Pleiotropic Effects of Vitamin D in Patients with Inflammatory Bowel Diseases
Abstract
1. Introduction
2. Vitamin D
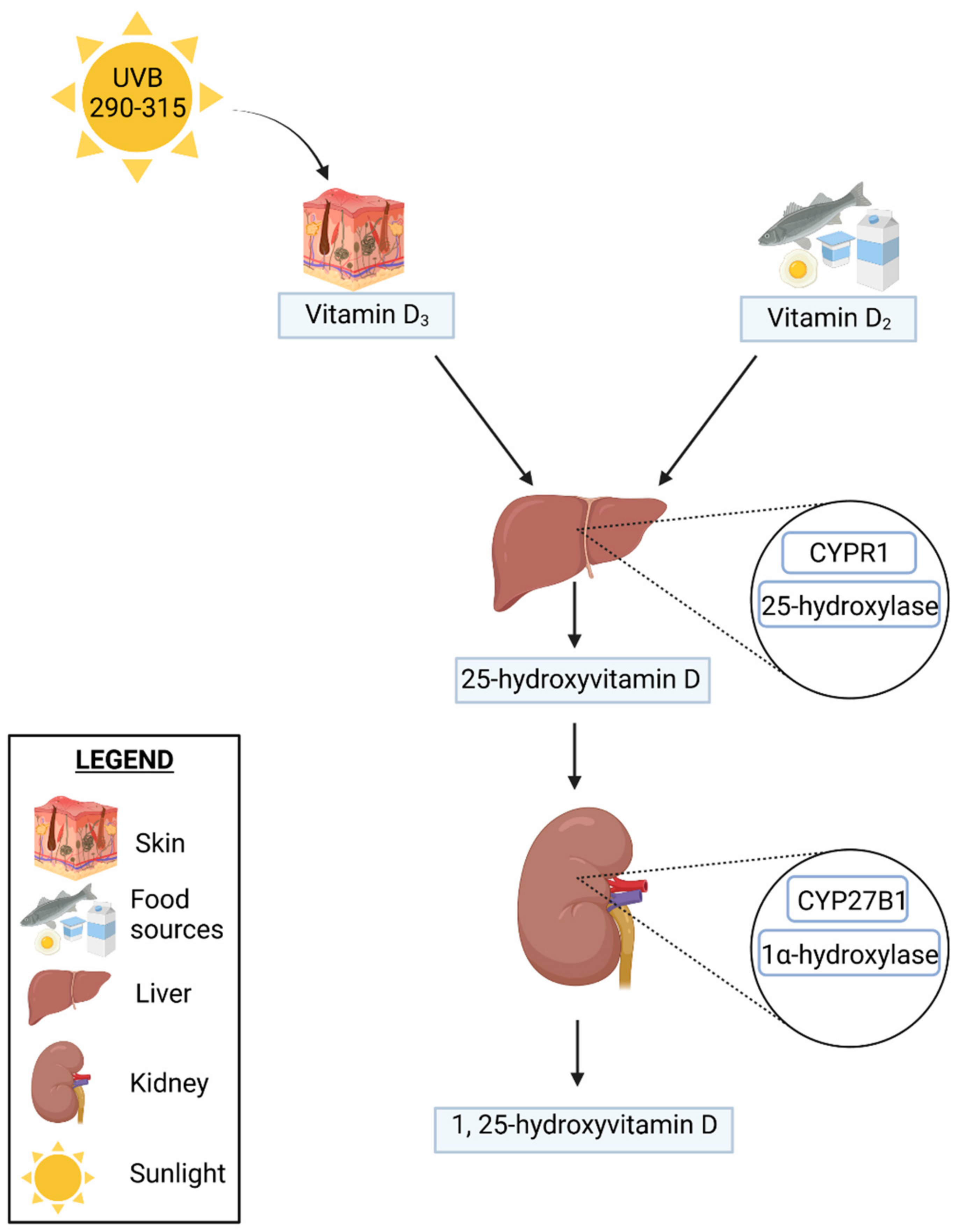
2.1. Vitamin D Metabolism
2.2. Natural Sources of Vitamin D
2.2.1. Food Sources of Vitamin D
2.2.2. Environmental Sources of Vitamin D
3. Inflammatory Bowel Diseases
Vitamin D Deficiency in the General Population and in IBD Patients
4. Genetic Determinants of Vitamin D Activity in IBDs
Vitamin D Receptor and Vitamin D Binding Protein
5. Vitamin D and the Immune System in IBD Patients
Proinflammatory Cytokines
6. Vitamin D, Microbiome, and IBD
7. Vitamin D and Osteoporosis in Patients with IBD
8. Vitamin D and Supplementation in Patients with IBD
9. Summary and Conclusions
| Topic | The Most Significant Information | References |
|---|---|---|
| Vitamin D deficiency | One billion people suffer from vitamin D deficiency worldwide | [43] |
| Vitamin D deficiency in IBD: 22–70% in Crohn’s disease 45% in ulcerative colitis | [48,49,50] | |
| Vitamin D, the risk and course of inflammatory bowel disease | Higher predicted plasma 25(OH)D concentrations significantly reduced the risk of CD and non-significantly reduced the risk of UC in women | [113] |
| 25(OH)D levels were inversely correlated with disease activity, and patients supplementing vitamin D presented a lower Crohn’s disease activity index and C-reactive protein as compared with patients with no supplementation | [159] | |
| Treatment with TNF-α and IL-6 resulted in a decreased expression of the vitamin D activating enzyme CYP27B1 | [115] | |
| Genetic factors, vitamin D, and inflammatory bowel disease | 49 IBD risk genes regulated by vitamin D signaling, 24 of which were reported to be upregulated, and 25 downregulated | [71] |
| Single-nucleotide polymorphisms in the human VDR (vitamin D receptor) gene were reported to be associated with an elevated susceptibility to IBD | [77,78] | |
| Vitamin D receptor and vitamin D-metabolizing hydroxylases are expressed in various immune cells, hence, the impact of vitamin D on both innate and acquired immunity | [85] | |
| A decreased 1,25 (OH)2D production or VDR expression may lead to intestinal inflammation and increased colonization by Proteobacteria | [129] | |
| Gut microbiota and vitamin D | Chlamydia trachomatis infection affects the gut microbiota and causes a decrease in VDR activity | [129] |
| Supplementation with the probiotic Lactobacillus reuteri increased serum 25(OH)D levels | [130] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fletcher, J.; Cooper, S.C.; Ghosh, S.; Hewison, M. The Role of Vitamin D in Inflammatory Bowel Disease: Mechanism to Management. Nutrients 2019, 11, 1019. [Google Scholar] [CrossRef]
- Nielsen, O.H.; Rejnmark, L.; Moss, A.C. Role of Vitamin D in the Natural History of Inflammatory Bowel Disease. J. Crohn’s Colitis 2018, 12, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.B.; Kim, H.W.; Lee, Y.S.; Yoon, S.M. Inflammatory Bowel Disease and Vitamin D. Korean J. Gastroenterol. 2020, 76, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, E.; Jørgensen, T.N. Immunological Effects of Vitamin D and Their Relations to Autoimmunity. J. Autoimmun. 2019, 100, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, O.H.; Hansen, T.I.; Gubatan, J.M.; Jensen, K.B.; Rejnmark, L. Managing Vitamin D Deficiency in Inflammatory Bowel Disease. Frontline Gastroenterol. 2019, 10, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; MacLaughlin, J.A.; Clark, M.B.; Holick, S.A.; Potts, J.T.; Anderson, R.R.; Blank, I.H.; Parrish, J.A.; Elias, P. Photosynthesis of Previtamin D3 in Human Skin and the Physiologic Consequences. Science 1980, 210, 203–205. [Google Scholar] [CrossRef]
- Płudowski, P.; Karczmarewicz, E.; Bayer, M.; Carter, G.; Chlebna-Sokół, D.; Czech-Kowalska, J.; Dębski, R.; Decsi, T.; Dobrzańska, A.; Franek, E.; et al. Practical Guidelines for the Supplementation of Vitamin D and the Treatment of Deficits in Central Europe—Recommended Vitamin D Intakes in the General Population and Groups at Risk of Vitamin D Deficiency. Endokrynol. Pol. 2013, 64, 319–327. [Google Scholar] [CrossRef]
- Yu, O.B.; Arnold, L.A. Calcitroic Acid—A Review. ACS Chem. Biol. 2016, 11, 2665–2672. [Google Scholar] [CrossRef]
- Cross, H.S.; Nittke, T.; Kallay, E. Colonic Vitamin D Metabolism: Implications for the Pathogenesis of Inflammatory Bowel Disease and Colorectal Cancer. Mol. Cell. Endocrinol. 2011, 347, 70–79. [Google Scholar] [CrossRef]
- Chang, S.-W.; Lee, H.-C. Vitamin D and Health—The Missing Vitamin in Humans. Pediatr. Neonatol. 2019, 60, 237–244. [Google Scholar] [CrossRef]
- Cardwell, G.; Bornman, J.F.; James, A.P.; Black, L.J. A Review of Mushrooms as a Potential Source of Dietary Vitamin D. Nutrients 2018, 10, 1498. [Google Scholar] [CrossRef]
- Bito, T.; Okumura, E.; Fujishima, M.; Watanabe, F. Potential of Chlorella as a Dietary Supplement to Promote Human Health. Nutrients 2020, 12, 2524. [Google Scholar] [CrossRef]
- Kühn, J.; Schröter, A.; Hartmann, B.M.; Stangl, G.I. Cocoa and Chocolate Are Sources of Vitamin D2. Food Chem. 2018, 269, 318–320. [Google Scholar] [CrossRef]
- Rogerson, D. Vegan Diets: Practical Advice for Athletes and Exercisers. J. Int. Soc. Sports Nutr. 2017, 14, 36. [Google Scholar] [CrossRef]
- Cashman, K.D. Vitamin D Deficiency: Defining, Prevalence, Causes, and Strategies of Addressing. Calcif. Tissue Int. 2020, 106, 14–29. [Google Scholar] [CrossRef]
- O’Mahony, L.; Stepien, M.; Gibney, M.J.; Nugent, A.P.; Brennan, L. The Potential Role of Vitamin D Enhanced Foods in Improving Vitamin D Status. Nutrients 2011, 3, 1023–1041. [Google Scholar] [CrossRef]
- Gruber, B.M. The Phenomenon of Vitamin D. Postępy Hig. Med. Dośw. 2015, 69, 127–139. [Google Scholar]
- Nair, R.; Maseeh, A. Vitamin D: The “Sunshine” Vitamin. J. Pharmacol. Pharmacother. 2012, 3, 118–126. [Google Scholar] [CrossRef]
- Mostafa, W.Z.; Hegazy, R.A. Vitamin D and the Skin: Focus on a Complex Relationship: A Review. J. Adv. Res. 2015, 6, 793–804. [Google Scholar] [CrossRef]
- Saraff, V.; Shaw, N. Sunshine and Vitamin D. Arch. Dis. Child. 2016, 101, 190–192. [Google Scholar] [CrossRef]
- Wacker, M.; Holick, M.F. Sunlight and Vitamin D. Dermatoendocrinology 2013, 5, 51–108. [Google Scholar] [CrossRef] [PubMed]
- Terushkin, V.; Bender, A.; Psaty, E.L.; Engelsen, O.; Wang, S.Q.; Halpern, A.C. Estimated Equivalency of Vitamin D Production from Natural Sun Exposure versus Oral Vitamin D Supplementation across Seasons at Two US Latitudes. J. Am. Acad. Dermatol. 2010, 62, 929.e1–929.e9. [Google Scholar] [CrossRef] [PubMed]
- Osmancevic, A.; Sandström, K.; Gillstedt, M.; Landin-Wilhelmsen, K.; Larkö, O.; Wennberg Larkö, A.-M.; Holick, M.F.; Krogstad, A.-L. Vitamin D Production after UVB Exposure—A Comparison of Exposed Skin Regions. J. Photochem. Photobiol. B Biol. 2015, 143, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Bogh, M.K.B.; Schmedes, A.V.; Philipsen, P.A.; Thieden, E.; Wulf, H.C. Interdependence between Body Surface Area and Ultraviolet B Dose in Vitamin D Production: A Randomized Controlled Trial. Br. J. Dermatol. 2011, 164, 163–169. [Google Scholar] [CrossRef]
- Passeron, T.; Bouillon, R.; Callender, V.; Cestari, T.; Diepgen, T.L.; Green, A.C.; van der Pols, J.C.; Bernard, B.A.; Ly, F.; Bernerd, F.; et al. Sunscreen Photoprotection and Vitamin D Status. Br. J. Dermatol. 2019, 181, 916–931. [Google Scholar] [CrossRef]
- Gallagher, J.C. Vitamin D and Aging. Endocrinol. Metab. Clin. N. Am. 2013, 42, 319–332. [Google Scholar] [CrossRef]
- Touvier, M.; Deschasaux, M.; Montourcy, M.; Sutton, A.; Charnaux, N.; Kesse-Guyot, E.; Assmann, K.E.; Fezeu, L.; Latino-Martel, P.; Druesne-Pecollo, N.; et al. Determinants of Vitamin D Status in Caucasian Adults: Influence of Sun Exposure, Dietary Intake, Sociodemographic, Lifestyle, Anthropometric, and Genetic Factors. J. Investig. Dermatol. 2015, 135, 378–388. [Google Scholar] [CrossRef]
- Podolsky, D.K. Inflammatory Bowel Disease. N. Engl. J. Med. 2002, 347, 417–429. [Google Scholar] [CrossRef]
- Ordás, I.; Eckmann, L.; Talamini, M.; Baumgart, D.C.; Sandborn, W.J. Ulcerative Colitis. Lancet 2012, 380, 1606–1619. [Google Scholar] [CrossRef]
- McDowell, C.; Farooq, U.; Haseeb, M. Inflammatory Bowel Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing Incidence and Prevalence of the Inflammatory Bowel Diseases with Time, Based on Systematic Review. Gastroenterology 2012, 142, 46–54. [Google Scholar] [CrossRef]
- M’Koma, A.E. Inflammatory Bowel Disease: An Expanding Global Health Problem. Clin. Med. Insights Gastroenterol. 2013, 6, 33–47. [Google Scholar] [CrossRef]
- Ye, Y.; Pang, Z.; Chen, W.; Ju, S.; Zhou, C. The Epidemiology and Risk Factors of Inflammatory Bowel Disease. Int. J. Clin. Exp. Med. 2015, 8, 22529–22542. [Google Scholar]
- Burisch, J.; Munkholm, P. The Epidemiology of Inflammatory Bowel Disease. Scand. J. Gastroenterol. 2015, 50, 942–951. [Google Scholar] [CrossRef]
- Alatab, S.; Sepanlou, S.G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Sadeghi, A.; Nixon, M.R.; Abdoli, A.; Abolhassani, H.; et al. The Global, Regional, and National Burden of Inflammatory Bowel Disease in 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef]
- Hamilton, B.; Green, H.; Heerasing, N.; Hendy, P.; Moore, L.; Chanchlani, N.; Walker, G.; Bewshea, C.; Kennedy, N.A.; Ahmad, T.; et al. Incidence and Prevalence of Inflammatory Bowel Disease in Devon, UK. Frontline Gastroenterol. 2020, 12, 461–470. [Google Scholar] [CrossRef]
- Burisch, J.; Pedersen, N.; Čuković-Čavka, S.; Brinar, M.; Kaimakliotis, I.; Duricova, D.; Shonová, O.; Vind, I.; Avnstrøm, S.; Thorsgaard, N.; et al. East-West Gradient in the Incidence of Inflammatory Bowel Disease in Europe: The ECCO-EpiCom Inception Cohort. Gut 2014, 63, 588–597. [Google Scholar] [CrossRef]
- Lovasz, B.D.; Golovics, P.A.; Vegh, Z.; Lakatos, P.L. New Trends in Inflammatory Bowel Disease Epidemiology and Disease Course in Eastern Europe. Dig. Liver Dis. 2013, 45, 269–276. [Google Scholar] [CrossRef]
- Loftus, E.V. Clinical Epidemiology of Inflammatory Bowel Disease: Incidence, Prevalence, and Environmental Influences. Gastroenterology 2004, 126, 1504–1517. [Google Scholar] [CrossRef]
- Shikhare, G.; Kugathasan, S. Inflammatory Bowel Disease in Children: Current Trends. J. Gastroenterol. 2010, 45, 673–682. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N. Environmental Risk Factors for Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2013, 9, 367–374. [Google Scholar] [CrossRef]
- Guan, Q. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J. Immunol. Res. 2019, 2019, 7247238. [Google Scholar] [CrossRef]
- Holick, M.F.; Chen, T.C. Vitamin D Deficiency: A Worldwide Problem with Health Consequences. Am. J. Clin. Nutr. 2008, 87, 1080S–1086S. [Google Scholar] [CrossRef]
- Hilger, J.; Friedel, A.; Herr, R.; Rausch, T.; Roos, F.; Wahl, D.A.; Pierroz, D.D.; Weber, P.; Hoffmann, K. A Systematic Review of Vitamin D Status in Populations Worldwide. Br. J. Nutr. 2014, 111, 23–45. [Google Scholar] [CrossRef]
- Holick, M.F. High Prevalence of Vitamin D Inadequacy and Implications for Health. Mayo Clin. Proc. 2006, 81, 353–373. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Bruyère, O.; Malaise, O.; Neuprez, A.; Collette, J.; Reginster, J.-Y. Prevalence of Vitamin D Inadequacy in European Postmenopausal Women. Curr. Med. Res. Opin. 2007, 23, 1939–1944. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.T.; Weaver, C.T. Linking Vitamin D Deficiency to Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2013, 19, 2245–2256. [Google Scholar] [CrossRef]
- Siffledeen, J.S.; Siminoski, K.; Steinhart, H.; Greenberg, G.; Fedorak, R.N. The Frequency of Vitamin D Deficiency in Adults with Crohn’s Disease. Can. J. Gastroenterol. 2003, 17, 473–478. [Google Scholar] [CrossRef]
- Jahnsen, J.; Falch, J.A.; Mowinckel, P.; Aadland, E. Vitamin D Status, Parathyroid Hormone and Bone Mineral Density in Patients with Inflammatory Bowel Disease. Scand. J. Gastroenterol. 2002, 37, 192–199. [Google Scholar] [CrossRef]
- Driscoll, R.H.; Meredith, S.C.; Sitrin, M.; Rosenberg, I.H. Vitamin D Deficiency and Bone Disease in Patients with Crohn’s Disease. Gastroenterology 1982, 83, 1252–1258. [Google Scholar] [CrossRef]
- Ratajczak, A.E.; Rychter, A.M.; Zawada, A.; Dobrowolska, A.; Krela-Kaźmierczak, I. Nutrients in the Prevention of Osteoporosis in Patients with Inflammatory Bowel Diseases. Nutrients 2020, 12, 1702. [Google Scholar] [CrossRef]
- Frigstad, S.O.; Høivik, M.; Jahnsen, J.; Dahl, S.R.; Cvancarova, M.; Grimstad, T.; Berset, I.P.; Huppertz-Hauss, G.; Hovde, Ø.; Torp, R.; et al. Vitamin D Deficiency in Inflammatory Bowel Disease: Prevalence and Predictors in a Norwegian Outpatient Population. Scand. J. Gastroenterol. 2017, 52, 100–106. [Google Scholar] [CrossRef]
- Li, X.-X.; Liu, Y.; Luo, J.; Huang, Z.-D.; Zhang, C.; Fu, Y. Vitamin D Deficiency Associated with Crohn’s Disease and Ulcerative Colitis: A Meta-Analysis of 55 Observational Studies. J. Transl. Med. 2019, 17, 323. [Google Scholar] [CrossRef]
- Kojecký, V.; Matouš, J.; Zádorová, Z.; Gřiva, M.; Kianička, B.; Uher, M. Vitamin D Supplementation Dose Needs to Be Higher in Patients with Inflammatory Bowel Disease: Interventional Study. Vnitr. Lek. 2019, 65, 470–474. [Google Scholar] [CrossRef]
- Bours, P.H.A.; Wielders, J.P.M.; Vermeijden, J.R.; van de Wiel, A. Seasonal Variation of Serum 25-Hydroxyvitamin D Levels in Adult Patients with Inflammatory Bowel Disease. Osteoporos. Int. 2011, 22, 2857–2867. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Y.; Gu, Q.; Du, Z.; Chen, W. The Association between Serum Vitamin D and Inflammatory Bowel Disease. Medicine 2019, 98, e15233. [Google Scholar] [CrossRef]
- Ulitsky, A.; Ananthakrishnan, A.N.; Naik, A.; Skaros, S.; Zadvornova, Y.; Binion, D.G.; Issa, M. Vitamin D Deficiency in Patients with Inflammatory Bowel Disease: Association with Disease Activity and Quality of Life. JPEN J. Parenter. Enter. Nutr. 2011, 35, 308–316. [Google Scholar] [CrossRef]
- Burrelli Scotti, G.; Afferri, M.T.; De Carolis, A.; Vaiarello, V.; Fassino, V.; Ferrone, F.; Minisola, S.; Nieddu, L.; Vernia, P. Factors Affecting Vitamin D Deficiency in Active Inflammatory Bowel Diseases. Dig. Liver Dis. 2019, 51, 657–662. [Google Scholar] [CrossRef]
- Eloranta, J.J.; Wenger, C.; Mwinyi, J.; Hiller, C.; Gubler, C.; Vavricka, S.R.; Fried, M.; Kullak-Ublick, G.A.; Swiss IBD Cohort Study Group. Association of a Common Vitamin D-Binding Protein Polymorphism with Inflammatory Bowel Disease. Pharm. Genom. 2011, 21, 559–564. [Google Scholar] [CrossRef]
- Fabisiak, N.; Fabisiak, A.; Watala, C.; Fichna, J. Fat-Soluble Vitamin Deficiencies and Inflammatory Bowel Disease: Systematic Review and Meta-Analysis. J. Clin. Gastroenterol. 2017, 51, 878–889. [Google Scholar] [CrossRef]
- Caviezel, D.; Maissen, S.; Niess, J.H.; Kiss, C.; Hruz, P. High Prevalence of Vitamin D Deficiency among Patients with Inflammatory Bowel Disease. Inflamm. Intest. Dis. 2018, 2, 200–210. [Google Scholar] [CrossRef]
- Sadeghian, M.; Saneei, P.; Siassi, F.; Esmaillzadeh, A. Vitamin D Status in Relation to Crohn’s Disease: Meta-Analysis of Observational Studies. Nutrition 2016, 32, 505–514. [Google Scholar] [CrossRef]
- Meyer, M.B.; Goetsch, P.D.; Pike, J.W. VDR/RXR and TCF4/β-Catenin Cistromes in Colonic Cells of Colorectal Tumor Origin: Impact on c-FOS and c-MYC Gene Expression. Mol. Endocrinol. 2012, 26, 37–51. [Google Scholar] [CrossRef]
- Basson, A. Vitamin D and Crohn’s Disease in the Adult Patient: A Review. JPEN J. Parenter. Enter. Nutr. 2014, 38, 438–458. [Google Scholar] [CrossRef]
- Singh, P.K.; van den Berg, P.R.; Long, M.D.; Vreugdenhil, A.; Grieshober, L.; Ochs-Balcom, H.M.; Wang, J.; Delcambre, S.; Heikkinen, S.; Carlberg, C.; et al. Integration of VDR Genome Wide Binding and GWAS Genetic Variation Data Reveals Co-Occurrence of VDR and NF-ΚB Binding That Is Linked to Immune Phenotypes. BMC Genom. 2017, 18, 132. [Google Scholar] [CrossRef]
- Hii, C.S.; Ferrante, A. The Non-Genomic Actions of Vitamin D. Nutrients 2016, 8, 135. [Google Scholar] [CrossRef]
- Wang, T.-T.; Dabbas, B.; Laperriere, D.; Bitton, A.J.; Soualhine, H.; Tavera-Mendoza, L.E.; Dionne, S.; Servant, M.J.; Bitton, A.; Seidman, E.G.; et al. Direct and Indirect Induction by 1,25-Dihydroxyvitamin D3 of the NOD2/CARD15-Defensin Β2 Innate Immune Pathway Defective in Crohn Disease. J. Biol. Chem. 2010, 285, 2227–2231. [Google Scholar] [CrossRef]
- Alleyne, D.; Witonsky, D.B.; Mapes, B.; Nakagome, S.; Sommars, M.; Hong, E.; Muckala, K.A.; Di Rienzo, A.; Kupfer, S.S. Colonic Transcriptional Response to 1α,25(OH)2 Vitamin D3 in African- and European-Americans. J. Steroid Biochem. Mol. Biol. 2017, 168, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Barral, A.; Costales-Carrera, A.; Buira, S.P.; Jung, P.; Ferrer-Mayorga, G.; Larriba, M.J.; Bustamante-Madrid, P.; Domínguez, O.; Real, F.X.; Guerra-Pastrián, L.; et al. Vitamin D Differentially Regulates Colon Stem Cells in Patient-Derived Normal and Tumor Organoids. FEBS J. 2020, 287, 53–72. [Google Scholar] [CrossRef] [PubMed]
- McGovern, D.P.B.; Gardet, A.; Törkvist, L.; Goyette, P.; Essers, J.; Taylor, K.D.; Neale, B.M.; Ong, R.T.H.; Lagacé, C.; Li, C.; et al. Genome-Wide Association Identifies Multiple Ulcerative Colitis Susceptibility Loci. Nat. Genet. 2010, 42, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Battistini, C.; Ballan, R.; Herkenhoff, M.E.; Saad, S.M.I.; Sun, J. Vitamin D Modulates Intestinal Microbiota in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2020, 22, 362. [Google Scholar] [CrossRef]
- Kellermann, L.; Jensen, K.B.; Bergenheim, F.; Gubatan, J.; Chou, N.D.; Moss, A.; Nielsen, O.H. Mucosal Vitamin D Signaling in Inflammatory Bowel Disease. Autoimmun. Rev. 2020, 19, 102672. [Google Scholar] [CrossRef]
- Liu, W.; Chen, Y.; Golan, M.A.; Annunziata, M.L.; Du, J.; Dougherty, U.; Kong, J.; Musch, M.; Huang, Y.; Pekow, J.; et al. Intestinal Epithelial Vitamin D Receptor Signaling Inhibits Experimental Colitis. J. Clin. Investig. 2013, 123, 3983–3996. [Google Scholar] [CrossRef]
- Leyssens, C.; Verlinden, L.; De Hertogh, G.; Kato, S.; Gysemans, C.; Mathieu, C.; Carmeliet, G.; Verstuyf, A. Impact on Experimental Colitis of Vitamin D Receptor Deletion in Intestinal Epithelial or Myeloid Cells. Endocrinology 2017, 158, 2354–2366. [Google Scholar] [CrossRef]
- Gallone, G.; Haerty, W.; Disanto, G.; Ramagopalan, S.V.; Ponting, C.P.; Berlanga-Taylor, A.J. Identification of Genetic Variants Affecting Vitamin D Receptor Binding and Associations with Autoimmune Disease. Hum. Mol. Genet. 2017, 26, 2164–2176. [Google Scholar] [CrossRef]
- Nobile, S.; Tenace, M.A.; Pappa, H.M. The Role of Vitamin D in the Pathogenesis of Inflammatory Bowel Disease. Gastrointest. Disord. 2019, 1, 231–240. [Google Scholar] [CrossRef]
- Bakke, D.; Sun, J. Ancient Nuclear Receptor VDR with New Functions: Microbiome and Inflammation. Inflamm. Bowel Dis. 2018, 24, 1149–1154. [Google Scholar] [CrossRef]
- Uitterlinden, A.G.; Fang, Y.; Van Meurs, J.B.J.; Pols, H.A.P.; Van Leeuwen, J.P.T.M. Genetics and Biology of Vitamin D Receptor Polymorphisms. Gene 2004, 338, 143–156. [Google Scholar] [CrossRef]
- Xue, L.-N.; Xu, K.-Q.; Zhang, W.; Wang, Q.; Wu, J.; Wang, X.-Y. Associations between Vitamin D Receptor Polymorphisms and Susceptibility to Ulcerative Colitis and Crohn’s Disease: A Meta-Analysis. Inflamm. Bowel Dis. 2013, 19, 54–60. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.T.; Hu, J.J.; Fan, R.; Zhou, J.; Zhong, J. Polymorphisms of the Vitamin D Receptor Gene and the Risk of Inflammatory Bowel Disease: A Meta-Analysis. Genet. Mol. Res. 2014, 13, 2598–2610. [Google Scholar] [CrossRef]
- Cho, Y.A.; Lee, J.; Oh, J.H.; Chang, H.J.; Sohn, D.K.; Shin, A.; Kim, J. Vitamin D Receptor FokI Polymorphism and the Risks of Colorectal Cancer, Inflammatory Bowel Disease, and Colorectal Adenoma. Sci. Rep. 2018, 8, 12899. [Google Scholar] [CrossRef]
- Zheng, S.-Z.; Zhang, D.-G.; Wu, H.; Jiang, L.-J.; Jin, J.; Lin, X.-Q.; Ding, R.; Jiang, Y. The Association between Vitamin D Receptor Polymorphisms and Serum 25-Hydroxyvitamin D Levels with Ulcerative Colitis in Chinese Han Population. Clin. Res. Hepatol. Gastroenterol. 2017, 41, 110–117. [Google Scholar] [CrossRef]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- Provvedini, D.M.; Tsoukas, C.D.; Deftos, L.J.; Manolagas, S.C. 1,25-Dihydroxyvitamin D3 Receptors in Human Leukocytes. Science 1983, 221, 1181–1183. [Google Scholar] [CrossRef]
- Matricon, J.; Barnich, N.; Ardid, D. Immunopathogenesis of Inflammatory Bowel Disease. Self Nonself 2010, 1, 299–309. [Google Scholar] [CrossRef]
- Gálvez, J. Role of Th17 Cells in the Pathogenesis of Human IBD. ISRN Inflamm. 2014, 2014, 928461. [Google Scholar] [CrossRef]
- Cantorna, M.T.; Snyder, L.; Lin, Y.-D.; Yang, L. Vitamin D and 1,25(OH)2D Regulation of T Cells. Nutrients 2015, 7, 3011–3021. [Google Scholar] [CrossRef]
- Cantorna, M.T. Mechanisms Underlying the Effect of Vitamin D on the Immune System. Proc. Nutr. Soc. 2010, 69, 286–289. [Google Scholar] [CrossRef]
- Meckel, K.; Li, Y.C.; Lim, J.; Kocherginsky, M.; Weber, C.; Almoghrabi, A.; Chen, X.; Kaboff, A.; Sadiq, F.; Hanauer, S.B.; et al. Serum 25-Hydroxyvitamin D Concentration Is Inversely Associated with Mucosal Inflammation in Patients with Ulcerative Colitis. Am. J. Clin. Nutr. 2016, 104, 113–120. [Google Scholar] [CrossRef]
- Bora, S.; Cantorna, M.T. The Role of UVR and Vitamin D on T Cells and Inflammatory Bowel Disease. Photochem. Photobiol. Sci. 2017, 16, 347–353. [Google Scholar] [CrossRef]
- Schardey, J.; Globig, A.-M.; Janssen, C.; Hofmann, M.; Manegold, P.; Thimme, R.; Hasselblatt, P. Vitamin D Inhibits Pro-Inflammatory T Cell Function in Patients With Inflammatory Bowel Disease. J. Crohn’s Colitis 2019, 13, 1546–1557. [Google Scholar] [CrossRef] [PubMed]
- Alhassan Mohammed, H.; Mirshafiey, A.; Vahedi, H.; Hemmasi, G.; Moussavi Nasl Khameneh, A.; Parastouei, K.; Saboor-Yaraghi, A.A. Immunoregulation of Inflammatory and Inhibitory Cytokines by Vitamin D3 in Patients with Inflammatory Bowel Diseases. Scand. J. Immunol. 2017, 85, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Mocanu, V.; Oboroceanu, T.; Zugun-Eloae, F. Current Status in Vitamin D and Regulatory T Cells—Immunological Implications. Rev. Med. Chir. Soc. Med. Nat. Iasi. 2013, 117, 965–973. [Google Scholar] [PubMed]
- Chen, S.; Sims, G.P.; Chen, X.X.; Gu, Y.Y.; Chen, S.; Lipsky, P.E. Modulatory Effects of 1,25-Dihydroxyvitamin D3 on Human B Cell Differentiation. J. Immunol. 2007, 179, 1634–1647. [Google Scholar] [CrossRef] [PubMed]
- Lisowska, K.A.; Bryl, E. The Role of Vitamin D in the Development of Autoimmune Diseases. Postępy Hig. Med. Dośw. 2017, 71, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Penna, G.; Adorini, L. 1 Alpha,25-Dihydroxyvitamin D3 Inhibits Differentiation, Maturation, Activation, and Survival of Dendritic Cells Leading to Impaired Alloreactive T Cell Activation. J. Immunol. 2000, 164, 2405–2411. [Google Scholar] [CrossRef]
- Moparthi, L.; Koch, S. Wnt Signaling in Intestinal Inflammation. Differentiation 2019, 108, 24–32. [Google Scholar] [CrossRef]
- Larriba, M.J.; González-Sancho, J.M.; Barbáchano, A.; Niell, N.; Ferrer-Mayorga, G.; Muñoz, A. Vitamin D Is a Multilevel Repressor of Wnt/b-Catenin Signaling in Cancer Cells. Cancers 2013, 5, 1242–1260. [Google Scholar] [CrossRef]
- Jo, S.; Yoon, S.; Lee, S.Y.; Kim, S.Y.; Park, H.; Han, J.; Choi, S.H.; Han, J.-S.; Yang, J.-H.; Kim, T.-H. DKK1 Induced by 1,25D3 Is Required for the Mineralization of Osteoblasts. Cells 2020, 9, 236. [Google Scholar] [CrossRef]
- Gubatan, J.; Chou, N.D.; Nielsen, O.H.; Moss, A.C. Systematic Review with Meta-Analysis: Association of Vitamin D Status with Clinical Outcomes in Adult Patients with Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2019, 50, 1146–1158. [Google Scholar] [CrossRef]
- Harries, A.D.; Brown, R.; Heatley, R.V.; Williams, L.A.; Woodhead, S.; Rhodes, J. Vitamin D Status in Crohn’s Disease: Association with Nutrition and Disease Activity. Gut 1985, 26, 1197–1203. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Cagan, A.; Gainer, V.S.; Cai, T.; Cheng, S.-C.; Savova, G.; Chen, P.; Szolovits, P.; Xia, Z.; De Jager, P.L.; et al. Normalization of Plasma 25-Hydroxy Vitamin D Is Associated with Reduced Risk of Surgery in Crohn’s Disease. Inflamm. Bowel Dis. 2013, 19, 1921–1927. [Google Scholar] [CrossRef]
- Cantorna, M.T.; Munsick, C.; Bemiss, C.; Mahon, B.D. 1,25-Dihydroxycholecalciferol Prevents and Ameliorates Symptoms of Experimental Murine Inflammatory Bowel Disease. J. Nutr. 2000, 130, 2648–2652. [Google Scholar] [CrossRef]
- Zhu, Y.; Mahon, B.D.; Froicu, M.; Cantorna, M.T. Calcium and 1 Alpha,25-Dihydroxyvitamin D3 Target the TNF-Alpha Pathway to Suppress Experimental Inflammatory Bowel Disease. Eur. J. Immunol. 2005, 35, 217–224. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Cheng, S.-C.; Cai, T.; Cagan, A.; Gainer, V.S.; Szolovits, P.; Shaw, S.Y.; Churchill, S.; Karlson, E.W.; Murphy, S.N.; et al. Association between Reduced Plasma 25-Hydroxy Vitamin D and Increased Risk of Cancer in Patients with Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2014, 12, 821–827. [Google Scholar] [CrossRef]
- Klampfer, L. Vitamin D and Colon Cancer. World J. Gastrointest. Oncol. 2014, 6, 430–437. [Google Scholar] [CrossRef]
- Padi, S.K.R.; Zhang, Q.; Rustum, Y.M.; Morrison, C.; Guo, B. MicroRNA-627 Mediates the Epigenetic Mechanisms of Vitamin D to Suppress Proliferation of Human Colorectal Cancer Cells and Growth of Xenograft Tumors in Mice. Gastroenterology 2013, 145, 437–446. [Google Scholar] [CrossRef]
- Pálmer, H.G.; González-Sancho, J.M.; Espada, J.; Berciano, M.T.; Puig, I.; Baulida, J.; Quintanilla, M.; Cano, A.; de Herreros, A.G.; Lafarga, M.; et al. Vitamin D(3) Promotes the Differentiation of Colon Carcinoma Cells by the Induction of E-Cadherin and the Inhibition of Beta-Catenin Signaling. J. Cell Biol. 2001, 154, 369–387. [Google Scholar] [CrossRef]
- Raman, M.; Milestone, A.N.; Walters, J.R.F.; Hart, A.L.; Ghosh, S. Vitamin D and Gastrointestinal Diseases: Inflammatory Bowel Disease and Colorectal Cancer. Ther. Adv. Gastroenterol. 2011, 4, 49–62. [Google Scholar] [CrossRef]
- Wang, T.J.; Zhang, F.; Richards, J.B.; Kestenbaum, B.; van Meurs, J.B.; Berry, D.; Kiel, D.P.; Streeten, E.A.; Ohlsson, C.; Koller, D.L.; et al. Common Genetic Determinants of Vitamin D Insufficiency: A Genome-Wide Association Study. Lancet 2010, 376, 180–188. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Cagan, A.; Cai, T.; Gainer, V.S.; Shaw, S.Y.; Churchill, S.; Karlson, E.W.; Murphy, S.N.; Kohane, I.; Liao, K.P.; et al. Common Genetic Variants Influence Circulating Vitamin D Levels in Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2015, 21, 2507–2514. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; Khalili, H.; Higuchi, L.M.; Bao, Y.; Korzenik, J.R.; Giovannucci, E.L.; Richter, J.M.; Fuchs, C.S.; Chan, A.T. Higher Predicted Vitamin D Status Is Associated with Reduced Risk of Crohn’s Disease. Gastroenterology 2012, 142, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Strober, W.; Fuss, I.J. Proinflammatory Cytokines in the Pathogenesis of Inflammatory Bowel Diseases. Gastroenterology 2011, 140, 1756–1767. [Google Scholar] [CrossRef] [PubMed]
- Hummel, D.M.; Fetahu, I.S.; Gröschel, C.; Manhardt, T.; Kállay, E. Role of Proinflammatory Cytokines on Expression of Vitamin D Metabolism and Target Genes in Colon Cancer Cells. J. Steroid Biochem. Mol. Biol. 2014, 144 Pt A, 91–95. [Google Scholar] [CrossRef]
- Du, J.; Chen, Y.; Shi, Y.; Liu, T.; Cao, Y.; Tang, Y.; Ge, X.; Nie, H.; Zheng, C.; Li, Y.C. 1,25-Dihydroxyvitamin D Protects Intestinal Epithelial Barrier by Regulating the Myosin Light Chain Kinase Signaling Pathway. Inflamm. Bowel Dis. 2015, 21, 2495–2506. [Google Scholar] [CrossRef] [PubMed]
- Sapone, A.; de Magistris, L.; Pietzak, M.; Clemente, M.G.; Tripathi, A.; Cucca, F.; Lampis, R.; Kryszak, D.; Cartenì, M.; Generoso, M.; et al. Zonulin Upregulation Is Associated with Increased Gut Permeability in Subjects with Type 1 Diabetes and Their Relatives. Diabetes 2006, 55, 1443–1449. [Google Scholar] [CrossRef]
- Almeida, A.; Mitchell, A.L.; Boland, M.; Forster, S.C.; Gloor, G.B.; Tarkowska, A.; Lawley, T.D.; Finn, R.D. A New Genomic Blueprint of the Human Gut Microbiota. Nature 2019, 568, 499–504. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the Human Gut Microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Adams, J.S.; Ren, S.; Liu, P.T.; Chun, R.F.; Lagishetty, V.; Gombart, A.F.; Borregaard, N.; Modlin, R.L.; Hewison, M. Vitamin D-Directed Rheostatic Regulation of Monocyte Antibacterial Responses. J. Immunol. 2009, 182, 4289–4295. [Google Scholar] [CrossRef]
- Anitha, M.; Vijay-Kumar, M.; Sitaraman, S.V.; Gewirtz, A.T.; Srinivasan, S. Gut Microbial Products Regulate Murine Gastrointestinal Motility via Toll-like Receptor 4 Signaling. Gastroenterology 2012, 143, 1006–1016.e4. [Google Scholar] [CrossRef]
- Lagishetty, V.; Misharin, A.V.; Liu, N.Q.; Lisse, T.S.; Chun, R.F.; Ouyang, Y.; McLachlan, S.M.; Adams, J.S.; Hewison, M. Vitamin D Deficiency in Mice Impairs Colonic Antibacterial Activity and Predisposes to Colitis. Endocrinology 2010, 151, 2423–2432. [Google Scholar] [CrossRef]
- Mouli, V.P.; Ananthakrishnan, A.N. Review Article: Vitamin D and Inflammatory Bowel Diseases. Aliment. Pharmacol. Ther. 2014, 39, 125–136. [Google Scholar] [CrossRef]
- Tajika, M.; Matsuura, A.; Nakamura, T.; Suzuki, T.; Sawaki, A.; Kato, T.; Hara, K.; Ookubo, K.; Yamao, K.; Kato, M.; et al. Risk Factors for Vitamin D Deficiency in Patients with Crohn’s Disease. J. Gastroenterol. 2004, 39, 527–533. [Google Scholar] [CrossRef]
- Yang, L.; Weaver, V.; Smith, J.P.; Bingaman, S.; Hartman, T.J.; Cantorna, M.T. Therapeutic Effect of Vitamin D Supplementation in a Pilot Study of Crohn’s Patients. Clin. Transl. Gastroenterol. 2013, 4, e33. [Google Scholar] [CrossRef]
- Zator, Z.A.; Cantu, S.M.; Konijeti, G.G.; Nguyen, D.D.; Sauk, J.; Yajnik, V.; Ananthakrishnan, A.N. Pretreatment 25-Hydroxyvitamin D Levels and Durability of Anti-Tumor Necrosis Factor-α Therapy in Inflammatory Bowel Diseases. JPEN J. Parenter. Enter. Nutr. 2014, 38, 385–391. [Google Scholar] [CrossRef]
- Cheng, J.; Fang, Z.-Z.; Kim, J.-H.; Krausz, K.W.; Tanaka, N.; Chiang, J.Y.L.; Gonzalez, F.J. Intestinal CYP3A4 Protects against Lithocholic Acid-Induced Hepatotoxicity in Intestine-Specific VDR-Deficient Mice. J. Lipid Res. 2014, 55, 455–465. [Google Scholar] [CrossRef]
- Kong, J.; Zhang, Z.; Musch, M.W.; Ning, G.; Sun, J.; Hart, J.; Bissonnette, M.; Li, Y.C. Novel Role of the Vitamin D Receptor in Maintaining the Integrity of the Intestinal Mucosal Barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G208–G216. [Google Scholar] [CrossRef]
- He, Q.; Ananaba, G.A.; Patrickson, J.; Pitts, S.; Yi, Y.; Yan, F.; Eko, F.O.; Lyn, D.; Black, C.M.; Igietseme, J.U.; et al. Chlamydial Infection in Vitamin D Receptor Knockout Mice Is More Intense and Prolonged Than in Wild-Type Mice. J. Steroid Biochem. Mol. Biol. 2013, 135, 7–14. [Google Scholar] [CrossRef]
- Jones, M.L.; Martoni, C.J.; Prakash, S. Oral Supplementation with Probiotic L. Reuteri NCIMB 30242 Increases Mean Circulating 25-Hydroxyvitamin D: A Post Hoc Analysis of a Randomized Controlled Trial. J. Clin. Endocrinol. Metab. 2013, 98, 2944–2951. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, C.; Chen, Z.; Chen, Z.; Zhang, W.; Ma, X.; Wang, L.; Yang, X.; Jiang, Z. Protective Effects of Lactobacillus Plantarum on Epithelial Barrier Disruption Caused by Enterotoxigenic Escherichia coli in Intestinal Porcine Epithelial Cells. Vet. Immunol. Immunopathol. 2016, 172, 55–63. [Google Scholar] [CrossRef]
- Kanis, J.A.; Cooper, C.; Rizzoli, R.; Reginster, J.-Y.; Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis (ESCEO); Committees of Scientific Advisors and National Societies of the International Osteoporosis Foundation (IOF). European Guidance for the Diagnosis and Management of Osteoporosis in Postmenopausal Women. Osteoporos. Int. 2019, 30, 3–44. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Prevention and Management of Osteoporosis. In WHO Technical Report Series; World Health Organization: Geneva, Switzerland, 2003; Volume 921, pp. 1–164. [Google Scholar]
- Kanis, J.A.; Melton, L.J.; Christiansen, C.; Johnston, C.C.; Khaltaev, N. The Diagnosis of Osteoporosis. J. Bone Miner. Res. 1994, 9, 1137–1141. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.; Lam, D.; Bronze, M.S.; Humphrey, M.B. Osteoporosis in Inflammatory Bowel Disease. Am. J. Med. 2009, 122, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Adriani, A.; Pantaleoni, S.; Luchino, M.; Ribaldone, D.G.; Reggiani, S.; Sapone, N.; Sguazzini, C.; Isaia, G.; Pellicano, R.; Astegiano, M. Osteopenia and Osteoporosis in Patients with New Diagnosis of Inflammatory Bowel Disease. Panminerva Med. 2014, 56, 145–149. [Google Scholar] [PubMed]
- Kärnsund, S.; Lo, B.; Bendtsen, F.; Holm, J.; Burisch, J. Systematic Review of the Prevalence and Development of Osteoporosis or Low Bone Mineral Density and Its Risk Factors in Patients with Inflammatory Bowel Disease. World J. Gastroenterol. 2020, 26, 5362–5374. [Google Scholar] [CrossRef] [PubMed]
- Krela-Kaźmierczak, I.; Michalak, M.; Szymczak-Tomczak, A.; Łykowska-Szuber, L.; Stawczyk-Eder, K.; Waszak, K.; Kucharski, M.A.; Dobrowolska, A.; Eder, P. Prevalence of Osteoporosis and Osteopenia in a Population of Patients with Inflammatory Bowel Diseases from the Wielkopolska Region. Pol. Arch. Intern. Med. 2018, 128, 447–454. [Google Scholar] [CrossRef]
- Lima, C.A.; Lyra, A.C.; Rocha, R.; Santana, G.O. Risk Factors for Osteoporosis in Inflammatory Bowel Disease Patients. World J. Gastrointest. Pathophysiol. 2015, 6, 210–218. [Google Scholar] [CrossRef]
- Sakellariou, G.T.; Moschos, J.; Berberidis, C.; Mpoumponaris, A.; Kadis, S.; Molyvas, E.; Kouklakis, G. Bone Density in Young Males with Recently Diagnosed Inflammatory Bowel Disease. Jt. Bone Spine 2006, 73, 725–728. [Google Scholar] [CrossRef]
- Nakamura, M.; Udagawa, N. Osteoporosis and RANKL signal. Clin. Calcium 2011, 21, 1149–1155. [Google Scholar]
- Nagy, V.; Penninger, J.M. The RANKL-RANK Story. Gerontology 2015, 61, 534–542. [Google Scholar] [CrossRef]
- Boyce, B.F.; Xing, L. The RANKL/RANK/OPG Pathway. Curr. Osteoporos. Rep. 2007, 5, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Atkins, G.J.; Findlay, D.M.; Anderson, P.H.; Morris, H.A. Chapter 23-Target Genes: Bone Proteins. In Vitamin D, 3rd ed.; Feldman, D., Pike, J.W., Adams, J.S., Eds.; Academic Press: San Diego, CA, USA, 2011; pp. 411–424. ISBN 978-0-12-381978-9. [Google Scholar]
- Sgambato, D.; Gimigliano, F.; De Musis, C.; Moretti, A.; Toro, G.; Ferrante, E.; Miranda, A.; De Mauro, D.; Romano, L.; Iolascon, G.; et al. Bone Alterations in Inflammatory Bowel Diseases. World J. Clin. Cases 2019, 7, 1908–1925. [Google Scholar] [CrossRef] [PubMed]
- Dadaei, T.; Safapoor, M.H.; Asadzadeh Aghdaei, H.; Balaii, H.; Pourhoseingholi, M.A.; Naderi, N.; Zojaji, H.; Azimzadeh, P.; Mohammadi, P.; Zali, M.R. Effect of Vitamin D3 Supplementation on TNF-α Serum Level and Disease Activity Index in Iranian IBD Patients. Gastroenterol. Hepatol. Bed. Bench. 2015, 8, 49–55. [Google Scholar] [PubMed]
- Pawlak-Buś, K.; Leszczyński, P. Inhibitory Wnt/b-Kateniny w Terapii Obniżonej Masy Kostnej—Nowe Perspektywy w Leczeniu Osteoporozy? Endokrynol. Otyłość Zaburzenia Przemiany Mater. 2011, 7, 11–15. [Google Scholar]
- Choi, H.Y.; Dieckmann, M.; Herz, J.; Niemeier, A. Lrp4, a Novel Receptor for Dickkopf 1 and Sclerostin, Is Expressed by Osteoblasts and Regulates Bone Growth and Turnover in Vivo. PLoS ONE 2009, 4, e7930. [Google Scholar] [CrossRef]
- Fretz, J.A.; Zella, L.A.; Kim, S.; Shevde, N.K.; Pike, J.W. 1,25-Dihydroxyvitamin D3 Induces Expression of the Wnt Signaling Co-Regulator LRP5 via Regulatory Elements Located Significantly Downstream of the Gene’s Transcriptional Start Site. J. Steroid. Biochem. Mol. Biol. 2007, 103, 440–445. [Google Scholar] [CrossRef]
- Cianferotti, L.; Demay, M.B. VDR-Mediated Inhibition of DKK1 and SFRP2 Suppresses Adipogenic Differentiation of Murine Bone Marrow Stromal Cells. J. Cell. Biochem. 2007, 101, 80–88. [Google Scholar] [CrossRef]
- Duque, G.; El Abdaimi, K.; Henderson, J.E.; Lomri, A.; Kremer, R. Vitamin D Inhibits Fas Ligand-Induced Apoptosis in Human Osteoblasts by Regulating Components of Both the Mitochondrial and Fas-Related Pathways. Bone 2004, 35, 57–64. [Google Scholar] [CrossRef]
- Walicka, M.; Czerwińska, E.; Marcinowska-Suchowierska, E. The Effecst of Vitamin D on Bone. Postępy Nauk. Med. 2012, XXV, 232–236. [Google Scholar]
- Ohnaka, K.; Tanabe, M.; Kawate, H.; Nawata, H.; Takayanagi, R. Glucocorticoid Suppresses the Canonical Wnt Signal in Cultured Human Osteoblasts. Biochem. Biophys. Res. Commun. 2005, 329, 177–181. [Google Scholar] [CrossRef]
- Humphrey, E.L.; Williams, J.H.H.; Davie, M.W.J.; Marshall, M.J. Effects of Dissociated Glucocorticoids on OPG and RANKL in Osteoblastic Cells. Bone 2006, 38, 652–661. [Google Scholar] [CrossRef]
- Shymanskyi, I.; Lisakovska, O.; Mazanova, A.; Labudzynskyi, D.; Veliky, M. Vitamin D3 Modulates Impaired Crosstalk between RANK and Glucocorticoid Receptor Signaling in Bone Marrow Cells after Chronic Prednisolone Administration. Front. Endocrinol. 2018, 9, 303. [Google Scholar] [CrossRef]
- Huybers, S.; Apostolaki, M.; van der Eerden, B.C.J.; Kollias, G.; Naber, T.H.J.; Bindels, R.J.M.; Hoenderop, J.G.J. Murine TNF(DeltaARE) Crohn’s Disease Model Displays Diminished Expression of Intestinal Ca2+ Transporters. Inflamm. Bowel Dis. 2008, 14, 803–811. [Google Scholar] [CrossRef]
- Ghishan, F.K.; Kiela, P.R. Advances in the Understanding of Mineral and Bone Metabolism in Inflammatory Bowel Diseases. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G191–G201. [Google Scholar] [CrossRef]
- Harbord, M.; Annese, V.; Vavricka, S.R.; Allez, M.; Barreiro-de Acosta, M.; Boberg, K.M.; Burisch, J.; De Vos, M.; De Vries, A.-M.; Dick, A.D.; et al. The First European Evidence-Based Consensus on Extra-Intestinal Manifestations in Inflammatory Bowel Disease. J. Crohn’s Colitis 2016, 10, 239–254. [Google Scholar] [CrossRef]
- Jørgensen, S.P.; Hvas, C.L.; Agnholt, J.; Christensen, L.A.; Heickendorff, L.; Dahlerup, J.F. Active Crohn’s Disease Is Associated with Low Vitamin D Levels. J. Crohn’s Colitis 2013, 7, e407–e413. [Google Scholar] [CrossRef]
- Stio, M.; Treves, C.; Martinesi, M.; d’Albasio, G.; Bagnoli, S.; Bonanomi, A.G. Effect of Anti-TNF Therapy and Vitamin D Derivatives on the Proliferation of Peripheral Blood Mononuclear Cells in Crohn’s Disease. Dig. Dis. Sci. 2004, 49, 328–335. [Google Scholar] [CrossRef]
- Winter, R.W.; Collins, E.; Cao, B.; Carrellas, M.; Crowell, A.M.; Korzenik, J.R. Higher 25-Hydroxyvitamin D Levels Are Associated with Greater Odds of Remission with Anti-Tumour Necrosis Factor-α Medications among Patients with Inflammatory Bowel Diseases. Aliment. Pharmacol. Ther. 2017, 45, 653–659. [Google Scholar] [CrossRef]
- Arihiro, S.; Nakashima, A.; Matsuoka, M.; Suto, S.; Uchiyama, K.; Kato, T.; Mitobe, J.; Komoike, N.; Itagaki, M.; Miyakawa, Y.; et al. Randomized Trial of Vitamin D Supplementation to Prevent Seasonal Influenza and Upper Respiratory Infection in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019, 25, 1088–1095. [Google Scholar] [CrossRef]
- Maconi, G.; Bosetti, C.; De Monti, A.; Boyapati, R.K.; Shelton, E.; Piazza, N.; Carvalhas Gabrielli, A.M.; Lenti, M.V.; Bezzio, C.; Ricci, C.; et al. Risk of COVID-19 in Patients with Inflammatory Bowel Diseases Compared to a Control Population. Dig. Liver Dis. 2021, 53, 263–270. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, D.; Deng, F. The Role of Vitamin D in Immune System and Inflammatory Bowel Disease. J. Inflamm. Res. 2022, 15, 3167–3185. [Google Scholar] [CrossRef] [PubMed]
- Scolaro, B.L.; Barretta, C.; Matos, C.H.; Malluta, E.F.; de Almeida, I.B.T.; Braggio, L.D.; Bobato, S.; Specht, C.M. Deficiency of vitamin D and its relation with clinical and laboratory activity of inflammatory bowel diseases. J. Coloproctol. 2018, 38, 99–104. [Google Scholar] [CrossRef]
- Dhawan, M.; Priyanka; Choudhary, O.P. Immunomodulatory and Therapeutic Implications of Vitamin D in the Management of COVID-19. Hum. Vaccines Immunother. 2022, 18, 2025734. [Google Scholar] [CrossRef] [PubMed]


| Interpretation | 25(OH)D Concentration (ng/mL) |
|---|---|
| Severe deficiency | 0–10 |
| Deficiency | 10–20 |
| Suboptimal concentration | 21–30 |
| Optimal concentration | 31–50 |
| High concentration | 50–100 |
| Toxic concentration | >100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymczak-Tomczak, A.; Ratajczak, A.E.; Kaczmarek-Ryś, M.; Hryhorowicz, S.; Rychter, A.M.; Zawada, A.; Słomski, R.; Dobrowolska, A.; Krela-Kaźmierczak, I. Pleiotropic Effects of Vitamin D in Patients with Inflammatory Bowel Diseases. J. Clin. Med. 2022, 11, 5715. https://doi.org/10.3390/jcm11195715
Szymczak-Tomczak A, Ratajczak AE, Kaczmarek-Ryś M, Hryhorowicz S, Rychter AM, Zawada A, Słomski R, Dobrowolska A, Krela-Kaźmierczak I. Pleiotropic Effects of Vitamin D in Patients with Inflammatory Bowel Diseases. Journal of Clinical Medicine. 2022; 11(19):5715. https://doi.org/10.3390/jcm11195715
Chicago/Turabian StyleSzymczak-Tomczak, Aleksandra, Alicja Ewa Ratajczak, Marta Kaczmarek-Ryś, Szymon Hryhorowicz, Anna Maria Rychter, Agnieszka Zawada, Ryszard Słomski, Agnieszka Dobrowolska, and Iwona Krela-Kaźmierczak. 2022. "Pleiotropic Effects of Vitamin D in Patients with Inflammatory Bowel Diseases" Journal of Clinical Medicine 11, no. 19: 5715. https://doi.org/10.3390/jcm11195715
APA StyleSzymczak-Tomczak, A., Ratajczak, A. E., Kaczmarek-Ryś, M., Hryhorowicz, S., Rychter, A. M., Zawada, A., Słomski, R., Dobrowolska, A., & Krela-Kaźmierczak, I. (2022). Pleiotropic Effects of Vitamin D in Patients with Inflammatory Bowel Diseases. Journal of Clinical Medicine, 11(19), 5715. https://doi.org/10.3390/jcm11195715






