Corneal Transplant Rejections in Patients Receiving Immune Checkpoint Inhibitors
Abstract
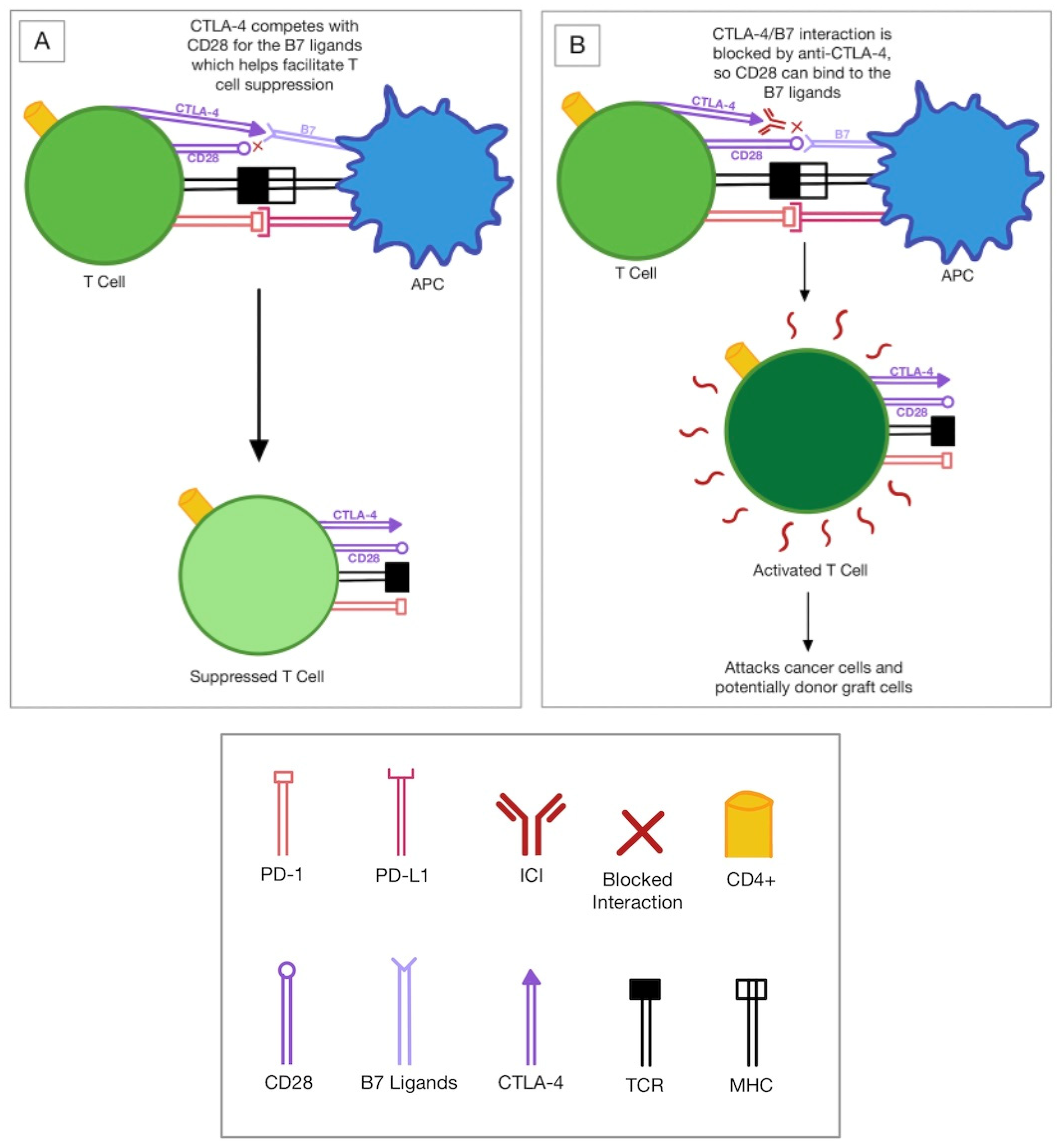
:- Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robert, C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 2020, 11, 3801. [Google Scholar] [CrossRef] [PubMed]
- Marin-Acevedo, J.A.; Kimbrough, E.M.O.; Lou, Y. Next generation of immune checkpoint inhibitors and beyond. J. Hematol. Oncol. 2021, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Dobosz, P.; Dzieciątkowski, T. The Intriguing History of Cancer Immunotherapy. Front. Immunol. 2019, 10, 2965. [Google Scholar] [CrossRef] [PubMed]
- Immunomodulators: Checkpoint Inhibitors, Cytokines, Agonists, Adjuvants—Cancer Research Institute (CRI). Available online: https://www.cancerresearch.org/en-us/immunotherapy/treatment-types/immunomodulators-checkpoint-inhibitors (accessed on 23 May 2022).
- Vaddepally, R.K.; Kharel, P.; Pandey, R.; Garje, R.; Chandra, A.B. Review of Indications of FDA-Approved Immune Checkpoint Inhibitors per NCCN Guidelines with the Level of Evidence. Cancers 2020, 12, 738. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Vinay, D.S.; Ryan, E.P.; Pawelec, G.; Talib, W.H.; Stagg, J.; Elkord, E.; Lichtor, T.; Decker, W.K.; Whelan, R.L.; Kumara, H.M.C.S.; et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2015; Volume 35, pp. S185–S198. [Google Scholar] [CrossRef]
- Linsley, P.S.; Greene, J.A.L.; Brady, W.; Bajorath, J.; Ledbetter, J.A.; Peach, R. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity 1994, 1, 793–801. [Google Scholar] [CrossRef]
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef]
- Niederkorn, J.Y. Immune mechanisms of corneal allograft rejection. Curr. Eye Res. 2007, 32, 1005–1016. [Google Scholar] [CrossRef]
- Responses to Alloantigens and Transplant Rejection—Immunobiology—NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK27163/ (accessed on 9 June 2022).
- Vigneron, N. Human Tumor Antigens and Cancer Immunotherapy. BioMed Res. Int. 2015, 2015, 948501. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.W.; Wang, Y.; Xia, R.L.; Liu, J.Y.; Ma, X.L. Immune checkpoint inhibitor-associated ophthalmic adverse events: Current understanding of its mechanisms, diagnosis, and management. Int. J. Ophthalmol. 2022, 15, 646–656. [Google Scholar] [CrossRef]
- Fang, T.; Maberley, D.A.; Etminan, M. Ocular adverse events with immune checkpoint inhibitors. J. Curr. Ophthalmol. 2019, 31, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.K.; Lee, J.K.; Huang, J.J. Bilateral drug (ipilimumab)-induced vitritis, choroiditis, and serious retinal detachments suggestive of vogt-koyanagi-harada syndrome. Retin. Cases Brief Rep. 2012, 6, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Dalvin, L.A.; Shields, C.L.; Orloff, M.; Sato, T.; Shields, J.A. Checkpoint Inhibitor Immune Therapy: Systemic Indications and Ophthalmic Side Effects. Retina 2018, 38, 1063–1078. [Google Scholar] [CrossRef] [PubMed]
- Gassmann, D.; Weiler, S.; Mertens, J.C.; Reiner, C.S.; Vrugt, B.; Nägeli, M.; Mangana, J.; Müllhaupt, B.; Jenni, F.; Misselwitz, B. Liver Allograft Failure After Nivolumab Treatment—A Case Report With Systematic Literature Research. Transplant. Direct 2018, 4, e376. [Google Scholar] [CrossRef]
- Lai, H.C.; Lin, J.F.; Hwang, T.I.S.; Liu, Y.F.; Yang, A.H.; Wu, C.K. Programmed Cell Death 1 (PD-1) Inhibitors in Renal Transplant Patients with Advanced Cancer: A Double-Edged Sword? Int. J. Mol. Sci. 2019, 20, 2194. [Google Scholar] [CrossRef]
- Ong, M.; Ibrahim, A.M.; Bourassa-Blanchette, S.; Canil, C.; Fairhead, T.; Knoll, G. Antitumor activity of nivolumab on hemodialysis after renal allograft rejection. J. Immunother. Cancer. 2016, 4, 64. [Google Scholar] [CrossRef]
- Owonikoko, T.K.; Kumar, M.; Yang, S.; Kamphorst, A.O.; Pillai, R.N.; Akondy, R.; Nautiyal, V.; Chatwal, M.S.; Book, W.M.; Sahu, A.; et al. Cardiac allograft rejection as a complication of PD-1 checkpoint blockade for cancer immunotherapy: A case report. Cancer Immunol. Immunother. 2017, 66, 45–50. [Google Scholar] [CrossRef]
- Kittai, A.S.; Oldham, H.; Cetnar, J.; Taylor, M. Immune Checkpoint Inhibitors in Organ Transplant Patients. J. Immunother. 2017, 40, 277–281. [Google Scholar] [CrossRef]
- Aguirre, L.E.; Guzman, M.E.; Lopes, G.; Hurley, J. Immune Checkpoint Inhibitors and the Risk of Allograft Rejection: A Comprehensive Analysis on an Emerging Issue. Oncologist 2019, 24, 394–401. [Google Scholar] [CrossRef] [Green Version]
- Spain, L.; Higgins, R.; Gopalakrishnan, K.; Turajlic, S.; Gore, M.; Larkin, J. Acute renal allograft rejection after immune checkpoint inhibitor therapy for metastatic melanoma. Ann. Oncol. 2016, 27, 1135–1137. [Google Scholar] [CrossRef]
- Yang, W.; Li, H.; Chen, P.W.; Alizadeh, H.; He, Y.; Hogan, R.N.; Niederkorn, J.Y. PD-L1 Expression on Human Ocular Cells and Its Possible Role in Regulating Immune-Mediated Ocular Inflammation. Investig. Opthalmology Vis. Sci. 2009, 50, 273. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, H.S.; Hemmati, H.D.; Dana, R. Immune checkpoint inhibitors and corneal transplant rejection: A call for awareness. Immunotherapy 2020, 12, 947–949. [Google Scholar] [CrossRef] [PubMed]
- Hori, J.; Kunishige, T.; Nakano, Y. Immune Checkpoints Contribute Corneal Immune Privilege: Implications for Dry Eye Associated with Checkpoint Inhibitors. Int. J. Mol. Sci. 2020, 21, 3962. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Shinagare, A.B.; Rennke, H.G.; Ghai, S.; Lorch, J.H.; Ott, P.A.; Rahma, O.E. The Safety and Efficacy of Checkpoint Inhibitors in Transplant Recipients: A Case Series and Systematic Review of Literature. Oncologist 2020, 25, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.; Vanathi, M.; Kumar, A.; Dash, Y.; Priya, S. Corneal graft rejection. Surv. Ophthalmol. 2007, 52, 375–396. [Google Scholar] [CrossRef]
- Nguyen, L.S.; Ortuno, S.; Lebrun-Vignes, B.; Johnson, D.B.; Moslehi, J.J.; Hertig, A.; Salem, J.-E. Transplant rejections associated with immune checkpoint inhibitors: A pharmacovigilance study and systematic literature review. Eur. J. Cancer 2021, 148, 36–47. [Google Scholar] [CrossRef]
- le Fournis, S.; Gohier, P.; Urban, T.; Jeanfaivre, T.; Hureaux, J. Corneal graft rejection in a patient treated with nivolumab for primary lung cancer. Lung Cancer 2016, 102, 28–29. [Google Scholar] [CrossRef]
- Vanhonsebrouck, E.; van de Walle, M.; Lybaert, W.; Kruse, V.; Roels, D. Bilateral Corneal Graft Rejection Associated With Pembrolizumab Treatment. Cornea 2020, 39, 1436–1438. [Google Scholar] [CrossRef]
- Azevedo Magalhaes, O.; Shalaby Bardan, A.; Zarei-Ghanavati, M.; Liu, C. Literature review and suggested protocol for prevention and treatment of corneal graft rejection. Eye 2020, 34, 442–450. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moshirfar, M.; Basharat, N.F.; Seitz, T.S.; Ply, B.K.; Ronquillo, Y.C.; Hoopes, P.C. Corneal Transplant Rejections in Patients Receiving Immune Checkpoint Inhibitors. J. Clin. Med. 2022, 11, 5647. https://doi.org/10.3390/jcm11195647
Moshirfar M, Basharat NF, Seitz TS, Ply BK, Ronquillo YC, Hoopes PC. Corneal Transplant Rejections in Patients Receiving Immune Checkpoint Inhibitors. Journal of Clinical Medicine. 2022; 11(19):5647. https://doi.org/10.3390/jcm11195647
Chicago/Turabian StyleMoshirfar, Majid, Noor F. Basharat, Tanner S. Seitz, Briana K. Ply, Yasmyne C. Ronquillo, and Phillip C. Hoopes. 2022. "Corneal Transplant Rejections in Patients Receiving Immune Checkpoint Inhibitors" Journal of Clinical Medicine 11, no. 19: 5647. https://doi.org/10.3390/jcm11195647




