Descemet Stripping Automated Endothelial Keratoplasty in Thick Corneas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients Selection
2.2. Donor Tissues
2.3. Surgical Procedure
2.4. Statistical Analysis
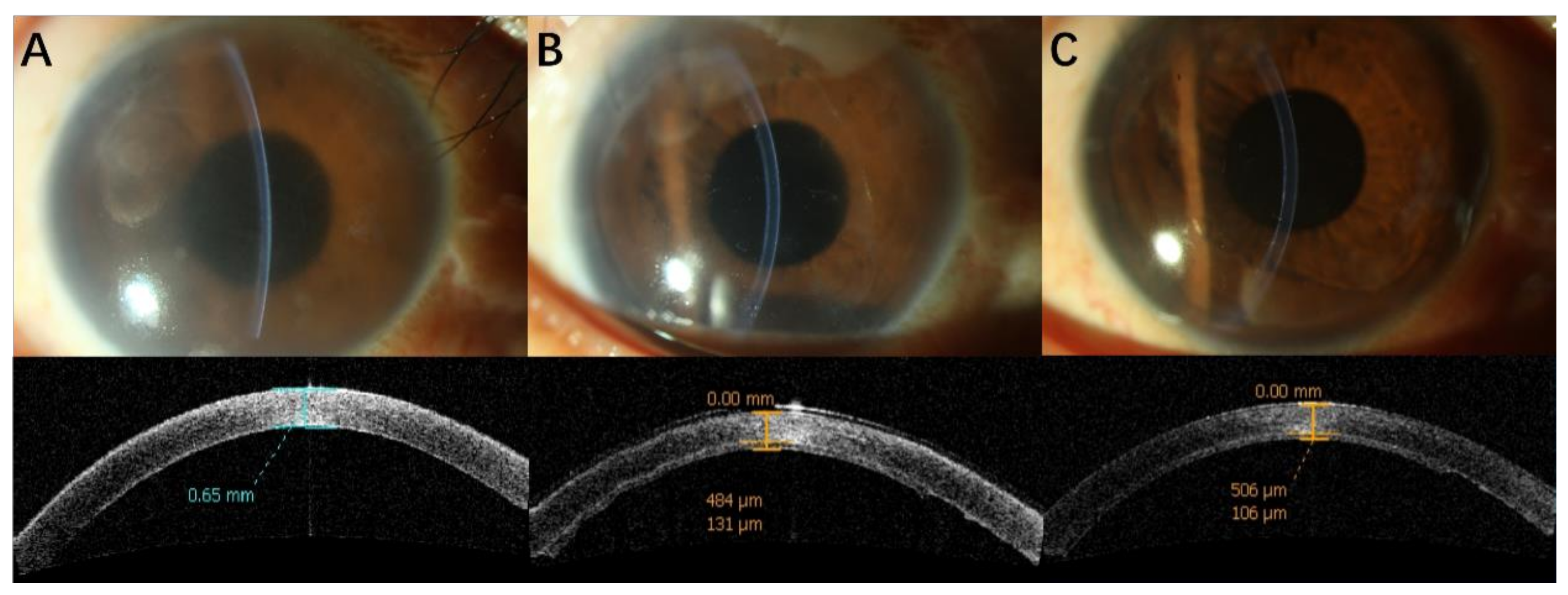
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, C.Y.; Lee, J.K.; Gore, P.K.; Lim, C.Y.; Chuck, R.S. Keratoplasty in the United States: A 10-Year Review from 2005 through 2014. Ophthalmology 2015, 122, 2432–2442. [Google Scholar] [CrossRef] [PubMed]
- Anshu, A.; Price, M.O.; Tan, D.T.; Price, F.W., Jr. Endothelial keratoplasty: A revolution in evolution. Surv. Ophthalmol. 2012, 57, 236–252. [Google Scholar] [CrossRef] [PubMed]
- Terry, M.A.; Chen, E.S.; Shamie, N.; Hoar, K.L.; Friend, D.J. Endothelial cell loss after Descemet’s stripping endothelial keratoplasty in a large prospective series. Ophthalmology 2008, 115, 488–496.e3. [Google Scholar] [CrossRef] [PubMed]
- Price, M.O.; Price, F.W., Jr. Endothelial cell loss after descemet stripping with endothelial keratoplasty influencing factors and 2-year trend. Ophthalmology 2008, 115, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Price, M.O.; Gorovoy, M.; Benetz, B.A.; Price, F.W., Jr.; Menegay, H.J.; Debanne, S.M.; Lass, J.H. Descemet’s stripping automated endothelial keratoplasty outcomes compared with penetrating keratoplasty from the Cornea Donor Study. Ophthalmology 2010, 117, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Guerra, F.P.; Anshu, A.; Price, M.O.; Price, F.W. Endothelial keratoplasty: Fellow eyes comparison of Descemet stripping automated endothelial keratoplasty and Descemet membrane endothelial keratoplasty. Cornea 2011, 30, 1382–1386. [Google Scholar] [CrossRef] [PubMed]
- Dickman, M.M.; Kruit, P.J.; Remeijer, L.; van Rooij, J.; Van der Lelij, A.; Wijdh, R.H.; van den Biggelaar, F.J.H.M.; Berendschot, T.T.J.M.; Nuijts, R.M. A Randomized Multicenter Clinical Trial of Ultrathin Descemet Stripping Automated Endothelial Keratoplasty (DSAEK) versus DSAEK. Ophthalmology 2016, 123, 2276–2284. [Google Scholar] [CrossRef] [PubMed]
- Gormsen, A.; Ivarsen, A.; Hjortdal, J. Retrospective Single-Center Registry Study on Graft Thickness 1 Year after Descemet Stripping Automated Endothelial Keratoplasty. Cornea 2019, 38, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Tourabaly, M.; Chetrit, Y.; Provost, J.; Georgeon, C.; Kallel, S.; Temstet, C.; Bouheraoua, N.; Borderie, V. Influence of graft thickness and regularity on vision recovery after endothelial keratoplasty. Br. J. Ophthalmol. 2020, 104, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, W.; Lin, C.C.; Austin, A.; Schubach, N.; Clover, J.; McLeod, S.D.; Porco, T.C.; Lietman, T.M.; Rose-Nussbaumer, J. Descemet Endothelial Thickness Comparison Trial: A Randomized Trial Comparing Ultrathin Descemet Stripping Automated Endothelial Keratoplasty with Descemet Membrane Endothelial Keratoplasty. Ophthalmology 2019, 126, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Rocha-de-Lossada, C.; Rachwani-Anil, R.; Borroni, D.; Sánchez-González, J.M.; Esteves-Marques, R.; Soler-Ferrández, F.L.; Gegúndez-Fernández, J.-A.; Romano, V.; Livny, E.; Rodríguez Calvo-de-Mora, M. New Horizons in the Treatment of Corneal Endothelial Dysfunction. J. Ophthalmol. 2021, 2021, 6644114. [Google Scholar] [CrossRef] [PubMed]
- Borroni, D.; Rocha-de-Lossada, C.; Parekh, M. Tips, Tricks, and Guides in Descemet Membrane Endothelial Keratoplasty Learning Curve. J. Ophthalmol. 2021, 2021, 1819454. [Google Scholar] [CrossRef] [PubMed]



| Group 1 (n = 15) | Group 2 (n = 15) | p-Value * | |
|---|---|---|---|
| Males, n (%) | 6 (40.0) | 6 (40.0) | 1.00 |
| Age (years), mean ± SD | 57.0 ± 16.1 | 59.5 ± 24.4 | 0.74 |
| Eye OD, n (%) | 8 (53.3) | 12 (80) | 0.12 |
| Diagnosis, n (%) | 0.55 | ||
| PBK | 12 (80.0) | 9 (60.0) | |
| Fuchs endothelial dystrophy | 2 (13.3) | 2 (13.3) | |
| Trauma | 1 (6.7) | 2 (13.3) | |
| CHED | 0 (0) | 2 (13.3) | |
| Preoperative BCVA (logMAR) | 1.21 ± 0.64 | 1.53 ± 0.70 | 0.16 |
| Preoperative corneal thickness (μm) | 707.6 ± 63.6 (range) | 924.0 ± 80.7 (range) | <0.001 |
| Triple-DSAEK, n (%) | 3 (20.0) | 3 (20.0) | 1.0 |
| Donor ECD (cells/mm2) | 3445 ± 348 | 3222 ± 343 | 0.08 |
| Group 1 (n = 15) Mean ± SD | Group 2 (n = 15) Mean ± SD | p-Value * | |
|---|---|---|---|
| BCVA (logMAR) | |||
| 1 month | 0.79 ± 0.37 | 0.80 ± 0.30 | 0.59 |
| 12 months | 0.53 ± 0.28 | 0.59 ± 0.25 | 0.28 |
| ECD (cells/mm2) | |||
| 1 month | 1637 ± 475 | 1613 ± 626 | 0.91 |
| 12 months | 1513 ± 537 | 1193 ± 600 | 0.13 |
| Recipient corneal thickness, RCT (μm) | |||
| 1 month | 508 ± 52 | 592 ± 126 | 0.03 |
| 12 months | 529 ± 53 | 539 ± 56 | 0.63 |
| Graft thickness, GT (μm) | |||
| 1 month | 117 ± 44 | 150 ± 59 | 0.08 |
| 12 months | 109 ± 33 | 110 ± 54 | 0.95 |
| Central cornea thickness (μm) | |||
| 1 month | 625 ± 60 | 742 ± 143 | 0.008 |
| 12 months | 638 ± 53 | 648 ± 75 | 0.806 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Wu, W.; Xiao, G.; Jhanji, V.; Hong, J.; Feng, Y. Descemet Stripping Automated Endothelial Keratoplasty in Thick Corneas. J. Clin. Med. 2022, 11, 5601. https://doi.org/10.3390/jcm11195601
Li C, Wu W, Xiao G, Jhanji V, Hong J, Feng Y. Descemet Stripping Automated Endothelial Keratoplasty in Thick Corneas. Journal of Clinical Medicine. 2022; 11(19):5601. https://doi.org/10.3390/jcm11195601
Chicago/Turabian StyleLi, Chendi, Wenyu Wu, Gege Xiao, Vishal Jhanji, Jing Hong, and Yun Feng. 2022. "Descemet Stripping Automated Endothelial Keratoplasty in Thick Corneas" Journal of Clinical Medicine 11, no. 19: 5601. https://doi.org/10.3390/jcm11195601
APA StyleLi, C., Wu, W., Xiao, G., Jhanji, V., Hong, J., & Feng, Y. (2022). Descemet Stripping Automated Endothelial Keratoplasty in Thick Corneas. Journal of Clinical Medicine, 11(19), 5601. https://doi.org/10.3390/jcm11195601






