Survival of Lung Cancer Patients by Histopathology in Taiwan from 2010 to 2016: A Nationwide Study
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
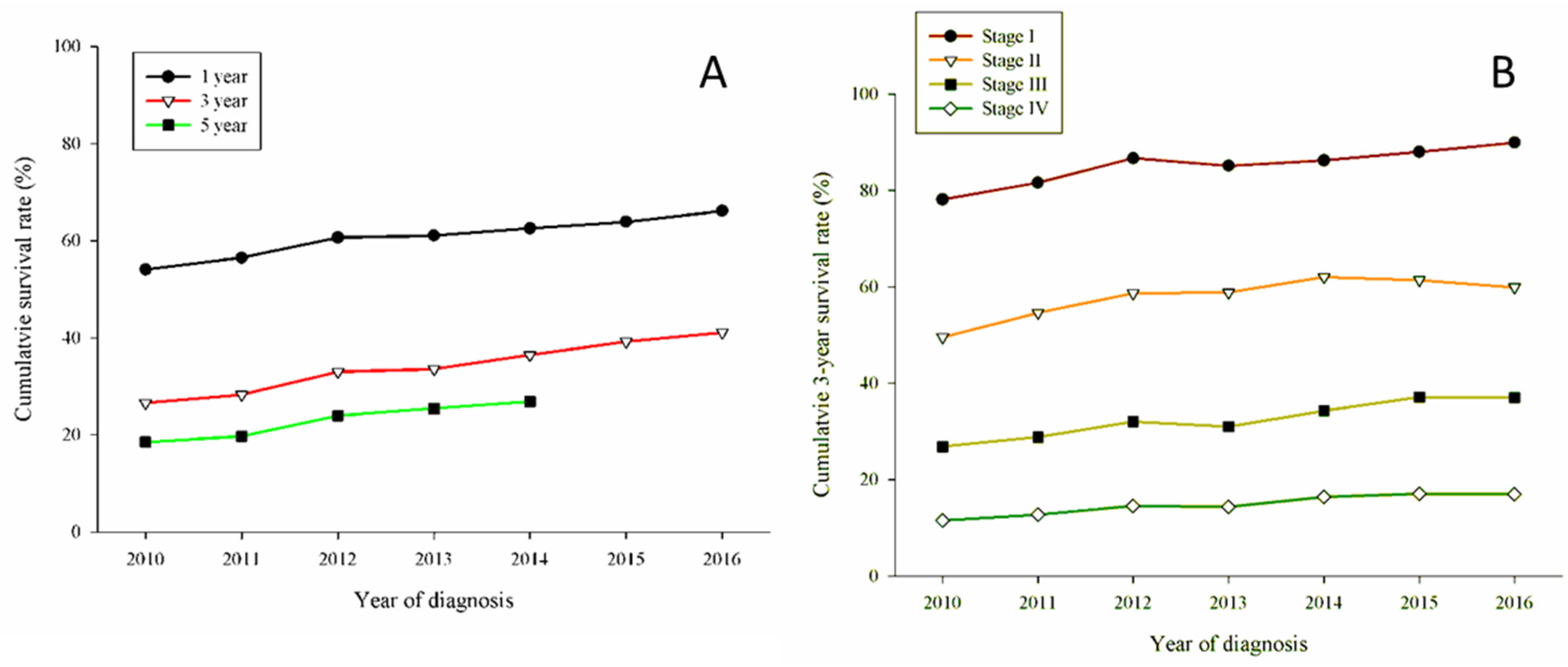
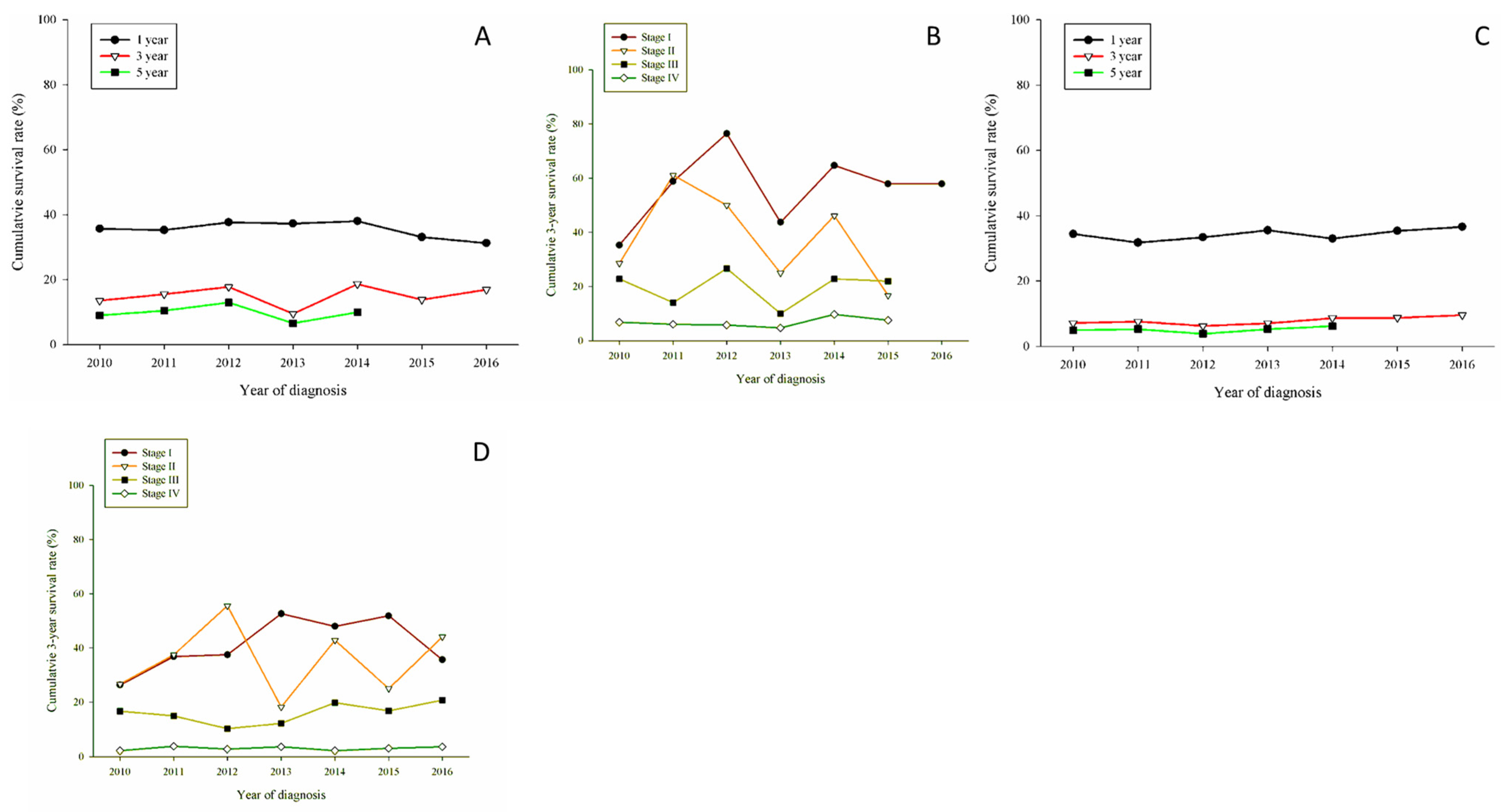
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Allemani, C.; Weir, H.K.; Carreira, H.; Harewood, R.; Spika, D.; Wang, X.-S.; Bannon, F.; Ahn, J.V.; Johnson, C.J.; Bonaventure, A. Global surveillance of cancer survival 1995–2009: Analysis of individual data for 25 676 887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 2015, 385, 977–1010. [Google Scholar] [CrossRef]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global surveillance of trends in cancer survival 2000–2014 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef]
- Zadnik, V.; Zagar, T.; Lokar, K.; Tomsic, S.; Konjevic, A.D.; Zakotnik, B. Trends in population-based cancer survival in Slovenia. Radiol. Oncol. 2021, 55, 42–49. [Google Scholar] [CrossRef]
- Wang, B.Y.; Huang, J.Y.; Cheng, C.Y.; Lin, C.H.; Ko, J.; Liaw, Y.P. Lung cancer and prognosis in taiwan: A population-based cancer registry. J. Thorac. Oncol. 2013, 8, 1128–1135. [Google Scholar] [CrossRef]
- Lin, H.T.; Liu, F.C.; Wu, C.Y.; Kuo, C.F.; Lan, W.C.; Yu, H.P. Epidemiology and Survival Outcomes of Lung Cancer: A Population-Based Study. BioMed Res. Int. 2019, 2019, 8148156. [Google Scholar] [CrossRef]
- Nicholson, A.G.; Tsao, M.S.; Beasley, M.B.; Borczuk, A.C.; Brambilla, E.; Cooper, W.A.; Dacic, S.; Jain, D.; Kerr, K.M.; Lantuejoul, S. The 2021 WHO classification of lung tumors: Impact of advances since 2015. J. Thorac. Oncol. 2022, 17, 362–387. [Google Scholar] [CrossRef]
- Howlader, N.; Forjaz, G.; Mooradian, M.J.; Meza, R.; Kong, C.Y.; Cronin, K.A.; Mariotto, A.B.; Lowy, D.R.; Feuer, E.J. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N. Engl. J. Med. 2020, 383, 640–649. [Google Scholar] [CrossRef]
- Chiang, C.-J.; You, S.-L.; Chen, C.-J.; Yang, Y.-W.; Lo, W.-C.; Lai, M.-S. Quality assessment and improvement of nationwide cancer registration system in Taiwan: A review. Jpn. J. Clin. Oncol. 2015, 45, 291–296. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, W.; Guo, Q.; Ye, J.; Zhang, D.; Zhang, Y.; Zeng, W.; Yu, D.; Peng, J.; Wei, Y. Prognosis and Survival Analysis of 922,317 Lung Cancer Patients from the US Based on the Most Recent Data from the SEER Database (April 15, 2021). Int. J. Gen. Med. 2021, 14, 9567. [Google Scholar] [CrossRef] [PubMed]
- Innos, K.; Oselin, K.; Laisaar, T.; Aareleid, T. Patterns of survival and surgical treatment in lung cancer patients in Estonia by histologic type and stage, 1996-2016. Acta Oncol. 2019, 58, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Brustugun, O.T.; Grønberg, B.H.; Fjellbirkeland, L.; Helbekkmo, N.; Aanerud, M.; Grimsrud, T.K.; Helland, Å.; Møller, B.; Nilssen, Y.; Strand, T.E.; et al. Substantial nation-wide improvement in lung cancer relative survival in Norway from 2000 to 2016. Lung Cancer 2018, 122, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Shen-Tu, Y.; Mao, F.; Pan, Y.; Wang, W.; Zhang, L.; Zhang, H.; Cheng, B.; Guo, H.; Wang, Z. Lymph node dissection and survival in patients with early stage nonsmall cell lung cancer: A 10-year cohort study. Medicine 2017, 96, e8356. [Google Scholar] [CrossRef] [PubMed]
- Midha, A.; Dearden, S.; McCormack, R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: A systematic review and global map by ethnicity (mutMapII). Am. J. Cancer Res. 2015, 5, 2892–2911. [Google Scholar]
- Sato, M.; Shames, D.S.; Gazdar, A.F.; Minna, J.D. A translational view of the molecular pathogenesis of lung cancer. J. Thorac. Oncol. 2007, 2, 327–343. [Google Scholar] [CrossRef]
- Bell, D.W.; Gore, I.; Okimoto, R.A.; Godin-Heymann, N.; Sordella, R.; Mulloy, R.; Sharma, S.V.; Brannigan, B.W.; Mohapatra, G.; Settleman, J.; et al. Inherited susceptibility to lung cancer may be associated with the T790M drug resistance mutation in EGFR. Nat. Genet. 2005, 37, 1315–1316. [Google Scholar] [CrossRef]
- Herbst, R.S.; Heymach, J.V.; Lippman, S.M. Lung cancer. N. Engl. J. Med. 2008, 359, 1367–1380. [Google Scholar] [CrossRef]
- Salomon, D.S.; Brandt, R.; Ciardiello, F.; Normanno, N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit. Rev. Oncol. Hematol. 1995, 19, 183–232. [Google Scholar] [CrossRef]
- Fontanini, G.; De Laurentiis, M.; Vignati, S.; Chinè, S.; Lucchi, M.; Silvestri, V.; Mussi, A.; De Placido, S.; Tortora, G.; Bianco, A.R.; et al. Evaluation of epidermal growth factor-related growth factors and receptors and of neoangiogenesis in completely resected stage I-IIIA non-small-cell lung cancer: Amphiregulin and microvessel count are independent prognostic indicators of survival. Clin. Cancer Res. 1998, 4, 241–249. [Google Scholar]
- Zimmermann, J. Interview with Jürg Zimmermann, global head of oncology & exploratory chemistry at Novartis. Future Med. Chem. 2009, 1, 1395–1398. [Google Scholar] [CrossRef] [PubMed]
- Attwood, M.M.; Fabbro, D.; Sokolov, A.V.; Knapp, S.; Schiöth, H.B. Trends in kinase drug discovery: Targets, indications and inhibitor design. Nat. Rev. Drug Discov. 2021, 20, 839–861. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, K.; Imanishi, N.; Ichiki, Y.; Tanaka, F. Treatment of Non-small Cell Lung Cancer with EGFR-mutations. J. UOEH 2019, 41, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Musika, W.; Kamsa-Ard, S.; Jirapornkul, C.; Santong, C.; Phunmanee, A. Lung Cancer Survival with Current Therapies and New Targeted Treatments: A Comprehensive Update from the Srinagarind Hospital-Based Cancer Registry from (2013 to 2017). Asian Pac. J. Cancer Prev. 2021, 22, 2501–2507. [Google Scholar] [CrossRef]
- Goss, G.; Tsai, C.M.; Shepherd, F.A.; Bazhenova, L.; Lee, J.S.; Chang, G.C.; Crino, L.; Satouchi, M.; Chu, Q.; Hida, T.; et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): A multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2016, 17, 1643–1652. [Google Scholar] [CrossRef]
- Sakata, Y.; Sakata, S.; Oya, Y.; Tamiya, M.; Suzuki, H.; Shibaki, R.; Okada, A.; Kobe, H.; Matsumoto, H.; Yokoi, T.; et al. Osimertinib as first-line treatment for advanced epidermal growth factor receptor mutation-positive non-small-cell lung cancer in a real-world setting (OSI-FACT). Eur. J. Cancer 2021, 159, 144–153. [Google Scholar] [CrossRef]
- Bergqvist, M.; Christensen, H.N.; Wiklund, F.; Bergström, S. Real world utilization of EGFR TKIs and prognostic factors for survival in NSCLC during 2010-2016 in Sweden: A nationwide observational study. Int. J. Cancer 2020, 146, 2510–2517. [Google Scholar] [CrossRef]
- Hsu, J.C.; Wei, C.F.; Yang, S.C.; Lin, P.C.; Lee, Y.C.; Lu, C.Y. Lung cancer survival and mortality in Taiwan following the initial launch of targeted therapies: An interrupted time series study. BMJ Open 2020, 10, e033427. [Google Scholar] [CrossRef]
- Lee, D.H. Treatments for EGFR-mutant non-small cell lung cancer (NSCLC): The road to a success, paved with failures. Pharmacol. Ther. 2017, 174, 1–21. [Google Scholar] [CrossRef]
- Song, Z.; Lin, B.; Shao, L.; Zhang, Y. Therapeutic efficacy of gefitinib and erlotinib in patients with advanced lung adenosquamous carcinoma. J. Chin. Med Assoc. 2013, 76, 481–485. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tochigi, N.; Dacic, S.; Nikiforova, M.; Cieply, K.M.; Yousem, S.A. Adenosquamous carcinoma of the lung: A microdissection study of KRAS and EGFR mutational and amplification status in a western patient population. Am. J. Clin. Pathol. 2011, 135, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Zhang, J.; Liang, W.; Huang, Y.; Yan, Y.; Wu, X.; Hu, Z.; Ma, Y.; Zhao, H.; Zhao, Y.; et al. Efficacy of epidermal growth factor receptor-tyrosine kinase inhibitors for Chinese patients with squamous cell carcinoma of lung harboring EGFR mutation. J. Thorac. Dis. 2013, 5, 585–592. [Google Scholar] [CrossRef]
- Liu, R.; Liu, J.; Shi, T.; Li, X.; Ren, D.; Chen, G.; Li, Y.; Liu, H.; Xu, S.; Chen, J. Clinicopathological and genetic characteristics of pulmonary large cell carcinoma under 2015 WHO classification: A pilot study. Oncotarget 2017, 8, 100754–100763. [Google Scholar] [CrossRef] [PubMed]
- Ogino, A.; Choi, J.; Lin, M.; Wilkens, M.K.; Calles, A.; Xu, M.; Adeni, A.E.; Chambers, E.S.; Capelletti, M.; Butaney, M.; et al. Genomic and pathological heterogeneity in clinically diagnosed small cell lung cancer in never/light smokers identifies therapeutically targetable alterations. Mol. Oncol. 2021, 15, 27–42. [Google Scholar] [CrossRef] [PubMed]

| Features | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 |
|---|---|---|---|---|---|---|---|
| Patients (N) | 9260 | 9105 | 9711 | 10,019 | 10,621 | 11,053 | 11,565 |
| Age (mean ± SD) | 68.03 ± 12.79 | 67.41 ± 12.61 | 67.09 ± 12.71 | 66.90 ± 12.62 | 66.71 ± 12.59 | 66.95 ± 12.40 | 66.61 ± 12.26 |
| Gender | |||||||
| Male | 5789 (62.5%) | 5603 (61.5%) | 5726 (59.0%) | 5949 (59.4%) | 6108 (57.5%) | 6325 (57.2%) | 6434 (55.6%) |
| Female | 3471 (37.5%) | 3502 (38.5%) | 3985 (41.1%) | 4070 (40.6%) | 4513 (42.5%) | 4728 (42.8%) | 5131 (44.4%) |
| CCI score | |||||||
| ≤2 | 1390 (15.1%) | 1396 (15.3%) | 1654 (17.0%) | 1776 (17.7%) | 1925 (18.1%) | 2073 (18.8%) | 2759 (23.9%) |
| 3–4 | 2446 (26.4%) | 2372 (26.1%) | 2552 (26.3%) | 2677 (26.7%) | 2720 (25.6%) | 2933 (26.5%) | 3213 (27.8%) |
| 5–8 | 2050 (22.1%) | 2023 (22.2%) | 2106 (21.7%) | 2139 (21.4%) | 2303 (21.7%) | 2305 (20.9%) | 2374 (20.5%) |
| >8 | 3374 (36.4%) | 3314 (36.4%) | 3399 (35.0%) | 3427 (34.2%) | 3673 (34.6%) | 3742 (33.9%) | 3219 (27.8%) |
| Location | |||||||
| Right | 5218 (56.4%) | 5233 (57.5%) | 5590 (57.6%) | 5681 (56.7%) | 6141 (57.8%) | 6305 (57.0%) | 6603 (57.1%) |
| Left | 3922 (42.3%) | 3790 (41.6%) | 4016 (41.3%) | 4235 (42.3%) | 4348 (40.9%) | 4639 (42.0%) | 4845 (41.9%) |
| Bilateral | 34 (0.4%) | 25 (0.3%) | 32 (0.3%) | 58 (0.6%) | 75 (0.7%) | 43 (0.4%) | 49 (0.4%) |
| Missing | 86 (0.9%) | 57 (0.6%) | 73 (0.8%) | 45 (0.5%) | 57 (0.5%) | 66 (0.6%) | 68 (0.6%) |
| Cell type | |||||||
| LUAD | 5421 (58.5%) | 5607 (61.6%) | 6323 (65.1%) | 6655 (66.4%) | 7192 (67.7%) | 7528 (68.1%) | 8060 (69.7%) |
| LUSC | 1646 (17.8%) | 1595 (17.5%) | 1540 (15.9%) | 1544 (15.4%) | 1581 (14.9%) | 1580 (14.3%) | 1519 (13.1%) |
| LUADSC | 76 (0.82%) | 104 (1.14%) | 133 (1.37%) | 127 (1.27%) | 142 (1.34%) | 136 (1.23%) | 151 (1.31%) |
| LCC | 311 (3.36%) | 258 (2.83%) | 231 (2.38%) | 212 (2.12%) | 263 (2.48%) | 275 (2.49%) | 224 (1.94%) |
| SCC | 787 (8.50%) | 793 (8.71%) | 751 (7.73%) | 794 (7.92%) | 794 (7.48%) | 802 (7.26%) | 844 (7.30%) |
| Others | 981 (10.59%) | 719 (7.90%) | 696 (7.17%) | 644 (6.43%) | 606 (5.71%) | 691 (6.25%) | 734 (6.35%) |
| Tumor size | |||||||
| <2 cm | 807 (8.7%) | 878 (9.6%) | 1138 (11.7%) | 1257 (12.6%) | 1514 (14.3%) | 1746 (15.8%) | 2131 (18.4%) |
| 2–3 cm | 1272 (13.7%) | 1294 (14.2%) | 1489 (15.3%) | 1544 (15.4%) | 1705 (16.1%) | 1804 (16.3%) | 1771 (15.3%) |
| 3–4 cm | 1361 (14.7%) | 1315 (14.4%) | 1433 (14.8%) | 1530 (15.3%) | 1579 (14.9%) | 1555 (14.1%) | 1646 (14.2%) |
| 4–5 cm | 1069 (11.5%) | 1125 (12.4%) | 1208 (12.4%) | 1177 (11.7%) | 1245 (11.7%) | 1281 (11.6%) | 1320 (11.4%) |
| 5–7 cm | 1527 (16.5%) | 1528 (16.8%) | 1475 (15.2%) | 1615 (16.1%) | 1673 (15.8%) | 1761 (15.9%) | 1712 (14.8%) |
| 7–9 cm | 778 (8.40%) | 883 (9.70%) | 865 (8.91%) | 831 (8.29%) | 908 (8.55%) | 938 (8.49%) | 961 (8.31%) |
| ≥9 cm | 485 (5.24%) | 508 (5.58%) | 518 (5.33%) | 515 (5.14%) | 524 (4.93%) | 566 (5.12%) | 592 (5.12%) |
| Missing | 1961 (21.2%) | 1574 (17.3%) | 1585 (16.3%) | 1550 (15.5%) | 1473 (13.9%) | 1402 (12.7%) | 1432 (12.4%) |
| Clinical stage | |||||||
| IA | 800 (8.6%) | 837 (9.2%) | 1120 (11.5%) | 1216 (12.1%) | 1487 (14.0%) | 1767 (16.0%) | 1981 (17.1%) |
| IB | 451 (4.9%) | 486 (5.3%) | 545 (5.6%) | 579 (5.8%) | 643 (6.1%) | 669 (6.1%) | 714 (6.2%) |
| IIA | 261 (2.8%) | 231 (2.5%) | 218 (2.2%) | 242 (2.4%) | 233 (2.2%) | 241 (2.2%) | 276 (2.4%) |
| IIB | 207 (2.2%) | 179 (2.0%) | 208 (2.1%) | 188 (1.9%) | 225 (2.1%) | 231 (2.1%) | 216 (1.9%) |
| IIIA | 826 (8.9%) | 734 (8.1%) | 760 (7.8%) | 751 (7.5%) | 754 (7.1%) | 754 (6.8%) | 730 (6.3%) |
| IIIB | 926 (10.0%) | 857 (9.4%) | 917 (9.4%) | 876 (8.7%) | 899 (8.5%) | 933 (8.4%) | 908 (7.9%) |
| IV | 5546 (59.9%) | 5635 (61.9%) | 5767 (59.4%) | 5873 (58.6%) | 6147 (57.9%) | 6184 (56.0%) | 6306 (54.5%) |
| Unknown | 243 (2.6%) | 146 (1.6%) | 176 (1.8%) | 294 (2.9%) | 233 (2.2%) | 274 (2.5%) | 434 (3.8%) |
| Treatment | |||||||
| Any | 7778 (84.0%) | 8396 (92.2%) | 8986 (92.5%) | 9340 (93.2%) | 9891 (93.1%) | 10,282 (93.0%) | 10,763 (93.1%) |
| C/T | 6163 (66.6%) | 5099 (56.0%) | 4958 (51.1%) | 4886 (48.8%) | 4901 (46.1%) | 4791 (43.4%) | 4907 (42.4%) |
| Surgery | 1945 (21.0%) | 2107 (23.1%) | 2580 (26.8%) | 2824 (28.2%) | 3174 (29.9%) | 3527 (31.9%) | 3974 (34.4%) |
| RT | 2612 (28.2%) | 2831 (31.1%) | 2879 (29.7%) | 2897 (28.9%) | 3029 (28.5%) | 3018 (27.3%) | 3046 (26.3%) |
| Target | 8 (0.1%) | 2552 (28.0%) | 2987 (30.8%) | 3164 (31.6%) | 3389 (31.9%) | 3364 (30.4%) | 3450 (29.8%) |
| 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | |
|---|---|---|---|---|---|---|---|
| All patients | |||||||
| 1-year OS | 54.07 (53.05–55.09) | 56.49 (55.47–57.51) | 60.63 (59.66–61.60) | 61.09 (60.14–62.04) | 62.56 (61.64–63.48) | 63.91 (63.01–64.81) | 66.14 (65.28–67.00) |
| 3-year OS | 26.57 (25.67–27.47) | 28.27 (27.34–29.20) | 32.98 (32.05–33.91) | 33.56 (32.63–34.49) | 36.48 (35.56–37.40) | 39.25 (38.34–40.16) | 41.12 (39.85–42.39) |
| 5-year OS | 18.5 3(17.74–19.32) | 19.78 (18.96–20.60) | 24.01 (23.16–24.86) | 25.45 (24.60–26.30) | 26.86 (25.61–28.11) | ||
| Median survival (months) | 13.75 (13.30–14.20) | 15.27 (14.73–15.82) | 17.92 (17.33–18.52) | 18.31 (17.71–18.92) | 19.85 (19.14–20.56) | 21.81 (21.01–22.61) | 24.68 (23.67–25.69) |
| 1-year OS | |||||||
| Clinical stage I | 92.57 (91.12–94.02) | 94.0 3(92.75–95.31) | 95.92 (94.97–96.87) | 94.54 (93.49–95.59) | 96.10 (95.28–96.92) | 96.39 (95.65–97.13) | 96.7 3(96.06–97.40) |
| Clinical stage II | 74.36 (70.40–78.32) | 79.51 (75.61–83.41) | 82.86 (79.27–86.45) | 83.95 (80.48–87.42) | 84.28 (80.95–87.61) | 85.59 (82.41–88.77) | 84.15 (80.92–87.38) |
| Clinical stage III | 58.7 3(56.42–61.04) | 61.28 (58.89–63.67) | 65.30 (63.03–67.57) | 61.52 (59.15–63.89) | 66.06 (63.79–68.33) | 65.68 (63.41–67.95) | 66.00 (63.71–68.29) |
| Clinical stage IV | 41.35 (40.05–42.65) | 44.10 (42.80–45.40) | 46.59 (45.30–47.88) | 47.64 (46.36–48.92) | 47.47 (46.22–48.72) | 47.85 (46.61–49.09) | 49.68 (48.45–50.91) |
| 3-year OS | |||||||
| Clinical stage I | 78.18 (75.89–80.47) | 81.6 3(79.55–83.71) | 86.7 3(85.10–88.36) | 85.1 3(83.48–86.78) | 86.24 (84.78–87.70) | 88.05 (86.76–89.34) | 89.9 3(88.29–91.57) |
| Clinical stage II | 49.57 (45.04–54.10) | 54.6 3(49.81–59.45) | 58.69 (54.01–63.37) | 58.84 (54.19–63.49) | 62.01 (57.56–66.46) | 61.44 (57.05–65.83) | 59.92 (52.14–67.70) |
| Clinical stage III | 26.8 3(24.75–28.91) | 28.79 (26.56–31.02) | 32.08 (29.85–34.31) | 30.98 (28.73–33.23) | 34.30 (32.01–36.59) | 37.11 (34.80–39.42) | 37.01 (33.85–40.17) |
| Clinical stage IV | 11.54 (10.70–12.38) | 12.76 (11.89–13.63) | 14.53 (13.62–15.44) | 14.39 (13.49–15.29) | 16.40 (15.47–17.33) | 17.09 (16.15–18.03) | 17.01 (15.49–18.53) |
| 5-year OS | |||||||
| Clinical stage I | 68.35 (65.76–70.94) | 71.28 (68.85–73.71) | 76.76 (74.72–78.80) | 76.55 (74.59–78.51) | 77.17 (74.94–79.40) | ||
| Clinical stage II | 39.53 (35.10–43.96) | 41.95 (37.17–46.73) | 43.90 (39.20–48.60) | 46.05 (41.35–50.75) | 42.08 (28.09–56.07) | ||
| Clinical stage III | 16.95 (15.19–18.71) | 19.74 (17.78–21.70) | 20.21 (18.29–22.13) | 21.39 (19.39–23.39) | 25.13 (22.82–27.44) | ||
| Clinical stage IV | 4.53 (3.98–5.08) | 5.16 (4.58–5.74) | 7.02 (6.36–7.68) | 7.0 3(6.38–7.68) | 7.10 (5.82–8.38) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, H.-C.; Huang, J.-Y.; Hsieh, M.-Y.; Wang, B.-Y. Survival of Lung Cancer Patients by Histopathology in Taiwan from 2010 to 2016: A Nationwide Study. J. Clin. Med. 2022, 11, 5503. https://doi.org/10.3390/jcm11195503
Tsai H-C, Huang J-Y, Hsieh M-Y, Wang B-Y. Survival of Lung Cancer Patients by Histopathology in Taiwan from 2010 to 2016: A Nationwide Study. Journal of Clinical Medicine. 2022; 11(19):5503. https://doi.org/10.3390/jcm11195503
Chicago/Turabian StyleTsai, Hsuan-Chih, Jing-Yang Huang, Ming-Yu Hsieh, and Bing-Yen Wang. 2022. "Survival of Lung Cancer Patients by Histopathology in Taiwan from 2010 to 2016: A Nationwide Study" Journal of Clinical Medicine 11, no. 19: 5503. https://doi.org/10.3390/jcm11195503
APA StyleTsai, H.-C., Huang, J.-Y., Hsieh, M.-Y., & Wang, B.-Y. (2022). Survival of Lung Cancer Patients by Histopathology in Taiwan from 2010 to 2016: A Nationwide Study. Journal of Clinical Medicine, 11(19), 5503. https://doi.org/10.3390/jcm11195503






