Ligament Alteration in Diabetes Mellitus
Abstract
1. Introduction
2. The Formation of Advanced Glycation End Products (AGEs) Is Considered to Be the Biggest Risk Factor for the Development of Diabetic Complications
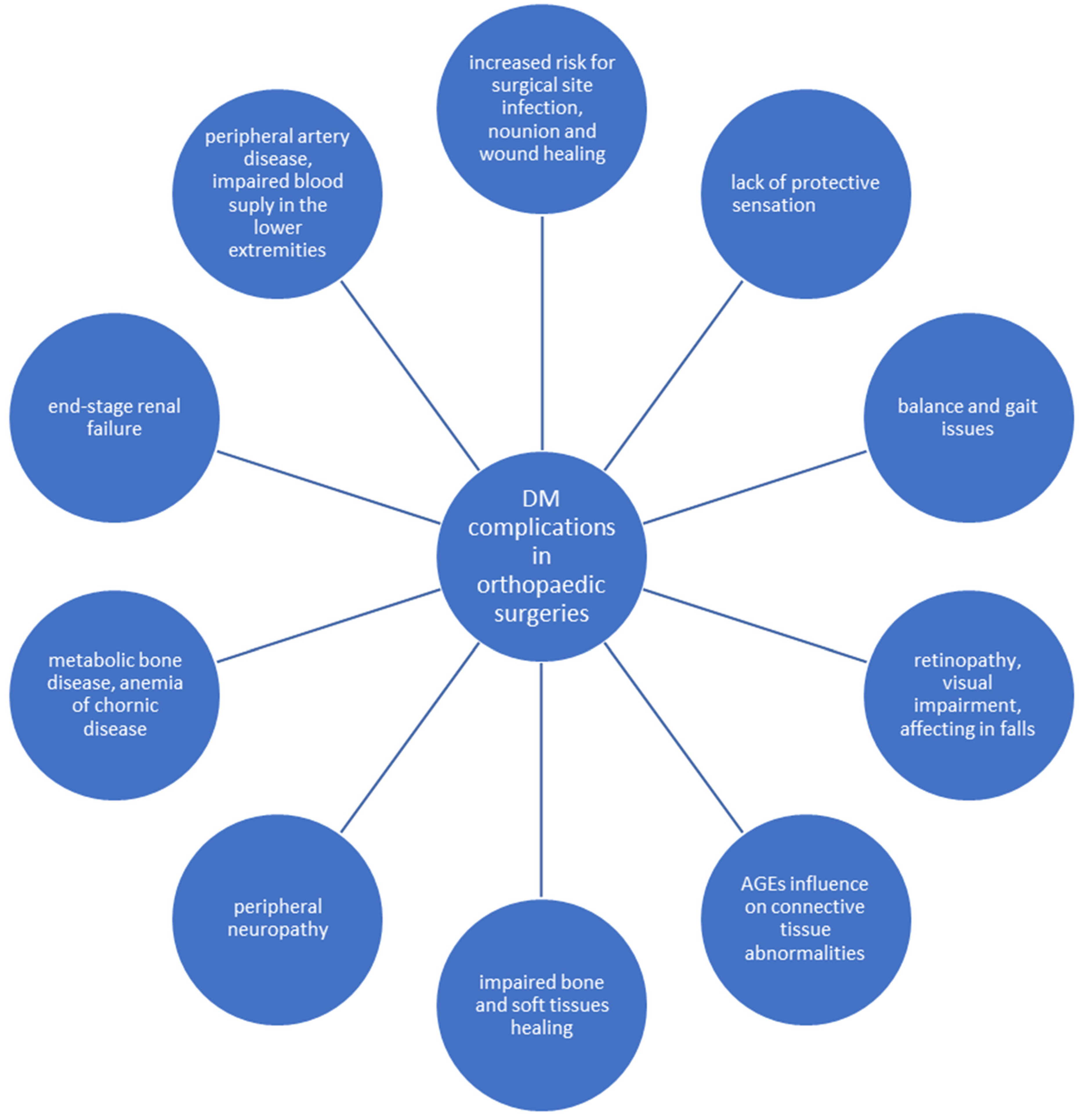
3. Negative Impact of Diabetes on the Musculoskeletal System
4. Heterogeneity in Connective Tissue Treatment Modalities—Tendons and Ligaments
5. Rheumatoid Arthritis (RA) and T1DM
6. Manifestation of Ligaments Pathologies in Diabetic Patients—Specific Findings
7. Histological and Biochemical Changes in Ligaments Specifically in Diabetic Patients
| Literature | Species Model | Groups | Duration of DM | Analysed Tissue | Found Correlations |
|---|---|---|---|---|---|
| Li K. et al., 1995 [27] | Sprague–Dawley rats | SCG (n = 22) DM (n = 28) DM-IT (n = 7) | 1 week | Ligament (MCL) | The length, thickness, and cross-sectional area of the DM MCL were significantly smaller than the control values—consistent with the reduced quantities of collagen in ligaments. MCL cell density was smaller in DM group compared with DM-IT, but DM-IT showed improvement in properties compared with untreated DM. |
| Vincente A. et al., 2020 [28] | Sprague–Dawley rats | CG (n = 20) DM (n = 20) DM-IT (n = 20) | 11 days | Ligament (PDL) | Force applied to PDL of DM rats caused higher inflammatory response, more oxidative stress, and a greater extent of orthodontic tooth movement than in normoglycemic rats. Stress produces a greater disorganisation of PDL in diabetic rats together with higher MMP-8 and MMP-9 expressions. Greater expression of it is observed in diabetic patients, which leads to increased collagen and gelatine degradation. This provokes poor regenerative features and worse prognosis of mechanical recovery after trauma and mechanical stress. |
| Njoto I. et al., 2018 [30] | Rattus norvegicus strain Wistar | CG (n = 3) DM (n = 15) | 61 days | Cartilage (chondrocytes and pericellular matrix) | Increases in glycemia of animal models interfere with chondrocyte shape and formation. Hyperglycaemia provokes production of pro-inflammatory mediators, such as AGEs, local toxicity to joint tissues, and apoptosis. |
| Njoto I. et al., 2019 [31] | Rattus norvegicus strain Wistar | CG (n = 5) DM (n = 15) DM-IT (n = 15) | 21 days; 28 days; 42 days | Ligament (ACL) | Protein expression of perlecan in ligaments gradually decreased over time within DM groups. Hyperglycaemia predisposes articular cartilage damage, higher severity of the osteoarthritis disease, and reaches into the intracellular compartment. |
| Xin L. et al., 2010 [57] | Sprague–Dawley rats | CG (n = 24) DM (n = 24) | 8 weeks | Ligament (PDL) | The DM group showed increased expression of MMP-1 and Col-III and decreased expression of Col-I in PDL. The DM group appeared to have worse recovery from damage caused by orthodontic movement. DM showed alterations in immune response, inflammation, extracellular matrix synthesis, and collagen destruction. |
| Tan J. et al., 2022 [47] | Genetically diabetic C57BLKS/J-Leprdb (db/db) mice and their C57BLKS/J wild-type littermates | DM (n = 10) IG (n = 10) CG (n = 8) | Ligament (PDL) | The mRNA expression levels of GRP78, ATF6, PERK, and XBP1 were highest in DM, followed by IG, and the lowest in CG. Hyperglycaemia activates ER stress. DM and IG microscopic observations showed disorganised cell arrangement in PDL, necrotic tissue, inflammatory cells, inflammation, granulation tissue hyperplasia, and disordered fibroblasts. | |
| Tang L. et al., 2022 [58] | Genetically diabetic C57BLKS/J-Leprdb (db/db) mice and their C57BLKS/J wild-type littermates | DM (n = ?) CG (n = ?) | 8 weeks | Ligament (PDL) | DM produced ROS with an increased MDA level indicating lipid peroxidation. SOD and GSH-Px levels, which refer to essential antioxidative scavengers of ROS, were significantly decreased in the serum of DM. The intracellular DNA damage measurement occurred based on markedly increased 8-OHdG expression in DM. The telomere oxidative damage (accelerated telomere shortening) was detected through an expression of 53BP1 and the colocalization of 53BP1 and TRF2 increase in the PDL of the DM group. |
| Li H. et al., 2008 [59] | Sprague–Dawley rats | DM (n = ?) CG (n = ?) | 12 weeks | Ligament (Posterior longitudinal ligament tissues of cervical spine) | Hyperglycaemia increases the gene expression and protein synthesis of collagen types I and III, particularly in cells of the posterior longitudinal ligament. |
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGEs | advanced glycation end products |
| DM | diabetes mellitus |
| ECM | extracellular matrix |
| RAGE | receptor for advanced glycation end products |
| Col1A1 | collagen type 1 |
| Col3A1 | collagen type 3 |
| MMP/s | metalloproteinase/s |
| VEGF | vascular endothelial growth factor |
| LSS | lumbar spinal stenosis |
| LF | ligamentum flavum |
| ROS | reactive oxygen species |
| PPARs | peroxisome proliferator-activated receptors |
| ER | endoplasmic reticulum |
| PDL | periodontal ligament |
| TMMPs | tissue inhibitors of MMPs |
| ATF6 | activating transcription factor 6 |
| PKR | double-stranded RNA-activated protein kinase |
| PERK | endoplasmic reticulum kinase |
| IRE1 | inositol requiring enzyme 1 |
References
- Colagiuri, S. Definition and Classification of Diabetes and Prediabetes and Emerging Data on Phenotypes. Endocrinol. Metab. Clin. N. Am. 2021, 50, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, S.; Sidon, E.; Kaisler, E.; Sheinis, D.; Velkes, S.; Ohana, N.; Benayahu, D. Diabetes mellitus is associated with increased elastin fiber loss in ligamentum flavum of patients with lumbar spinal canal stenosis: Results of a pilot histological study. Eur. Spine J. 2018, 27, 1614–1622. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.; Khamis, T.; Enan, G.; El-Didamony, G.; Sitohy, B.; Abdel-Fattah, G. The Healing Capability of Clove Flower Extract (CFE) in Streptozotocin-Induced (STZ-Induced) Diabetic Rat Wounds Infected with Multidrug Resistant Bacteria. Molecules 2022, 27, 2270. [Google Scholar] [CrossRef]
- Passarelli, M.; Machado, U.F.F. AGEs-Induced and Endoplasmic Reticulum Stress/Inflammation-Mediated Regulation of GLUT4 Expression and Atherogenesis in Diabetes Mellitus. Cells 2021, 11, 104. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Rui, Y.; Li, G.; Wang, C. Alterations of tendons in diabetes mellitus: What are the current findings? Int. Orthop. 2015, 39, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Leong, N.L.; Kator, J.L.; Clemens, T.L.; James, A.; Enamoto-Iwamoto, M.; Jiang, J. Tendon and Ligament Healing and Current Approaches to Tendon and Ligament Regeneration. J. Orthop. Res. 2020, 38, 7–12. [Google Scholar] [CrossRef]
- WHO. About Diabetes. 2014. Available online: https://www.who.int/diabetes/action_online/basics/en/index3.html (accessed on 21 September 2022).
- Gustafson, B.; Hedjazifar, S.; Gogg, S.; Hammarstedt, A.; Smith, U. Insulin resistance and impaired adipogenesis. Trends Endocrinol. Metab. 2015, 26, 193–200. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation WHO. Global Report on Diabetes; Working Papers; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Cui, A.; Li, H.; Wang, D.; Zhong, J.; Chen, Y.; Lu, H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine 2020, 29–30, 100587. [Google Scholar] [CrossRef]
- Liao, K.P.; Gunnarsson, M.; Källberg, H.; Ding, B.; Plenge, R.M.; Padyukov, L.; Karlson, E.W.; Klareskog, L.; Askling, J.; Alfredsson, L. Specific association of type 1 diabetes mellitus with anti-cyclic citrullinated peptide-positive rheumatoid arthritis. Arthritis Rheum. 2009, 60, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Armitage, L.H.; Wallet, M.A.; Mathews, C.E. Influence of PTPN22 Allotypes on Innate and Adaptive Immune Function in Health and Disease. Front. Immunol. 2021, 25, 636618. [Google Scholar] [CrossRef]
- Arivazhagan, L.; López-Díez, R.; Shekhtman, A.; Ramasamy, R.; Schmidt, A.M. Glycation and a Spark of ALEs (Advanced Lipoxidation End Products)—Igniting RAGE/Diaphanous-1 and Cardiometabolic Disease. Front. Cardiovasc. Med. 2022, 9, 937071. [Google Scholar] [CrossRef] [PubMed]
- Hudson, D.M.; Archer, M.; King, K.B.; Eyre, D.R. Glycation of type I collagen selectively targets the same helical domain lysine sites as lysyl oxidase-mediated cross-linking. J. Biol. Chem. 2018, 293, 15620–15627. [Google Scholar] [CrossRef] [PubMed]
- Velayoudom-Cephise, F.L.; Cano-Sanchez, M.; Bercion, S.; Tessier, F.; Yu, Y.; Boulanger, E.; Neviere, R. Receptor for advanced glycation end products modulates oxidative stress and mitochondrial function in the soleus muscle of mice fed a high-fat diet. Appl. Physiol. Nutr. Metab. 2020, 45, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. 2014, 18, 1. [Google Scholar] [CrossRef]
- Wukich, D.K. Diabetes and its negative impact on outcomes in orthopaedic surgery. World J. Orthop. 2015, 6, 331–339. [Google Scholar] [CrossRef]
- Stolarczyk, A.; Sarzyńska, S.; Gondek, A.; Cudnoch-Jędrzejewska, A. Influence of diabetes on tissue healing in orthopaedic injuries. Clin. Exp. Pharmacol. Physiol. 2018, 45, 619–627. [Google Scholar] [CrossRef]
- Galway, U.; Chahar, P.; Schmidt, M.T.; Araujo-Duran, J.A.; Shivakumar, J.; Turan, A.; Ruetzler, K. Perioperative challenges in management of diabetic patients undergoing non-cardiac surgery. World J. Diabetes 2021, 12, 1255–1266. [Google Scholar] [CrossRef]
- Duggan, E.; Chen, Y. Glycemic Management in the Operating Room: Screening, Monitoring, Oral Hypoglycemics, and Insulin Therapy. Curr. Diab. Rep. 2019, 19, 134. [Google Scholar] [CrossRef]
- Lai, J.; Li, Q.; He, Y.; Zou, S.; Bai, X.; Rastogi, S. Glycemic Control Regimens in the Prevention of Surgical Site Infections: A Meta-Analysis of Randomized Clinical Trials. Front. Surg. 2022, 9, 855409. [Google Scholar] [CrossRef]
- Lenguerrand, E.; Beswick, A.D.; Whitehouse, M.R.; Wylde, V.; Blom, A.W. Outcomes following hip and knee replacement in diabetic versus nondiabetic patients and well versus poorly controlled diabetic patients: A prospective cohort study. Acta Orthop. 2018, 89, 399–405. [Google Scholar] [CrossRef]
- Kato, S.; Saito, M.; Funasaki, H.; Marumo, K. Distinctive collagen maturation process in fibroblasts derived from rabbit anterior cruciate ligament, medial collateral ligament, and patellar tendon in vitro. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 1384–1392. [Google Scholar] [CrossRef] [PubMed]
- Loots, M.A.; Lamme, E.N.; Mekkes, J.R.; Bos, J.D.; Middelkoop, E. Cultured fibroblasts from chronic diabetic wounds on the lower extremity (non-insulin-dependent diabetes mellitus) show disturbed proliferation. Arch. Dermatol. Res. 1999, 291, 93–99. [Google Scholar] [CrossRef]
- Black, E.; Vibe-Petersen, J.; Jorgensen, L.N.; Madsen, S.M.; Agren, M.S.; Holstein, P.E.; Perrild, H.; Gottrup, F. Decrease of collagen deposition in wound repair in type 1 diabetes independent of glycemic control. Arch. Surg. 2003, 138, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Lerman, O.Z.; Galiano, R.D.; Armour, M.; Levine, J.P.; Gurtner, G.C. Cellular dysfunction in the diabetic fibroblast: Impairment in migration, vascular endothelial growth factor production, and response to hypoxia. Am. J. Pathol. 2003, 162, 303–312. [Google Scholar] [CrossRef]
- Li, K.C.; Zernicke, R.F.; Barnard, R.J.; Li, A.F. Response of immature diabetic rat bone-ligament junctions to insulin and exercise. Clin. Biomech. 1995, 10, 331–336. [Google Scholar] [CrossRef]
- Vicente, A.; Bravo-González, L.A.; Navarro, J.A.; Buendía, A.J.; Camacho-Alonso, F. Effects of diabetes on oxidative stress, periodontal ligament fiber orientation, and matrix metalloproteinase 8 and 9 expressions during orthodontic tooth movement. Clin. Oral Investig. 2021, 25, 1383–1394. [Google Scholar] [CrossRef]
- Willett, T.L.; Kandel, R.; De Croos, J.N.; Avery, N.C.; Grynpas, M.D. Enhanced levels of non-enzymatic glycation and pentosidine crosslinking in spontaneous osteoarthritis progression. Osteoarthr. Cartil. 2012, 20, 736–744. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Njoto, I.; Soekanto, A.; Ernawati, E.; Abdurrachman, A.; Kalim, H.; Handono, K.; Soeatmadji, D.W.; Fatchiyah, F. Chondrocyte Intracellular Matrix Strain Fields of Articular Cartilage Surface in Hyperglycemia Model of Rat: Cellular Morphological Study. Med. Arch. 2018, 72, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Njoto, I.; Kalim, H.; Soeatmadji, D.W.; Handono, K.; Fatchiyah, F. Effect of Hyperglycemia to the mRNA Level and Protein Expression of Perlecan at Rat Model of Osteoarthritis with Diabetes Mellitus Type 1. Med. Arch. 2019, 73, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Radojčić, M.R.; Thudium, C.S.; Henriksen, K.; Tan, K.; Karlsten, R.; Dudley, A.; Chessell, I.; Karsdal, M.A.; Bay-Jensen, A.C.; Crema, M.D.; et al. Biomarker of extracellular matrix remodelling C1M and proinflammatory cytokine interleukin 6 are related to synovitis and pain in end-stage knee osteoarthritis patients. Pain 2017, 158, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Asadian, L.; Haddadi, K.; Aarabi, M.; Zare, A. Diabetes Mellitus, a New Risk Factor for Lumbar Spinal Stenosis: A Case-Control Study. Clin. Med. Insights Endocrinol. Diabetes 2016, 9, CMED-S39035. [Google Scholar] [CrossRef]
- Anekstein, Y.; Smorgick, Y.; Lotan, R.; Agar, G.; Shalmon, E.; Floman, Y.; Mirovsky, Y. Diabetes mellitus as a risk factor for the development of lumbar spinal stenosis. Isr. Med. Assoc. J. 2010, 12, 16–20. [Google Scholar]
- Lotan, R.; Oron, A.; Anekstein, Y.; Shalmon, E.; Mirovsky, Y. Lumbar stenosis and systemic diseases: Is there any relevance? J. Spinal Disord. Tech. 2008, 21, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Abate, M.; Schiavone, C.; Pelotti, P.; Salini, V. Limited joint mobility in diabetes and ageing: Recent advances in pathogenesis and therapy. Int. J. Immunopathol. Pharmacol. 2010, 23, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Maruf, M.H.; Suzuki, A.; Hayashi, K.; Habibi, H.; Salimi, H.; Terai, H.; Tamai, K.; Hoshino, M.; Toyoda, H.; Yamada, K.; et al. Increased advanced glycation end products in hypertrophied ligamentum flavum of diabetes mellitus patients. Spine J. 2019, 19, 1739–1745. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, K.W.; Cho, H.G.; Kang, K.T.; Chang, B.S.; Lee, C.K.; Yeom, J.S. Indirect effects of decompression surgery on glycemic homeostasis in patients with type 2 diabetes mellitus and lumbar spinal stenosis. Spine J. 2015, 15, 25–33. [Google Scholar] [CrossRef]
- Kim, K.T.; Cho, D.C.; Sung, J.K.; Kim, C.H.; Kang, H.; Kim, D.H. Changes in HbA1c levels and body mass index after successful decompression surgery in patients with type 2 diabetes mellitus and lumbar spinal stenosis: Results of a 2-year follow-up study. Spine J. 2017, 17, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Li, Y.J.; Rui, Y.F.; Dai, G.C.; Shi, L.; Xu, H.L.; Ni, M.; Zhao, S.; Chen, H.; Wang, C.; et al. The effects of high glucose on tendon-derived stem cells: Implications of the pathogenesis of diabetic tendon disorders. Oncotarget 2017, 8, 17518–17528. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.F.; Wang, H.K.; Chang, H.W.; Sun, J.; Sun, J.S.; Chao, Y.H. High glucose alters tendon homeostasis through downregulation of the AMPK/Egr1 pathway. Sci. Rep. 2017, 7, 44199. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, R.C.; Knowles, H.J.; Carr, A.J.; Hulley, P.A. Cell differentiation versus cell death: Extracellular glucose is a key determinant of cell fate following oxidative stress exposure. Cell Death Dis. 2014, 5, e1074. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, M.; Bardiot, A.; Lesur, C.; Moulharat, N.; Thomas, M.; Richard, I.; Fradin, A. Effects of agonists of peroxisome proliferator-activated receptor gamma on proteoglycan degradation and matrix metalloproteinase production in rat cartilage in vitro. Osteoarthr. Cartil. 2002, 10, 673–679. [Google Scholar] [CrossRef]
- Damerau, A.; Gaber, T.; Ohrndorf, S.; Hoff, P. JAK/STAT Activation: A General Mechanism for Bone Development, Homeostasis, and Regeneration. Int. J. Mol. Sci. 2020, 21, 9004. [Google Scholar] [CrossRef]
- Mei, Y.M.; Li, L.; Wang, X.Q.; Zhang, M.; Zhu, L.F.; Fu, Y.W.; Xu, Y. AGEs induces apoptosis and autophagy via reactive oxygen species in human periodontal ligament cells. J. Cell. Biochem. 2019, 121, 3764–3779. [Google Scholar] [CrossRef] [PubMed]
- Kjaer, M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol. Rev. 2004, 84, 649–698. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Zhou, Y.; Luo, J.; Wu, X.; Liu, H.; Wang, W.; Li, Z.; Zhong, M.; Wu, L.; Li, X. High glucose inhibits the osteogenic differentiation of periodontal ligament stem cells in periodontitis by activating endoplasmic reticulum stress. Ann. Transl. Med. 2022, 10, 204. [Google Scholar] [CrossRef] [PubMed]
- Oakes, S.A.; Papa, F.R. The role of endoplasmic reticulum stress in human pathology. Annu Rev Pathol. 2015, 10, 173–194. [Google Scholar] [CrossRef]
- Elvira, B.; Vandenbempt, V.; Bauzá-Martinez, J.; Crutzen, R.; Negueruela, J.; Ibrahim, H.; Winder, M.L.; Brahma, M.K.; Vekeriotaite, B.; Martens, P.J.; et al. PTPN2 Regulates the Interferon Signaling and Endoplasmic Reticulum Stress Response in Pancreatic β-Cells in Autoimmune Diabetes. Diabetes 2022, 71, 653–668. [Google Scholar] [CrossRef] [PubMed]
- Snyder, J.T.; Darko, C.; Sharma, R.B.; Alonso, L.C. Endoplasmic Reticulum Stress Induced Proliferation Remains Intact in Aging Mouse β-Cells. Front. Endocrinol. 2021, 12, 734079. [Google Scholar] [CrossRef]
- Tan, J.; Zhou, L.; Xue, P.; An, Y.; Luo, L.; Zhang, R.; Wu, G.; Wang, Y.; Zhu, H.; Wang, Q. Tumor Necrosis Factor-α Attenuates the Osteogenic Differentiation Capacity of Periodontal Ligament Stem Cells by Activating PERK Signaling. J. Periodontol. 2016, 87, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Van Doren, S.R. Matrix metalloproteinase interactions with collagen and elastin. Matrix Biol. 2015, 44–46, 224–231. [Google Scholar] [CrossRef]
- Xiao, D.; Yang, R.; Gong, L.; Zhang, Y.; Xie, Y.; Ni, S. Plantamajoside inhibits high glucose-induced oxidative stress, inflammation, and extracellular matrix accumulation in rat glomerular mesangial cells through the inactivation of Akt/NF-κB pathway. J. Recept. Signal Transduct. Res. 2021, 41, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Zuo, Z.H.; Luo, P.; Pang, F.S.; Hu, J.T. The effect of cyclic tensile force on the actin cytoskeleton organization and morphology of human periodontal ligament cells. Biochem. Biophys. Res. Commun. 2018, 506, 950–955. [Google Scholar] [CrossRef]
- Vander Horst, M.A.; Raeman, C.H.; Dalecki, D.; Hocking, D.C. Time- and Dose-Dependent Effects of Pulsed Ultrasound on Dermal Repair in Diabetic Mice. Ultrasound Med. Biol. 2021, 47, 1054–1066. [Google Scholar] [CrossRef]
- Lobmann, R.; Ambrosch, A.; Schultz, G.; Waldmann, K.; Schiweck, S.; Lehnert, H. Expression of matrix-metalloproteinases and their inhibitors in the wounds of diabetic and nondiabetic patients. Diabetologia 2002, 45, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Lin, Z.; Na, W.; Xiaohong, F.; Liangjia, B. Periodontal Ligament Remodeling and Alveolar Bone Resorption During Orthodontic Tooth Movement in Rats with Diabetes. Diabetes Technol. Ther. 2010, 12, 65–73. [Google Scholar]
- Tang, L.; Li, T.; Chang, Y.; Wang, Z.; Li, Y.; Wang, F.; Sui, L. Diabetic oxidative stress-induced telomere damage aggravates periodontal bone loss in periodontitis. Biochem. Biophys. Res. Commun. 2022, 614, 22–28. [Google Scholar] [CrossRef]
- Li, H.; Liu, D.; Zhao, C.Q.; Jiang, L.S.; Dai, L.Y. High glucose promotes collagen synthesis by cultured cells from rat cervical posterior longitudinal ligament via transforming growth factor-β1. Eur. Spine J. 2008, 17, 873–881. [Google Scholar] [CrossRef][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamska, O.; Stolarczyk, A.; Gondek, A.; Maciąg, B.; Świderek, J.; Czuchaj, P.; Modzelewski, K. Ligament Alteration in Diabetes Mellitus. J. Clin. Med. 2022, 11, 5719. https://doi.org/10.3390/jcm11195719
Adamska O, Stolarczyk A, Gondek A, Maciąg B, Świderek J, Czuchaj P, Modzelewski K. Ligament Alteration in Diabetes Mellitus. Journal of Clinical Medicine. 2022; 11(19):5719. https://doi.org/10.3390/jcm11195719
Chicago/Turabian StyleAdamska, Olga, Artur Stolarczyk, Agata Gondek, Bartosz Maciąg, Jakub Świderek, Paweł Czuchaj, and Krzysztof Modzelewski. 2022. "Ligament Alteration in Diabetes Mellitus" Journal of Clinical Medicine 11, no. 19: 5719. https://doi.org/10.3390/jcm11195719
APA StyleAdamska, O., Stolarczyk, A., Gondek, A., Maciąg, B., Świderek, J., Czuchaj, P., & Modzelewski, K. (2022). Ligament Alteration in Diabetes Mellitus. Journal of Clinical Medicine, 11(19), 5719. https://doi.org/10.3390/jcm11195719






