google Scholar] [CrossRef]
- Müller-Seubert, W.; Ostermaier, P.; Horch, R.E.; Distel, L.; Frey, B.; Cai, A.; Arkudas, A. Intra- and Early Postoperative Evaluation of Malperfused Areas in an Irradiated Random Pattern Skin Flap Model Using Indocyanine Green Angiography and Near-Infrared Reflectance-Based Imaging and Infrared Thermography. J. Pers. Med. 2022, 12, 237. [Google Scholar] [CrossRef] [PubMed]
- Auger, F.A.; Guignard, R.; López Valle, C.A.; Germain, L. Role and innocuity of Tisseel®, a tissue glue, in the grafting process and in vivo evolution of human cultured epidermis. Br. J. Plast. Surg. 1993, 46, 136–142. [Google Scholar] [CrossRef]
- Hunyadi, J.; Farkas, B.; Bertényi, C.; Oláh, J.; Dobozy, A. Keratinocyte Grafting: A New Means of Transplantation for Full-Thickness Wounds. J. Dermatol. Surg. Oncol. 1988, 14, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Kirsner, R.S.; Marston, W.A.; Snyder, R.J.; Lee, T.D.; Cargill, D.I.; Slade, H.B. Spray-applied cell therapy with human allogeneic fi bro-blasts and keratinocytes for the treatment of chronic venous leg ulcers: A phase 2, multicentre, double-blind, randomised, placebo-controlled trial. Lancet 2012, 380, 977–985. [Google Scholar] [CrossRef]
- Navarro, F.A.; Stoner, M.L.; Park, C.S.; Huertas, J.C.; Lee, H.B.; Wood, F.M.; Orgill, D.P. Sprayed keratinocyte suspensions accelerate epidermal coverage in a porcine microwound model. J. Burn Care Rehabil. 2000, 21, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Wood, F.M.; Giles, N.; Stevenson, A.; Rea, S.; Fear, M. Characterisation of the cell suspension harvested from the dermal epidermal junction using a ReCell® kit. Burns 2012, 38, 44–51. [Google Scholar] [CrossRef]
- Wen, X.; Zheng, Y.; Wu, J.; Yue, L.; Wang, C.; Luan, J.; Wu, Z.; Wang, K. In vitro and in vivo investigation of bacterial cellulose dressing containing uniform silver sulfadiazine nanoparticles for burn wound healing. Prog. Nat. Sci. 2015, 25, 197–203. [Google Scholar] [CrossRef]
- Voigt, M.; Schauer, M.; Schaefer, D.; Andree, C.; Horch, R.; Stark, G. Cultured Epidermal Keratinocytes on a Microspherical Transport System Are Feasible to Reconstitute the Epidermis in Full-Thickness Wounds. Tissue Eng. 1999, 5, 563–572. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sörgel, C.A.; Schmid, R.; Kengelbach-Weigand, A.; Promny, T.; Horch, R.E. Air-Pressure-Supported Application of Cultured Human Keratinocytes in a Fibrin Sealant Suspension as a Potential Clinical Tool for Large-Scale Wounds. J. Clin. Med. 2022, 11, 5032. https://doi.org/10.3390/jcm11175032
Sörgel CA, Schmid R, Kengelbach-Weigand A, Promny T, Horch RE. Air-Pressure-Supported Application of Cultured Human Keratinocytes in a Fibrin Sealant Suspension as a Potential Clinical Tool for Large-Scale Wounds. Journal of Clinical Medicine. 2022; 11(17):5032. https://doi.org/10.3390/jcm11175032
Chicago/Turabian StyleSörgel, Celena A., Rafael Schmid, Annika Kengelbach-Weigand, Theresa Promny, and Raymund E. Horch. 2022. "Air-Pressure-Supported Application of Cultured Human Keratinocytes in a Fibrin Sealant Suspension as a Potential Clinical Tool for Large-Scale Wounds" Journal of Clinical Medicine 11, no. 17: 5032. https://doi.org/10.3390/jcm11175032
APA StyleSörgel, C. A., Schmid, R., Kengelbach-Weigand, A., Promny, T., & Horch, R. E. (2022). Air-Pressure-Supported Application of Cultured Human Keratinocytes in a Fibrin Sealant Suspension as a Potential Clinical Tool for Large-Scale Wounds. Journal of Clinical Medicine, 11(17), 5032. https://doi.org/10.3390/jcm11175032







