Short- and Long-Term Effect of Cochlear Implantation on Disabling Tinnitus in Single-Sided Deafness Patients: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Quality Assessment
2.4. Data Extraction
3. Results
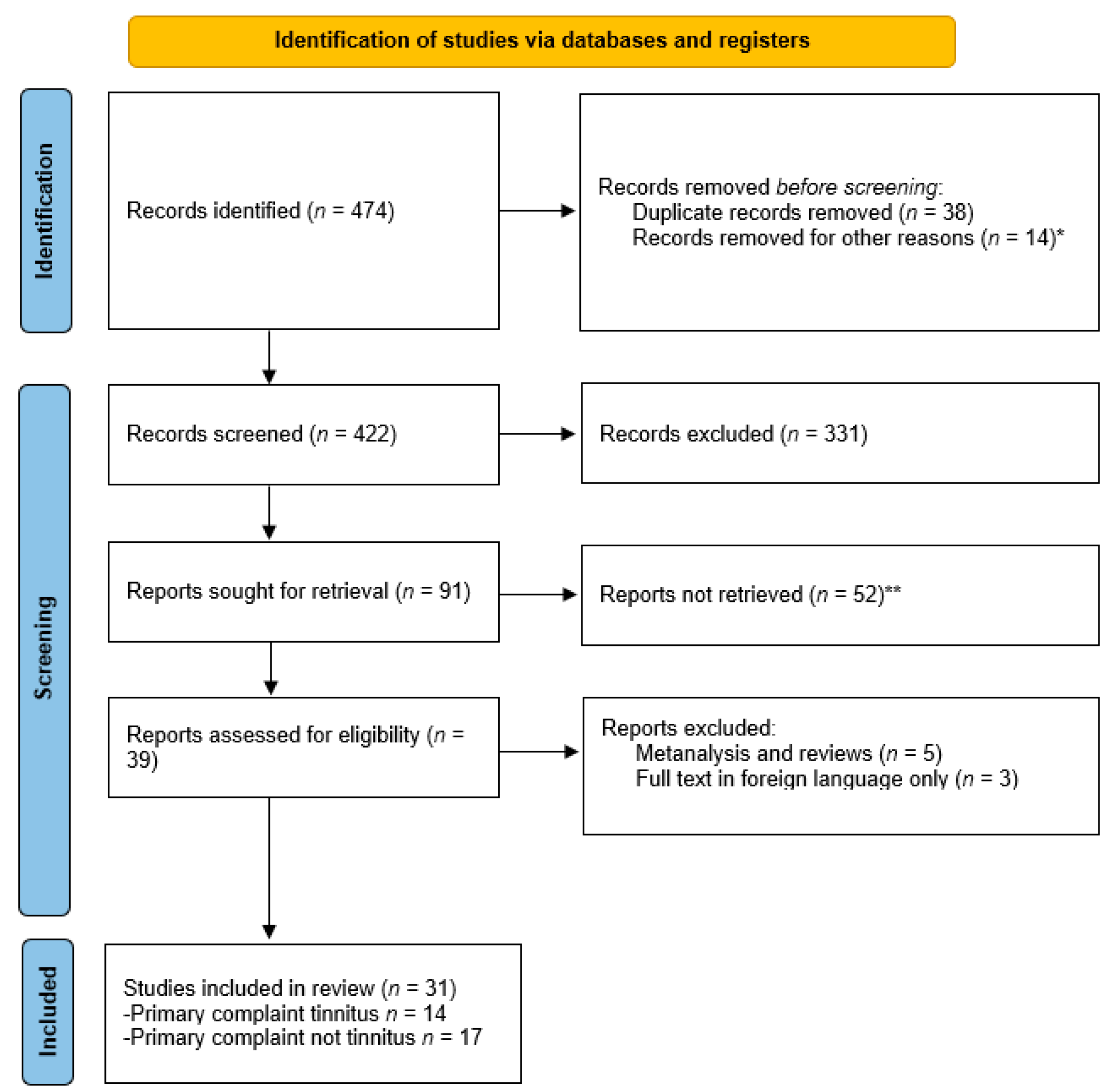
3.1. Search Strategy and Study Selection
3.2. Quality Assessment of Included Studies
| ROBINS-I tool | Risk of Bias (RoB) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Study Design | Sample Size | Bias Due to Confounding | Bias in Selection of Participants | Bias in Classification of Interventions | Deviation from Intended Intervention | Bias Due to Missing Data | Bias in Measurement of Outcomes | Bias in Selection of Reported Result |
| Ahmed et al. [63] | PCS | 13 | ● | O | O | Ø | Ø | ◐ | O |
| Arts et al. [62] | PCS | 10 | ● | O | O | ● | O | O | O |
| Holder et al. [64] | PCS | 12 | ● | ● | Ø | Ø | Ø | ◐ | O |
| Kleinjung et al. [60] | CR | 1 | ● | NA | Ø | O | Ø | NA | Ø |
| Macias et al. [66] | PCS | 16 | ● | O | O | O | O | ◐ | O |
| Mertens et al. [55] | RCT | 23 | ● | ● | ● | Ø | Ø | ◐ | O |
| Mertens et al. [56] | PCS | 11 | ● | O | O | ● | Ø | ◐ | O |
| Poncet-Wallet et al. [65] | PCS | 26 | ● | O | O | ● | ● | ◐ | ◐ |
| Punte et al. [59] | PCS | 26 | Ø | O | O | Ø | O | ◐ | ● |
| Punte et al. [68] | PCS | 7 | ● | O | O | ● | O | ◐ | O |
| Ramos et al. [61] | PCS | 6 | ● | O | O | ● | O | ◐ | O |
| Song et al. [58] | PCS | 9 | O | O | O | Ø | ● | ◐ | ● |
| Van de Heyning et al. [54] | PCS | 22 | ● | O | O | O | ● | ◐ | ◐ |
| Zeng et al. [67] | CR | 1 | ● | NA | Ø | ● | Ø | NA | Ø |
| ROBINS-I Tool | Risk of Bias (RoB) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Study Design | Sample Size | Bias Due to Confounding | Bias in Selection of Participants | Bias in Classification of Interventions | Deviation from Intended Intervention | Bias Due to Missing Data | Bias in Measurement of Outcomes | Bias in Selection of Reported Result |
| Arndt et al. [44] | PCS | 11 | O | O | O | Ø | O | ◐ | O |
| Buechner et al. [73] | PCS | 5 | ● | ● | O | O | O | ◐ | O |
| Dillon et al. [8] | PCS | 20 | ● | O | O | O | Ø | ◐ | O |
| Dorbeau et al. [82] | PCS | 18 | ● | O | O | O | O | ◐ | O |
| Finke et al. [75] | RCS | 14 | ● | O | ● | Ø | ● | ◐ | Ø |
| Friedman et al. [71] | RCS | 16 | O | O | ● | Ø | ● | ◐ | ◐ |
| Gartrell et al. [77] | CR | 1 | ● | NA | Ø | Ø | Ø | NA | Ø |
| Harkonen et al. [80] | PCS | 7 | ● | O | O | Ø | O | ◐ | O |
| Haubler et al. [72] | PCS | 20 | O | O | ● | Ø | O | ◐ | O |
| Kitoh et al. [81] | PCS | 5 | ● | O | O | Ø | ● | ◐ | O |
| Macias et al. [78] | PCS | 16 | ● | O | O | Ø | O | ◐ | O |
| Mertens et al. [57] | PCS | 15 | ● | O | Ø | ● | O | ◐ | O |
| Peters et al. [83] | PCS | 28 | ● | O | O | O | O | ◐ | ◐ |
| Sladen et al. [74] | RCS | 23 | ● | ● | ● | Ø | ● | ◐ | ◐ |
| Sullivan et al. [76] | RCS | 60 | ● | O | ● | Ø | ● | ◐ | ◐ |
| Tavora-Vieira et al. [69] | PCS | 9 | O | O | O | O | O | ◐ | O |
| Tavora-Vieira et al. [70] | PCS | 28 | O | O | O | O | O | ◐ | O |
3.3. Data Extraction and Study Outcomes
3.3.1. Tinnitus Evaluated as a Primary Complaint
3.3.2. Tinnitus Evaluated as an Additional Complaint
| Study | Patients’ Criteria | n | Evaluation | Interval Studied | Results | Conclusion |
|---|---|---|---|---|---|---|
| Arndt et al. [44] | CI in SSD and tinnitus refractory to conventional treatment | 11 | Tests
Questionnaires
| 6 months |
|
|
| Buechner et al. [73] | CI in SSD and tinnitus | 5 | Tests
Questionnaires
| 12 months |
|
|
| Dillon et al. [8] | CI in SSD and tinnitus | 20 | Questionnaires
| 12 months |
|
|
| Dorbeau et al. [82] | CI in SSD and tinnitus | 18 | Tests
Questionnaires
| 12 months |
|
|
| Finke et al. [75] | CI in SSD and tinnitus | 14 | Tests
Questionnaires
| 53 months |
|
|
| Friedman et al. [71] | CI in SSD and tinnitus | 16 | Tests
Subjective assessments
| 12 months |
|
|
| Gartrell et al. [77] | CI in SSD and severe tinnitus refractory to medical therapies | 1 | Tests
Questionnaires
| 18 months |
|
|
| Härkönen et al. [80] | CI in SSD and tinnitus | 7 | Tests
Questionnaires
| 28 months |
|
|
| Häußler et al. [72] | CI in SSD and tinnitus refractory to conventional treatment | 20 | Tests
Questionnaires
| 36 months |
|
|
| Kitoh et al. [81] | CI in SSD patients | 5 | Tests
Questionnaires
| 12 months |
|
|
| Macias et al. [78] | CI in SSD and disabling tinnitus and hyperacusis refractory to conventional treatment | 16 | Questionnaires
| 12 months |
|
|
| Mertens et al. [57] | CI in SSD and disabling tinnitus | 15 | Tests
Questionnaires
| 36 months |
|
|
| Peters et al. [83] | CI and bone conduction devices in SSD and tinnitus | 28 | Tests
Questionnaires
| 6 months |
|
|
| Sladen et al. [74] | CI in SSD and tinnitus | 23 | Tests
Other
| 6 months |
|
|
| Sullivan et al. [76] | CI in SSD patients and tinnitus | 60 | Tests
Questionnaires
| 72 months |
|
|
| Tavora-Vieira et al. [69] | CI in SSD and tinnitus | 9 | Tests
Questionnaires
| 3 months |
|
|
| Tavora-Vieira et al. [70] | CI in SSD with tinnitus | 28 | Tests
Questionnaires
| 24 months |
|
|
3.3.3. Effect of Cochlear Implant on Other Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Snapp, H.A.; Ausili, S.A. Hearing with one ear: Consequences and treatments for profound unilateral hearing loss. J. Clin. Med. 2020, 9, 1010. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, S.; Sano, H.; Nishio, S.; Takumi, Y.; Okamoto, M.; Usami, S.I.; Ogawa, K. Hearing handicap in adults with unilateral deafness and bilateral hearing loss. Otol. Neurotol. 2013, 34, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Wie, O.B.; Pripp, A.H.; Tvete, O. Unilateral deafness in adults: Effects on communication and social interaction. Ann. Otol. Rhinol. Laryngol. 2010, 119, 772–781. [Google Scholar] [PubMed]
- Douglas, S.A.; Yeung, P.; Daudia, A.; Gatehouse, S.; O’Donoghue, G.M. Spatial hearing disability after acoustic neuroma removal. Laryngoscope 2007, 117, 1648–1651. [Google Scholar] [CrossRef]
- Marx, M.; Mosnier, I.; Venail, F.; Mondain, M.; Uziel, A.; Bakhos, D.; Lescanne, E.; N’Guyen, Y.; Bernardeschi, D.; Sterkers, O.; et al. Cochlear Implantation and Other Treatments in Single-Sided Deafness and Asymmetric Hearing Loss: Results of a National Multicenter Study including a Randomized Controlled Trial. Audiol. Neurotol. 2021, 26, 414–424. [Google Scholar] [CrossRef]
- Henderson-Sabes, J.; Shang, Y.; Perez, P.L.; Chang, J.L.; Pross, S.E.; Findlay, A.M.; Mizuiri, D.; Hinkley, L.B.; Nagarajan, S.S.; Cheung, S.W. Corticostriatal functional connectivity of bothersome tinnitus in single-sided deafness. Sci. Rep. 2019, 9, 19552. [Google Scholar] [CrossRef]
- König, O.; Schaette, R.; Kempter, R.; Gross, M. Course of hearing loss and occurrence of tinnitus. Hear. Res. 2006, 221, 59–64. [Google Scholar] [CrossRef]
- Dillon, M.T.; Buss, E.; Rooth, M.A.; King, E.R.; Deres, E.J.; Buchman, C.A.; Pillsbury, H.C.; Brown, K.D. Effect of Cochlear Implantation on Quality of Life in Adults with Unilateral Hearing Loss. Audiol. Neurotol. 2017, 22, 259–271. [Google Scholar] [CrossRef]
- Ciminelli, P.; Machado, S.; Palmeira, M.; Carta, M.G.; Beirith, S.C.; Nigri, M.L.; Mezzasalma, M.A.; Nardi, A.E. Tinnitus: The Sound of Stress? Clin. Pract. Epidemiol. Ment. Health 2018, 14, 264–269. [Google Scholar] [CrossRef]
- Hallam, R.S.; Jakes, S.C.; Hinchcliffe, R. Cognitive variables in tinnitus annoyance. Br. J. Clin. Psychol. 1988, 27, 213–222. [Google Scholar] [CrossRef]
- Zeman, F.; Koller, M.; Schecklmann, M.; Langguth, B.; Landgrebe, M.; Figueired, R.; Aazevedo, A.; Rates, M.; Binetti, C.; Elgoyhen, A.B.; et al. Tinnitus assessment by means of standardized self-report questionnaires: Psychometric properties of the Tinnitus Questionnaire (TQ), the Tinnitus Handicap Inventory (THI), and their short versions in an international and multi-lingual sample. Health Qual. Life Outcomes 2012, 10, 128. [Google Scholar] [CrossRef] [PubMed]
- Newman, C.W.; Jacobson, G.P.; Spitzer, J.B.; Surgery, N.; Ford Hospital, H.; Newman, M. Development of the Tinnitus Handicap Inventory. Arch. Otolaryngol. Head Neck Surg. 1996, 122, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.; Henry, J.; Bowen, M.; Haralambous, G. Tinnitus reaction questionnaire: Psychometric properties of a measure of distress associated with tinnitus. J. Speech Hear Res. 1991, 34, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Raj-Koziak, D.; Gos, E.; Swierniak, W.; Rajchel, J.J.; Karpiesz, L.; Niedzialek, I.; Wlodarczyk, E.; Skarzynski, H.; Skarzynski, P.H. Visual analogue scales as a tool for initial assessment of tinnitus severity: Psychometric evaluation in a clinical population. Audiol. Neurotol. 2018, 23, 229–237. [Google Scholar] [CrossRef]
- Croft, C.; Brown, R.F.; Thorsteinsson, E.B.; Noble, W. Development of the Tinnitus response scales: Factor analyses, subscale reliability and validity analyses. Int. Tinnitus J. 2013, 18, 45–56. [Google Scholar] [CrossRef]
- Halford, J.; Anderson, S. Tinnitus severity measured by a subjective scale, audiometry and clinical judgement. J. Laryngol. Otol. 1991, 105, 89–93. [Google Scholar] [CrossRef]
- Meikle, M.B.; Stewart, B.J.; Griest, S.E.; Henry, J.A. Tinnitus Outcomes Assessment. Trends Amplif. 2008, 12, 223–235. [Google Scholar] [CrossRef]
- McCombe, A.; Baguley, D.; Coles, R.; McKenna, L.; McKinney, C.; Windle-Taylor, P. Guidelines for the grading of tinnitus severity: The results of a working group commissioned by the British Association of Otolaryngologists, Head and Neck Surgeons, 1999. Clin. Otolaryngol. Allied Sci. 2001, 26, 388–393. [Google Scholar] [CrossRef]
- Han, B.I.; Lee, H.W.; Kim, T.Y.; Lim, J.S.; Shin, K.S. Tinnitus: Characteristics, causes, mechanisms, and treatments. J. Clin. Neurol. 2009, 5, 11–19. [Google Scholar] [CrossRef]
- Auerbach, B.D.; Rodrigues, P.V.; Salvi, R.J. Central Gain Control in Tinnitus and Hyperacusis. Front. Neurol. 2014, 5, 206. [Google Scholar] [CrossRef] [Green Version]
- Gomaa, M.A.M.; Elmagd, M.H.A.; Elbadry, M.M.; Kader, R.M.A. Depression, Anxiety and Stress Scale in patients with tinnitus and hearing loss. Eur. Arch. Oto-Rhino-Laryngol. 2014, 271, 2177–2184. [Google Scholar] [CrossRef]
- Zirke, N.; Goebel, G.; Mazurek, B. Tinnitus und psychische Komorbiditäten. HNO 2010, 58, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Simões, J.P.; Neff, P.K.A.; Langguth, B.; Schlee, W.; Schecklmann, M. The progression of chronic tinnitus over the years. Sci. Rep. 2021, 11, 4162. [Google Scholar] [CrossRef] [PubMed]
- Cima, R.F.F.; Mazurek, B.; Haider, H.; Kikidis, D.; Lapira, A.; Noreña, A.; Hoare, D.J. A multidisciplinary European guideline for tinnitus: Diagnostics, assessment, and treatment. HNO 2019, 67, 10–42. [Google Scholar] [CrossRef]
- Tunkel, D.E.; Bauer, C.A.; Sun, G.H.; Rosenfeld, R.M.; Chandrasekhar, S.S.; Cunningham, E.R.; Archer, S.M.; Blakley, B.W.; Carter, J.M.; Granieri, E.C.; et al. Clinical practice guideline: Tinnitus. Otolaryngol.–Head Neck Surg. 2014, 151, S1–S40. [Google Scholar] [CrossRef]
- Baldo, P.; Doree, C.; Lazzarini, R.; Molin, P.; McFerran, D.J. Antidepressants for patients with tinnitus. Cochrane Database Syst. Rev. 2012, 2012, CD003853. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, C.E.L.; Rynja, S.P.; Van Zanten, G.A.; Rovers, M. Anticonvulsants for tinnitus. Cochrane Database Syst. Rev. 2011, 2011, CD007960. [Google Scholar] [CrossRef] [PubMed]
- Sereda, M.; Xia, J.; Scutt, P.; Hilton, M.P.; El Refaie, A.; Hoare, D.J. Ginkgo biloba for tinnitus. Cochrane Database Syst. Rev. 2019, 2019, CD013514. [Google Scholar] [CrossRef]
- Person, O.C.; Puga, M.E.; da Silva, E.M.; Torloni, M.R. Zinc supplementation for tinnitus. Cochrane Database Syst. Rev. 2016, 11, CD009832. [Google Scholar] [CrossRef]
- Londero, A.; Bonfils, P.; Lefaucheur, J.P. Transcranial magnetic stimulation and subjective tinnitus. A review of the literature, 2014–2016. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2018, 135, 51–58. [Google Scholar] [CrossRef]
- Ferreira, M.C.; De Matos, I.L.; De Toledo, I.P.; Honório, H.M.; Mondelli, M.F.C.G.; Gallun, F.E.; Rasetshwane, D. Effects of low-level laser therapy as a therapeutic strategy for patients with tinnitus: A systematic review. J. Speech Lang. Hear. Res. 2021, 64, 279–298. [Google Scholar] [CrossRef] [PubMed]
- Rogha, M.; Rezvani, M.; Khodami, A.R. The effects of acupuncture on the inner ear originated tinnitus. J. Res. Med. Sci. 2011, 16, 1217–1223. [Google Scholar] [PubMed]
- Jun, H.J.; Park, M.K. Cognitive behavioral therapy for tinnitus: Evidence and efficacy. Korean J. Audiol. 2013, 17, 101–104. [Google Scholar] [CrossRef]
- Cima, R.F.F.; Maes, I.H.; Joore, M.A.; Scheyen, D.J.W.W.; El Refaie, A.; Baguley, D.M.; Anteunis, L.J.C.; Van Breukelen, G.J.P.; Vlaeyen, J.W.S. Specialised treatment based on cognitive behaviour therapy versus usual care for tinnitus: A randomised controlled trial. Lancet 2012, 379, 1951–1959. [Google Scholar] [CrossRef]
- Scherer, R.W.; Formby, C.; Gold, S.; Erdman, S.; Rodhe, C.; Carlson, M.; Shade, D.; Tucker, M.; Sensinger, L.M.C.; Hughes, G.; et al. The Tinnitus Retraining Therapy Trial (TRTT): Study protocol for a randomized controlled trial. Trials 2014, 15, 396. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.M.; Hall, D.A.; Walker, D.-M.; Hoare, D.J. Psychological Therapy for People with Tinnitus: A scoping review of treatment components. Ear Hear. 2017, 38, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Arif, M.; Sadlier, M.; Rajenderkumar, D.; James, J.; Tahir, T. A randomised controlled study of mindfulness meditation versus relaxation therapy in the management of tinnitus. J. Laryngol. Otol. 2017, 131, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Gunjawate, D.R.; Ravi, R. Effect of yoga and meditation on tinnitus: A systematic review. J. Laryngol. Otol. 2021, 135, 284–287. [Google Scholar] [CrossRef]
- Attias, J.; Shemesh, Z.; Shoham, C.; Shahar, A.; Sohmer, H. Efficacy of self-hypnosis for tinnitus relief. Scand. Audiol. 1990, 19, 245–249. [Google Scholar] [CrossRef]
- House, J.W. Treatment of severe tinnitus with biofeedback training. Laryngoscope 1978, 88, 406–412. [Google Scholar] [CrossRef]
- Chen, J.; Zhong, P.; Meng, Z.; Pan, F.; Qi, L.; He, T.; Lu, J.; He, P.; Zheng, Y. Investigation on chronic tinnitus efficacy of combination of non-repetitive preferred music and educational counseling: A preliminary study. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 2745–2752. [Google Scholar] [CrossRef] [PubMed]
- Roland, L.T.; Lenze, E.J.; Hardin, F.M.; Kallogjeri, D.; Nicklaus, J.; Wineland, A.M.; Fendell, G.; Peelle, J.E.; Piccirillo, J.F. Effects of mindfulness based stress reduction therapy on subjective bother and neural connectivity in chronic tinnitus. Otolaryngol.–Head Neck Surg. 2015, 152, 919–926. [Google Scholar] [CrossRef]
- Tyler, R.S.; Perreau, A.; Powers, T.; Watts, A.; Owen, R.; Ji, H.; Mancini, P.C. Tinnitus sound therapy trial shows effectiveness for those with tinnitus. J. Am. Acad. Audiol. 2020, 31, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Arndt, S.; Aschendorff, A.; Laszig, R.; Beck, R.; Schild, C.; Kroeger, S.; Ihorst, G.; Wesarg, T. Comparison of pseudobinaural hearing to real binaural hearing rehabilitation after cochlear implantation in patients with unilateral deafness and tinnitus. Otol. Neurotol. 2011, 32, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Marx, M.; Costa, N.; Lepage, B.; Taoui, S.; Molinier, L.; Deguine, O.; Fraysse, B. Cochlear implantation as a treatment for single-sided deafness and asymmetric hearing loss: A randomized controlled evaluation of cost-utility. BMC Ear Nose Throat Disord. 2019, 19, 1–10. [Google Scholar] [CrossRef]
- Donato, M.; Santos, R.; Correia, F.; Escada, P. Single-sided deafness: Bone conduction devices or cochlear implantation? A systematic review with meta-analysis. Acta Otorrinolaringol. Engl. Ed. 2021, 72, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Nucleus 24 Cochlear Implant System—P970051/S205. Available online: https://www.fda.gov/medical-devices/recently-approved-devices/nucleus-24-cochlear-implant-system-p970051s205 (accessed on 3 March 2022).
- Recommandation pour la Pratique Clinique—Indications de L’implant Cochléaire chez L’adulte et chez L’enfant; Société Française d’ORL Chirurgie la Face du Cou: France, 2018.
- Peter, N.; Liyanage, N.; Pfiffner, F.; Huber, A.; Kleinjung, T. The Influence of Cochlear Implantation on Tinnitus in Patients with Single-Sided Deafness: A Systematic Review. Otolaryngol.–Head Neck Surg. 2019, 161, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.A.; Lee, J.A.; Nguyen, S.A.; McRackan, T.R.; Meyer, T.A.; Lambert, P.R. Cochlear Implantation for Treatment of Tinnitus in Single-sided Deafness: A Systematic Review and Meta-analysis. Otol. Neurotol. 2020, 41, e1004–e1012. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C. The primary outcome measure and its importance in clinical trials. J. Clin. Psychiatry 2015, 76, e1320–e1323. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Sterne, J.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Van De Heyning, P.; Vermeire, K.; Diebl, M.; Nopp, P.; Anderson, I.; De Ridder, D. Incapacitating unilateral tinnitus in single-sided deafness treated by cochlear implantation. Ann. Otol. Rhinol. Laryngol. 2008, 117, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Mertens, G.; De Bodt, M.; Van de Heyning, P. Cochlear implantation as a long-term treatment for ipsilateral incapacitating tinnitus in subjects with unilateral hearing loss up to 10 years. Hear. Res. 2016, 331, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mertens, G.; Van Rompaey, V.; Van de Heyning, P. Electric-acoustic stimulation suppresses tinnitus in a subject with high-frequency single-sided deafness. Cochlear Implants Int. 2018, 19, 292–296. [Google Scholar] [CrossRef]
- Mertens, G.; Punte, A.K.; De Ridder, D.; Van De Heyning, P. Tinnitus in a single-sided deaf ear reduces speech reception in the nontinnitus ear. Otol. Neurotol. 2013, 34, 662–666. [Google Scholar] [CrossRef]
- Song, J.J.; Punte, A.K.; De Ridder, D.; Vanneste, S.; Van de Heyning, P. Neural substrates predicting improvement of tinnitus after cochlear implantation in patients with single-sided deafness. Hear. Res. 2013, 299, 1–9. [Google Scholar] [CrossRef]
- Punte, A.K.; Vermeire, K.; Hofkens, A.; De Bodt, M.; De Ridder, D.; Van de Heyning, P. Cochlear implantation as a durable tinnitus treatment in single-sided deafness. Cochlear Implants Int. 2011, 12 (Suppl. 1), S26–S29. [Google Scholar] [CrossRef]
- Kleinjung, T.; Steffens, T.; Strutz, J.; Langguth, B. Curing tinnitus with a Cochlear Implant in a patient with unilateral sudden deafness: A case report. Cases J. 2009, 2, 7462. [Google Scholar] [CrossRef]
- Ramos, Á.; Polo, R.; Masgoret, E.; Artiles, O.; Lisner, I.; Zaballos, M.L.; Moreno, C.; Osorio, Á. Cochlear Implant in Patients With Sudden Unilateral Sensorineural Hearing Loss and Associated Tinnitus. Acta Otorrinolaringol. Engl. Ed. 2012, 63, 15–20. [Google Scholar] [CrossRef]
- Arts, R.A.G.J.; George, E.L.J.; Janssen, M.; Griessner, A.; Zierhofer, C.; Stokroos, R.J. Tinnitus suppression by intracochlear electrical stimulation in single sided deafness—A prospective clinical trial: Follow-up. PLoS ONE 2016, 11, e0153131. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.F.M.; Khater, A. Tinnitus suppression after cochlear implantation in patients with single-sided deafness. Egypt. J. Otolaryngol. 2017, 33, 61–66. [Google Scholar] [CrossRef]
- Holder, J.T.; O’Connell, B.; Hedley-Williams, A.; Wanna, G. Cochlear implantation for single-sided deafness and tinnitus suppression. Am. J. Otolaryngol.–Head Neck Med. Surg. 2017, 38, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Poncet-Wallet, C.; Mamelle, E.; Godey, B.; Truy, E.; Guevara, N.; Ardoint, M.; Gnansia, D.; Hoen, M.; Saaï, S.; Mosnier, I.; et al. Prospective Multicentric Follow-up Study of Cochlear Implantation in Adults with Single-Sided Deafness: Tinnitus and Audiological Outcomes. Otol. Neurotol. 2020, 41, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Ramos Macías, A.; Falcón-González, J.C.; Manrique Rodríguez, M.; Morera Pérez, C.; García-Ibáñez, L.; Cenjor Español, C.; Coudert-Koall, C.; Killian, M. One-Year Results for Patients with Unilateral Hearing Loss and Accompanying Severe Tinnitus and Hyperacusis Treated with a Cochlear Implant. Audiol. Neurotol. 2018, 23, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.G.; Tang, Q.; Dimitrijevic, A.; Starr, A.; Larky, J.; Blevins, N.H. Tinnitus suppression by low-rate electric stimulation and its electrophysiological mechanisms. Hear. Res. 2011, 277, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Kleine Punte, A.; De Ridder, D.; Van De Heyning, P. On the necessity of full length electrical cochlear stimulation to suppress severe tinnitus in single-sided deafness. Hear. Res. 2013, 295, 24–29. [Google Scholar] [CrossRef]
- Távora-Vieira, D.; Marino, R.; Krishnaswamy, J.; Kuthbutheen, J.; Rajan, G.P. Cochlear implantation for unilateral deafness with and without tinnitus: A case series. Laryngoscope 2013, 123, 1251–1255. [Google Scholar] [CrossRef]
- Távora-Vieira, D.; Marino, R.; Acharya, A.; Rajan, G.P. The impact of cochlear implantation on speech understanding, subjective hearing performance, and tinnitus perception in patients with unilateral severe to profound hearing loss. Otol. Neurotol. 2015, 36, 430–436. [Google Scholar] [CrossRef]
- Friedmann, D.R.; Ahmed, O.H.; McMenomey, S.O.; Shapiro, W.H.; Waltzman, S.B.; Thomas Roland, J. Single-sided deafness cochlear implantation: Candidacy, evaluation, and outcomes in children and adults. Otol. Neurotol. 2016, 37, e154–e160. [Google Scholar] [CrossRef]
- Häußler, S.M.; Knopke, S.; Dudka, S.; Gräbel, S.; Ketterer, M.C.; Battmer, R.D.; Ernst, A.; Olze, H. Improvement in tinnitus distress, health-related quality of life and psychological comorbidities by cochlear implantation in single-sided deaf patients. HNO 2020, 68, 1–10. [Google Scholar] [CrossRef]
- Buechner, A.; Brendel, M.; Lesinski-Schiedat, A.; Wenzel, G.; Frohne-Buechner, C.; Jaeger, B.; Lenarz, T. Cochlear implantation in unilateral deaf subjects associated with ipsilateral tinnitus. Otol. Neurotol. 2010, 31, 1381–1385. [Google Scholar] [CrossRef] [PubMed]
- Sladen, D.P.; Frisch, C.D.; Carlson, M.L.; Driscoll, C.L.W.; Torres, J.H.; Zeitler, D.M. Cochlear implantation for single-sided deafness: A multicenter study. Laryngoscope 2017, 127, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Finke, M.; Bönitz, H.; Lyxell, B.; Illg, A. Cochlear implant effectiveness in postlingual single-sided deaf individuals: What’s the point? Int. J. Audiol. 2017, 56, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, C.B.; Al-Qurayshi, Z.; Zhu, V.; Liu, A.; Dunn, C.; Gantz, B.J.; Hansen, M.R. Long-term audiologic outcomes after cochlear implantation for single-sided deafness. Laryngoscope 2020, 130, 1805–1811. [Google Scholar] [CrossRef]
- Gartrell, B.C.; Jones, H.G.; Kan, A.; Buhr-Lawler, M.; Gubbels, S.P.; Litovsky, R.Y. Investigating Long-Term Effects of Cochlear Implantation in Single-Sided Deafness. Otol. Neurotol. 2014, 35, 1525–1532. [Google Scholar] [CrossRef]
- Ramos Macías, A.; Falcón González, J.C.; Manrique, M.; Morera, C.; García-Ibáñez, L.; Cenjor, C.; Coudert-Koall, C.; Killian, M. Cochlear implants as a treatment option for unilateral hearing loss, severe tinnitus and hyperacusis. Audiol. Neurotol. 2015, 20, 60–66. [Google Scholar] [CrossRef]
- Tavora-Vieira, D.; De Ceulaer, G.; Govaerts, P.J.; Rajan, G.P. Cochlear Implantation Improves Localization Ability in Patients with Unilateral Deafness. Ear Hear. 2015, 36, e93–e98. [Google Scholar] [CrossRef]
- Härkönen, K.; Kivekäs, I.; Rautiainen, M.; Kotti, V.; Sivonen, V.; Vasama, J.P. Single-Sided Deafness: The Effect of Cochlear Implantation on Quality of Life, Quality of Hearing, and Working Performance. Orl 2015, 77, 339–345. [Google Scholar] [CrossRef]
- Kitoh, R.; Moteki, H.; Nishio, S.; Shinden, S.; Kanzaki, S.; Iwasaki, S.; Ogawa, K.; Usami, S.I. The effects of cochlear implantation in Japanese single-sided deafness patients: Five case reports. Acta Otolaryngol. 2016, 136, 460–464. [Google Scholar] [CrossRef]
- Dorbeau, C.; Galvin, J.; Fu, Q.J.; Legris, E.; Marx, M.; Bakhos, D. Binaural Perception in Single-Sided Deaf Cochlear Implant Users with Unrestricted or Restricted Acoustic Hearing in the Non-Implanted Ear. Audiol. Neurotol. 2018, 23, 187–197. [Google Scholar] [CrossRef]
- Peters, J.P.M.; van Heteren, J.A.A.; Wendrich, A.W.; van Zanten, G.A.; Grolman, W.; Stokroos, R.J.; Smit, A.L. Short-term outcomes of cochlear implantation for single-sided deafness compared to bone conduction devices and contralateral routing of sound hearing aids—Results of a Randomised controlled trial (CINGLE-trial). PLoS ONE 2021, 16, e0257447. [Google Scholar] [CrossRef] [PubMed]
| Study | Patients’ Criteria | n | Evaluation | Interval Studied | Results | Conclusion |
|---|---|---|---|---|---|---|
| Ahmed et al. [63] | CI in SSD and disabling tinnitus | 13 | Questionnaires
| 3 months |
|
|
| Arts et al. [62] | CI in SSD and tinnitus (Intracochlear electrical stimulation vs. standard clinical CI). | 10 | Tests
Questionnaires
| 3 months |
|
|
| Holder et al. [64] | CI in SSD and tinnitus | 12 | Tests
Questionnaires
| 12 months |
|
|
| Kleinjung et al. [60] | CI in SSD and severe tinnitus refractory to treatment | 1 | Questionnaires
| 3 months |
|
|
| Macias et al. [66] | CI in SDD and severe tinnitus | 16 | Questionnaires
| 12 months |
|
|
| Mertens et al. [55] | CI in SSD and disabling tinnitus | 23 | Questionnaires
| 36 months |
|
|
| Mertens et al. [56] | CI in SSD and disabling tinnitus | 11 | Questionnaires
| 3 months |
|
|
| Poncet-Wallet et al. [65] | CI in SSD and disabling tinnitus | 26 | Tests
Questionnaires
| 13 months |
|
|
| Punte et al. [59] | CI in SSD and severe tinnitus | 26 | Tests
Questionnaires
| 6 months |
|
|
| Punte et al. [68] | CI in SSD and severe tinnitus | 7 | Tests
Questionnaires
| 6 months |
|
|
| Ramos et al. [61] | CI in SSD and disabling tinnitus refractory to prior treatment | 6 | Tests
Questionnaires
| 3 months |
|
|
| Song et al. [58] | CI in SSD and intractable tinnitus | 9 | Tests
Questionnaires
| 6 months |
|
|
| Van de Heyning et al. [54] | CI in SSD and severe intractable tinnitus unresponsive to treatment | 22 | Questionnaires
| 24 months |
|
|
| Zeng et al. [67] | CI in SSD and debilitating tinnitus refractory to treatment | 1 | Tests
Questionnaires
| 720 s |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idriss, S.A.; Reynard, P.; Marx, M.; Mainguy, A.; Joly, C.-A.; Ionescu, E.C.; Assouly, K.K.S.; Thai-Van, H. Short- and Long-Term Effect of Cochlear Implantation on Disabling Tinnitus in Single-Sided Deafness Patients: A Systematic Review. J. Clin. Med. 2022, 11, 5664. https://doi.org/10.3390/jcm11195664
Idriss SA, Reynard P, Marx M, Mainguy A, Joly C-A, Ionescu EC, Assouly KKS, Thai-Van H. Short- and Long-Term Effect of Cochlear Implantation on Disabling Tinnitus in Single-Sided Deafness Patients: A Systematic Review. Journal of Clinical Medicine. 2022; 11(19):5664. https://doi.org/10.3390/jcm11195664
Chicago/Turabian StyleIdriss, Samar A., Pierre Reynard, Mathieu Marx, Albane Mainguy, Charles-Alexandre Joly, Eugen Constant Ionescu, Kelly K. S. Assouly, and Hung Thai-Van. 2022. "Short- and Long-Term Effect of Cochlear Implantation on Disabling Tinnitus in Single-Sided Deafness Patients: A Systematic Review" Journal of Clinical Medicine 11, no. 19: 5664. https://doi.org/10.3390/jcm11195664






