Urgent Transcatheter Edge-to-Edge Repair for Severe Mitral Regurgitation in Patients with Refractory Cardiogenic Shock
Abstract
1. Introduction
2. Methods
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Engstrm, A.E.; Vis, M.M.; Bouma, B.J.; Claessen, B.E.P.M.; Sjauw, K.D.; Baan, J.; Meuwissen, M.; Koch, K.T.; De Winter, R.J.; Tijssen, J.G.; et al. Mitral regurgitation is an independent predictor of 1-year mortality in ST-elevation myocardial infarction patients presenting in cardiogenic shock on admission. Acute Card. Care 2010, 12, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N. Acute mitral regurgitation. Heart 2019, 105, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Stout, K.K.; Verrier, E.D. Acute valvular regurgitation. Circulation 2009, 119, 3232–3241. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Lindenfeld, J.; Abraham, W.T.; Kar, S.; Lim, D.S.; Mishell, J.M.; Whisenant, B.; Grayburn, P.A.; Rinaldi, M.; Kapadia, S.R.; et al. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N. Engl. J. Med. 2018, 379, 2307–2318. [Google Scholar] [CrossRef] [PubMed]
- Coats, A.J.S.; Anker, S.D.; Baumbach, A.; Alfieri, O.; von Bardeleben, R.S.; Bauersachs, J.; Bax, J.J.; Boveda, S.; Čelutkienė, J.; Cleland, J.G.; et al. The management of secondary mitral regurgitation in patients with heart failure: A joint position statement from the Heart Failure Association (HFA), European Association of Cardiovascular Imaging (EACVI), European Heart Rhythm Association (EHRA), and European Association of Percutaneous Cardiovascular Interventions (EAPCI) of the ESC. Eur. Heart J. 2021, 42, 1254–1269. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart diseaseDeveloped by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Baran, D.A.; Grines, C.L.; Bailey, S.; Burkhoff, D.; Hall, S.A.; Henry, T.D.; Hollenberg, S.M.; Kapur, N.K.; O’Neill, W.; Ornato, J.P.; et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: This document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter. Cardiovasc. Interv. 2019, 94, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.T.; Saric, M.; Faletra, F.F.; Garg, R.; Gillam, L.D.; Horton, K.; Khalique, O.K.; Little, S.H.; Mackensen, G.B.; Oh, J.; et al. Recommended Standards for the Performance of Transesophageal Echocardiographic Screening for Structural Heart Intervention: From the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2022, 35, 1–76. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Rev. Esp. De Cardiol. Engl. Ed 2021, 74, 544. [Google Scholar] [CrossRef]
- Tang, G.H.L.; Estevez-Loureiro, R.; Yu, Y.; Prillinger, J.B.; Zaid, S.; Psotka, M.A. Survival Following Edge-to-Edge Transcatheter Mitral Valve Repair in Patients With Cardiogenic Shock: A Nationwide Analysis. J. Am. Heart Assoc. 2021, 10, e019882. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.G.; Simard, T.; Kovach, C.; Flint, K.; Don, C.; di Santo, P.; Adamo, M.; Branca, L.; Valentini, F.; Benito-González, T.; et al. Transcatheter Mitral Valve Repair in Cardiogenic Shock and Mitral Regurgitation: A Patient-Level, Multicenter Analysis. JACC Cardiovasc. Interv. 2021, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Falasconi, G.; Melillo, F.; Pannone, L.; Adamo, M.; Ronco, F.; Latib, A.; Rahgozar, K.; Carrabba, N.; Valenti, R.; Citro, R.; et al. Use of edge-to-edge percutaneous mitral valve repair for severe mitral regurgitation in cardiogenic shock: A multicenter observational experience (MITRA-SHOCK study). Catheter. Cardiovasc. Interv. 2021, 98, E163–E170. [Google Scholar] [CrossRef] [PubMed]
- Henry, T.D.; Tomey, M.I.; Tamis-Holland, J.E.; Thiele, H.; Rao, S.V.; Menon, V.; Klein, D.G.; Naka, Y.; Piña, I.L.; Kapur, N.K.; et al. Invasive Management of Acute Myocardial Infarction Complicated by Cardiogenic Shock: A Scientific Statement From the American Heart Association. Circulation 2021, 143, E815–E829. [Google Scholar] [CrossRef] [PubMed]
- Osman, M.; Syed, M.; Patibandla, S.; Sulaiman, S.; Kheiri, B.; Shah, M.K.; Bianco, C.; Balla, S.; Patel, B. Fifteen-Year Trends in Incidence of Cardiogenic Shock Hospitalization and In-Hospital Mortality in the United States. J. Am. Heart Assoc. 2021, 10, e021061. [Google Scholar] [CrossRef] [PubMed]
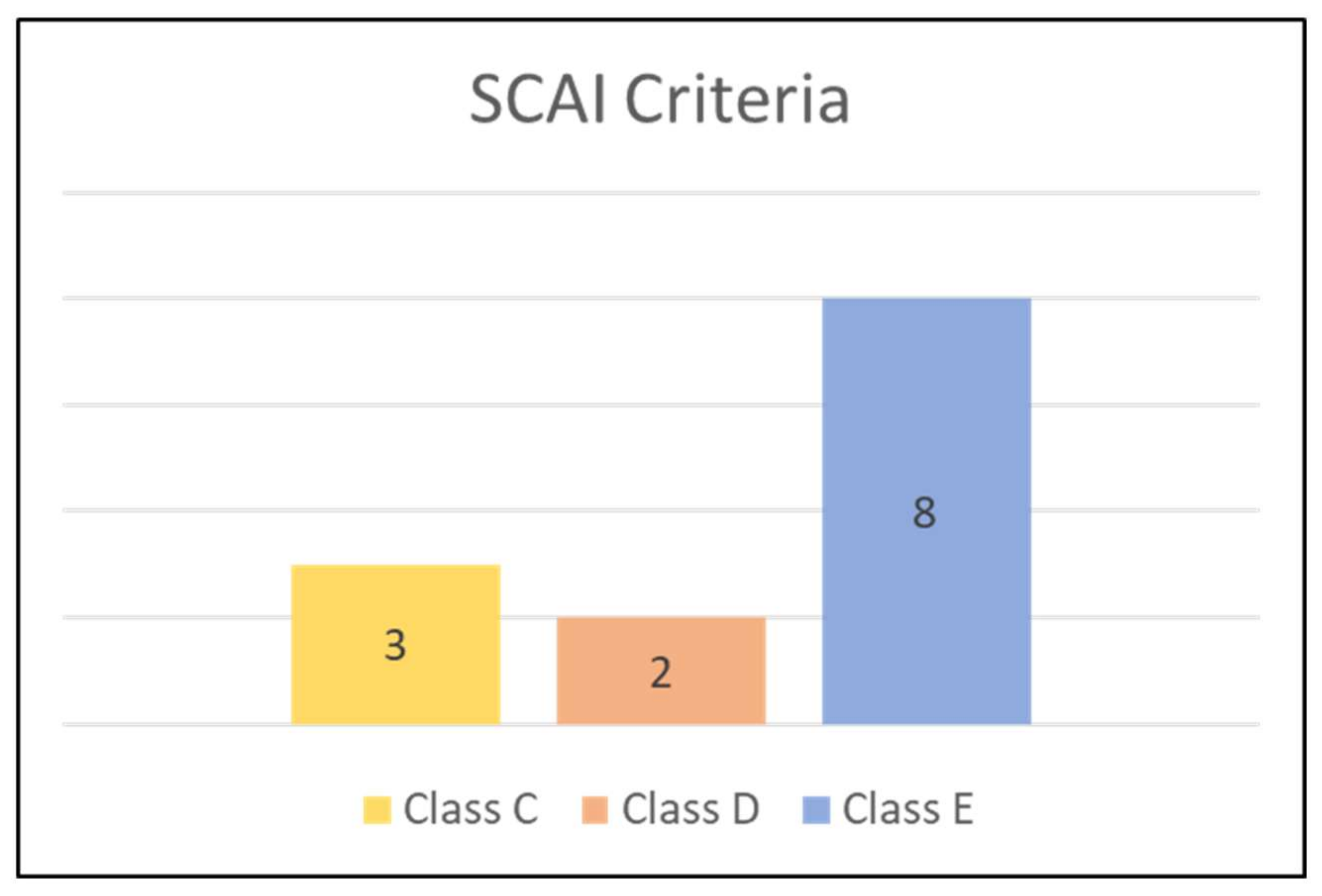
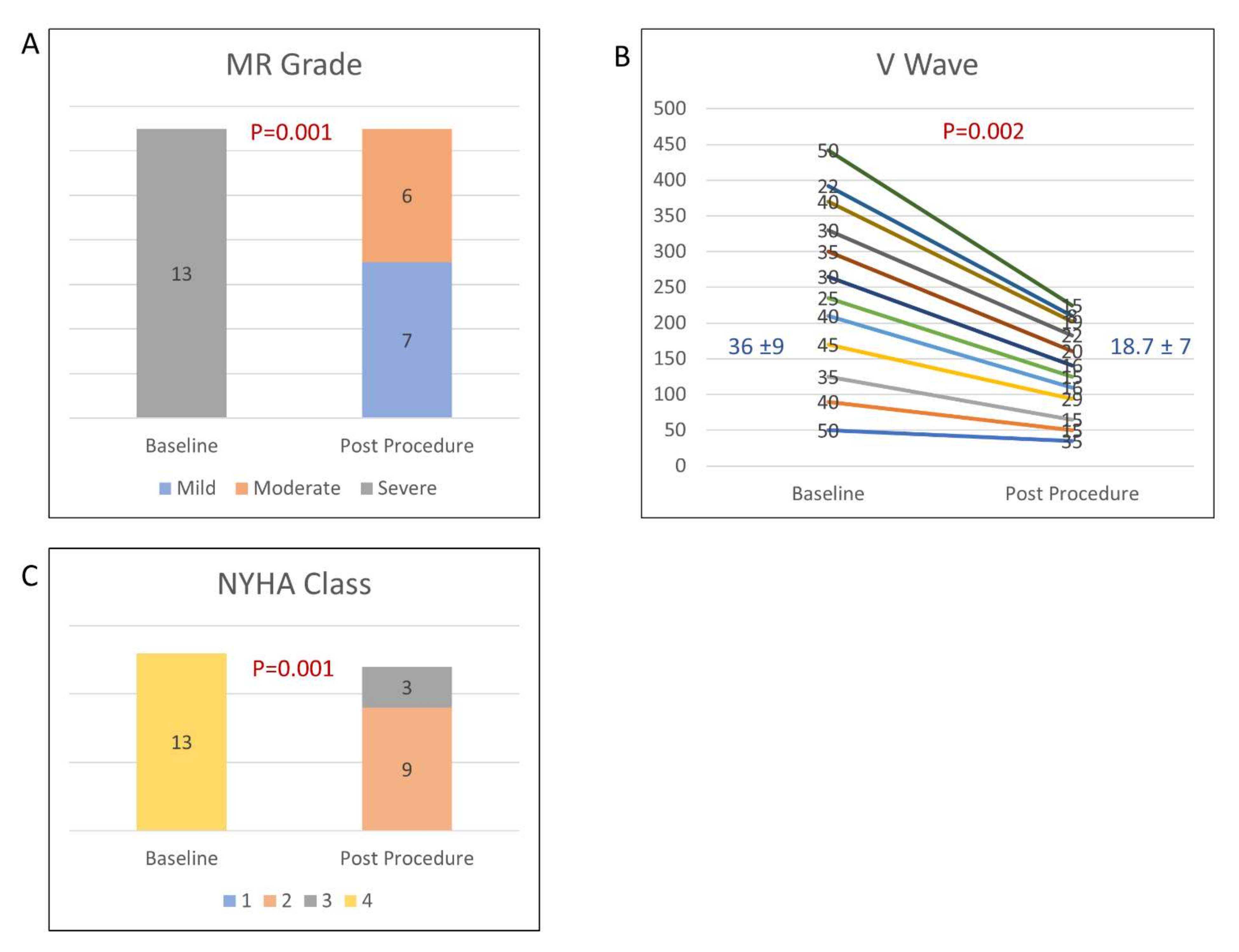
| Baseline | Post-Procedure | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient # | Age | Sex | MR Grade | V-Wave pre | MR TYPE | HTN | HPL | DM | AF | CKD | IHD | SCAI | EuroScore II | Mechanical Ventilation | Vasopressors | MCS | 30D Mortality | 6M Mortality | V-Wave after Clip | MR Grade 1 D Post-Procedure | NYHA |
| 1 | 80 | M | Severe | 50 | Secondary | YES | YES | YES | YES | YES | YES | C | 37 | NO | Nor | IABP | Alive | Alive | 35 | Moderate | 2 |
| 2 | 90 | M | Severe | 40 | Secondary | YES | NO | NO | NO | YES | YES | C | 35 | NO | No | No | Alive | Alive | 15 | Moderate | 2 |
| 3 | 74 | M | Severe | 35 | Secondary | YES | YES | YES | NO | NO | YES | D | 27 | YES | Nor | IABP | Alive | Alive | 15 | Moderate | 2 |
| 4 | 82 | M | Severe | 45 | Secondary | YES | NO | NO | YES | NO | YES | E | 27 | YES | Nor+Phen | No | Alive | Alive | 29 | Moderate | 2 |
| 5 | 62 | M | Severe | 40 | Secondary | NO | YES | NO | NO | NO | YES | E | 16 | YES | Nor+Dobu+Phen | Impella+IABP | Alive | Alive | 16 | Mild | 3 |
| 6 | 71 | F | Severe | 25 | Secondary | NO | NO | NO | NO | NO | YES | C | 23 | NO | Nor | No | Alive | Alive | 15 | Mild | 2 |
| 7 | 63 | M | Severe | 30 | Secondary | YES | NO | NO | YES | NO | YES | E | 16 | YES | Noe+Phen | IABP | Dead | Dead | 16 | Moderate | - |
| 8 | 64 | M | Severe | 35 | Secondary | YES | YES | NO | YES | NO | YES | E | 16 | YES | Nor+Dob | No | Alive | Alive | 20 | Mild | 2 |
| 9 | 78 | M | Severe | N/A | Secondary | YES | YES | YES | NO | NO | YES | D | 29 | NO | Nor+Phen | No | Alive | Alive | N/A | Mild | 2 |
| 10 | 51 | M | Severe | 30 | Secondary | NO | NO | NO | NO | NO | YES | E | 15 | YES | Nor | IABP | Alive | Alive | 22 | Mild | 3 |
| 11 | 66 | M | Severe | 40 | Primary | YES | NO | YES | NO | NO | YES | E | 20 | YES | Nor | IABP+ECMO | Alive | Alive | 19 | Mild | 3 |
| 12 | 66 | M | Severe | 22 | Secondary | NO | YES | NO | NO | NO | YES | E | 17 | YES | Nor+Dob | IABP+ECMO | Alive | Alive | 8 | Mild | 2 |
| 13 | 67 | M | Severe | 50 | Secondary | YES | YES | NO | NO | NO | YES | E | 18 | YES | Nor | IABP | Alive | Alive | 15 | Moderate | 2 |
| Mean/Rate | 70 | 92% | 100% | 36.8 | 92% | 69% | 54% | 31% | 31% | 15% | 100% | 62% | 23 | 77% | 85% | 61% | 8% | 8% | 18.8 | 54% | 2.3 |
| Male | severe | Functional | E Category | Mortality | mild | ||||||||||||||||
| Patient # | LVEDD (cm) | LVESD (cm) | LVEDV Biplane (mL) | LVESV Biplane (mL) | LVSV Biplane (mL) | EF Biplane (mL) | LVSV Continuity (mL) | EROA (cm2) | Regurgitant Volume (mL) * | Vena Contracta (mm) | PV Flow Pattern | LASd (cm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5.4 | 3.1 | 100 | 35 | 65 | 53% | 53 | 0.41 | 42 | 6 | 5.1 | |
| 2 | 6.7 | 5 | 116 | 72 | 44 | 38% | 0.59 | 65 | 9 | S reverse | 5.5 | |
| 3 | 5.7 | 4.8 | 204 | 154 | 131 | 64% | 39 | 0.28 | 41 | 9 | 5.9 | |
| 4 | 6.1 | 5 | 195 | 118 | 77 | 39% | 40 | 37 | 8 | S reverse | 4.9 | |
| 5 | 5.6 | 4.5 | 127 | 72 | 55 | 43% | 47 | 0.28 | 58 | 8 | 4.8 | |
| 6 | 5.0 | 4.2 | 160 | 107 | 53 | 33% | 37 | 0.25 | 40 | 9 | S reverse | 4.3 |
| 7 | 6.3 | 5 | 237 | 153 | 84 | 35% | 63 | 0.53 | 56 | 7 | S Blunting | 5.3 |
| 8 | 5.7 | 3.5 | 169 | 112 | 57 | 34% | 34 | 23 | 5 | S Blunting | 4.9 | |
| 9 | 6.0 | 5.3 | 144 | 90 | 54 | 38% | 34 | 20 | 7 | S Blunting | 3.8 | |
| 10 | 6.0 | 5.1 | 162 | 104 | 58 | 36% | 34 | 0.27 | 29 | 12 | S Blunting | 5.4 |
| 11 | 5.6 | 5.2 | 142 | 90 | 52 | 37% | 35 | 27 | 10 | S Blunting | 5.1 | |
| 12 | 5.5 | 4.7 | 106 | 52 | 54 | 51% | 28 | 26 | 6 | S reverse | 4.1 | |
| 13 | 4.7 | 3.4 | 140 | 55 | 85 | 61% | 44 | 0.64 | 83 | 8 | S Blunting | 5.3 |
| Average | 5.7 ± 0.5 | 4.5 ± 0.7 | 154 ± 40 | 93 ± 37 | 67 ± 24 | 43 ± 11 | 41 ± 10 | 0.4 ± 0.2 | 42 ± 19 | 8 ± 2 | 5.0 ± 0.6 | |
| Patient # | LVEDD (cm) | LVESD (cm) | LVEDV Biplane (mL) | LVESV Biplane (mL) | LVSV Biplane (mL) | EF Biplane (mL) | LVSV Continuity (mL) | EROA (cm2) | Regurgitant Volume (mL)** | Vena Contracta(mm) | PV Flow Pattern | LASd (cm) |
| 1 | 4.8 | 3.3 | 77 | 37 | 40 | 55% | 51 | 4 | 3 | 4.8 | ||
| 2 | 5.5 | 4.8 | 100 | 57 | 43 | 43% | 0.24 | 37 | 4 | SD equal | 4.2 | |
| 3 | 5.5 | 4 | 227 | 175 | 52 | 23% | 42 | 10 | 3 | SD equal | 6 | |
| 4 | 6.1 | 50 | 197 | 119 | 78 | 40% | 47 | 31 | 5 | S Dominance | 4.5 | |
| 5 | 5.4 | 4.3 | 156 | 86 | 70 | 45% | 53 | 17 | 2 | 4.5 | ||
| 6 | 5.5 | 4.6 | 165 | 91 | 74 | 45% | 68 | 6 | 3 | 3.7 | ||
| 7 | 6.3 | 4.7 | 217 | 135 | 82 | 38% | 72 | 10 | 3 | S Dominance | 5.1 | |
| 8 | 5.1 | 3.9 | 146 | 88 | 58 | 40% | 57 | 1 | 1 | S Dominance | 4.7 | |
| 9 | 6.1 | 5.7 | 157 | 115 | 42 | 27% | 34 | 8 | 3 | SD equal | 4.9 | |
| 10 | 5.9 | 4.9 | 157 | 84 | 73 | 46% | 45 | 28 | 2 | S Blunting | 5.2 | |
| 11 | 6.3 | 5.3 | 142 | 68 | 74 | 52% | 57 | 20 | 2 | SD equal | 4.2 | |
| 12 | 5.2 | 3.9 | 118 | 52 | 66 | 56% | 53 | 13 | 2 | S Dominance | 4.1 | |
| 13 | 6.1 | 4.1 | 146 | 63 | 83 | 57% | 72 | 11 | 4 | SD equal | 5.1 | |
| Average | 5.6 ± 0.5 | 5.0 ± 0.7 | 154 ± 43 | 90 ± 38 | 64 ± 15 | 44 ± 12 | 54 ± 11 | 15 ± 11 | 3 ± 1 | 4.7 ± 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perel, N.; Asher, E.; Taha, L.; Levy, N.; Steinmetz, Y.; Karameh, H.; Karmi, M.; Maller, T.; Harari, E.; Dvir, D.; et al. Urgent Transcatheter Edge-to-Edge Repair for Severe Mitral Regurgitation in Patients with Refractory Cardiogenic Shock. J. Clin. Med. 2022, 11, 5617. https://doi.org/10.3390/jcm11195617
Perel N, Asher E, Taha L, Levy N, Steinmetz Y, Karameh H, Karmi M, Maller T, Harari E, Dvir D, et al. Urgent Transcatheter Edge-to-Edge Repair for Severe Mitral Regurgitation in Patients with Refractory Cardiogenic Shock. Journal of Clinical Medicine. 2022; 11(19):5617. https://doi.org/10.3390/jcm11195617
Chicago/Turabian StylePerel, Nimrod, Elad Asher, Luoay Taha, Nir Levy, Yoed Steinmetz, Hani Karameh, Mohammad Karmi, Tomer Maller, Emanuel Harari, Danny Dvir, and et al. 2022. "Urgent Transcatheter Edge-to-Edge Repair for Severe Mitral Regurgitation in Patients with Refractory Cardiogenic Shock" Journal of Clinical Medicine 11, no. 19: 5617. https://doi.org/10.3390/jcm11195617
APA StylePerel, N., Asher, E., Taha, L., Levy, N., Steinmetz, Y., Karameh, H., Karmi, M., Maller, T., Harari, E., Dvir, D., Glikson, M., Carasso, S., & Shuvy, M. (2022). Urgent Transcatheter Edge-to-Edge Repair for Severe Mitral Regurgitation in Patients with Refractory Cardiogenic Shock. Journal of Clinical Medicine, 11(19), 5617. https://doi.org/10.3390/jcm11195617








