The Value of Hemoglobin Glycation Index–Diabetes Mellitus System in Evaluating and Predicting Incident Stroke in the Chinese Population
Abstract
1. Introduction
2. Methods
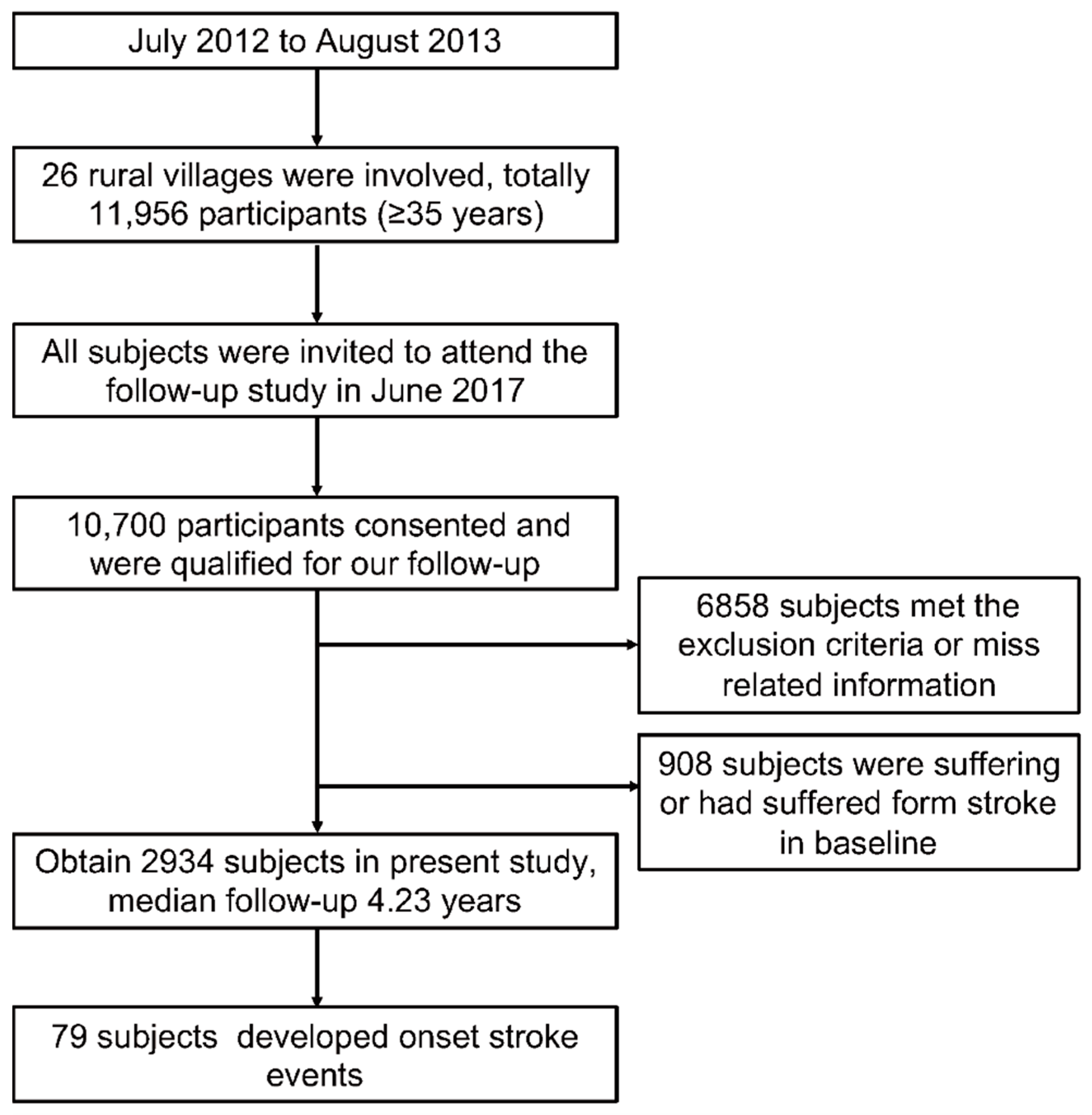
2.1. Study Population
2.2. Ethics Approval and Informed Consent
2.3. Data Collection and Lifestyle Risk Factors
2.4. Anthropometrics, Biochemical Parameters, and Blood Pressure Measurements
2.5. Definition
2.6. Judgment and Definition of Clinical Outcomes
2.7. Statistical Analysis
2.8. Independent Data Access and Analysis
2.9. Materials and Data Availability
3. Results
3.1. Baseline Characteristics of the Study
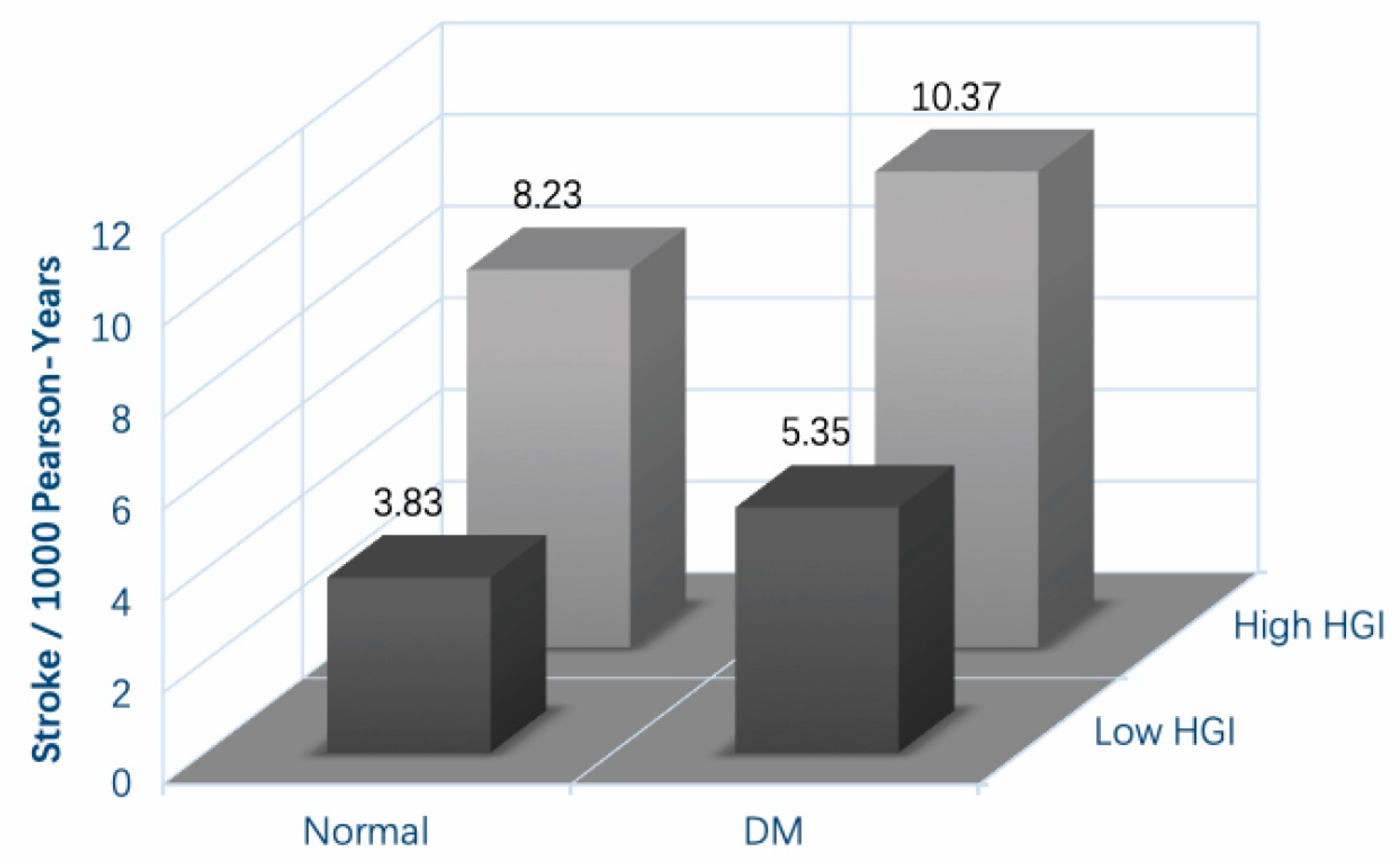
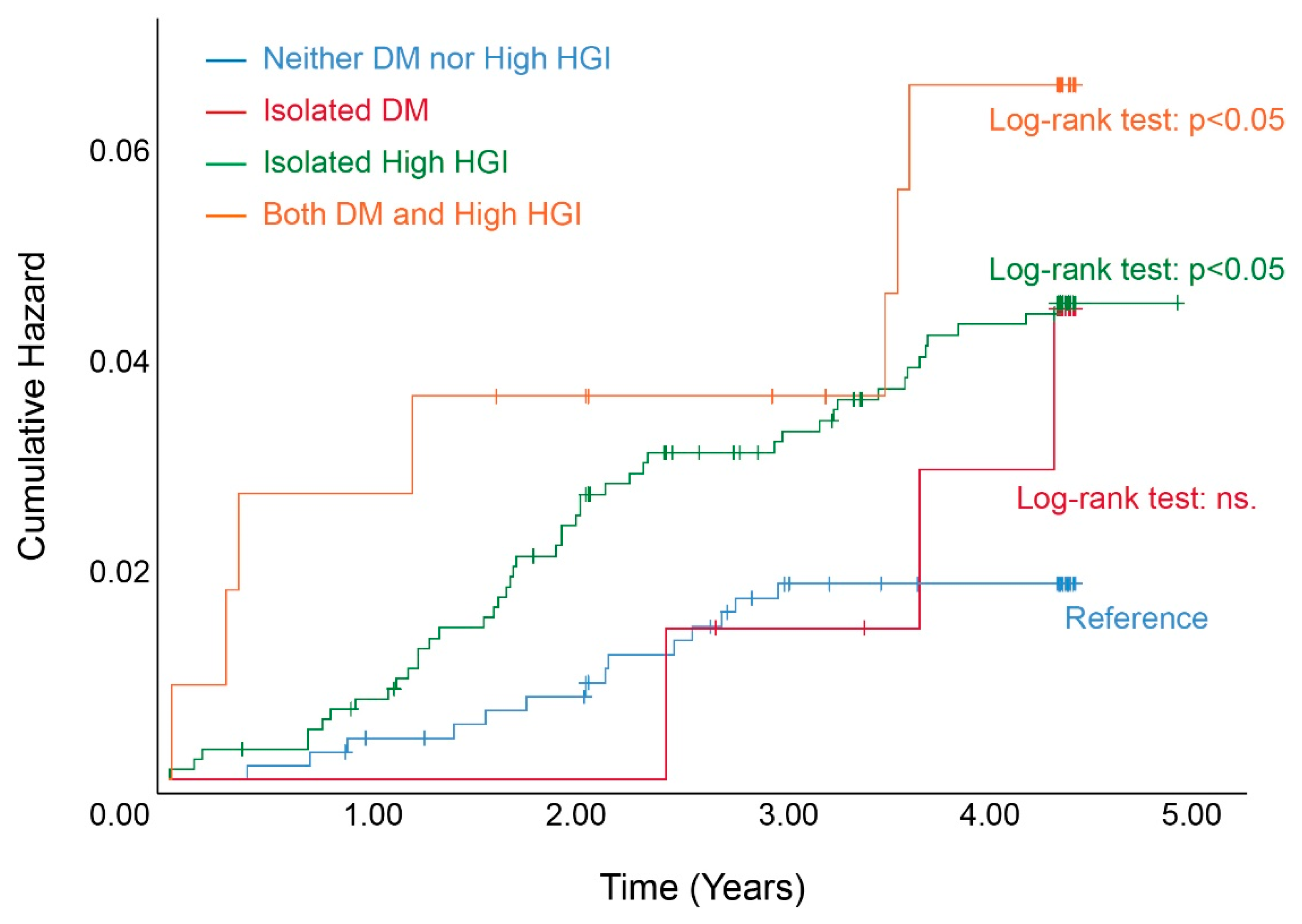
3.2. The Incidence and Cumulative Hazard of Onset Stroke Based on the HGI–DM System
3.3. High HGI Can Elevate the Risk of Incident Stroke and Promote Adverse Outcomes
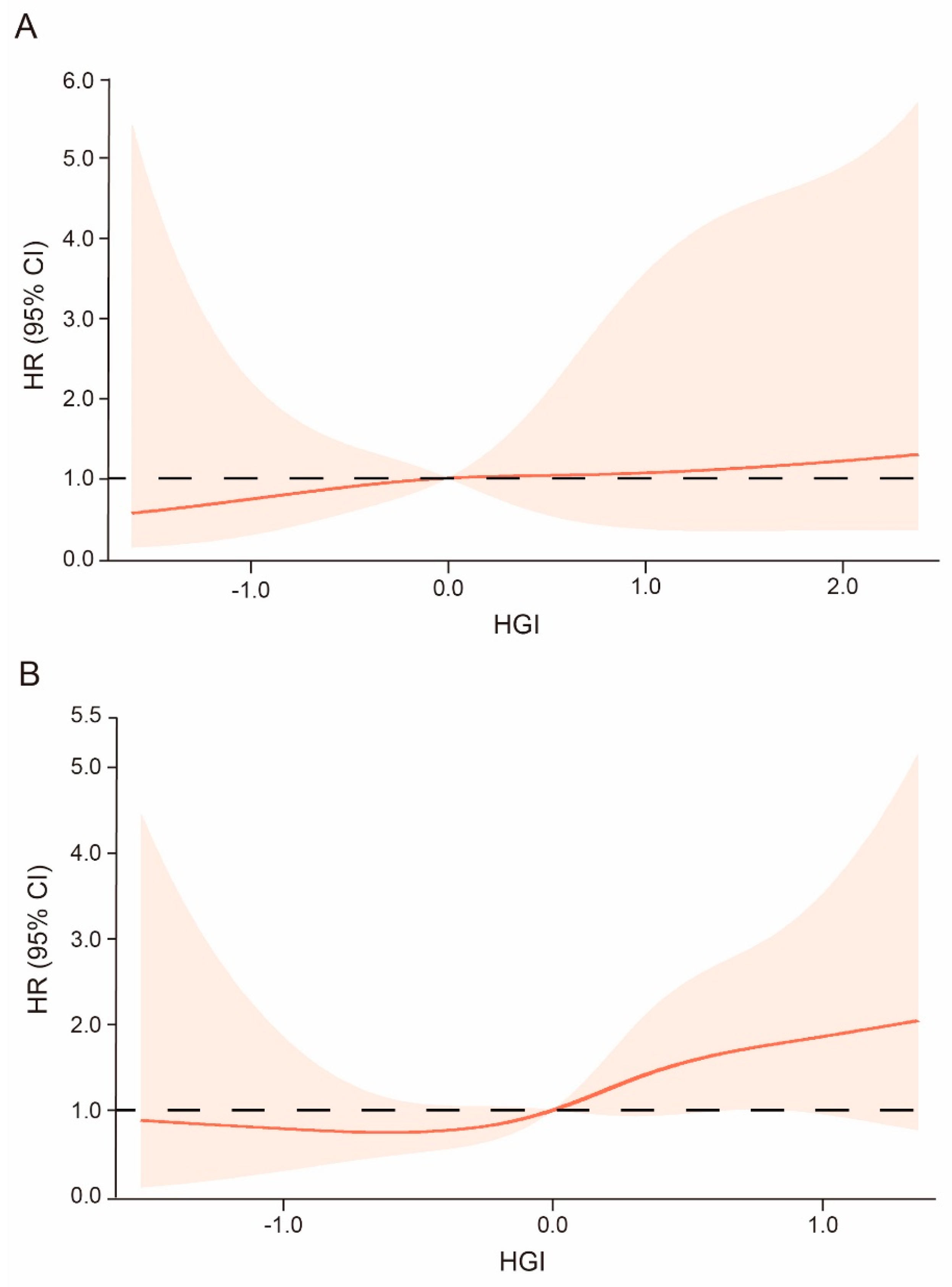
3.4. High HGI Trend Increases the Risk of Incident Stroke Regardless of DM
3.5. HGI Can Improve the Predictive Effect of the Traditional Model for Stroke
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mansfield, A.; Inness, E.L.; McIlroy, W.E. Stroke. Handb. Clin. Neurol. 2018, 159, 205–228. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jiang, B.; Sun, H.; Ru, X.; Sun, D.; Wang, L.; Wang, L.; Jiang, Y.; Li, Y.; Wang, Y.; et al. Prevalence, Incidence, and Mortality of Stroke in China: Results from a Nationwide Population-Based Survey of 480 687 Adults. Circulation 2017, 135, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Kyu, H.H.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1859–1922. [Google Scholar] [CrossRef]
- Krishnamurthi, R.V.; Ikeda, T.; Feigin, V.L. Global, Regional and Country-Specific Burden of Ischaemic Stroke, Intracerebral Haemorrhage and Subarachnoid Haemorrhage: A Systematic Analysis of the Global Burden of Disease Study 2017. Neuroepidemiology 2020, 54, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Wu, S.; Wu, B.; Liu, M.; Chen, Z.; Wang, W.; Anderson, C.S.; Sandercock, P.; Wang, Y.; Huang, Y.; Cui, L.; et al. Stroke in China: Advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019, 18, 394–405. [Google Scholar] [CrossRef]
- Xia, X.; Yue, W.; Chao, B.; Li, M.; Cao, L.; Wang, L.; Shen, Y.; Li, X. Prevalence and risk factors of stroke in the elderly in Northern China: Data from the National Stroke Screening Survey. J. Neurol. 2019, 266, 1449–1458. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Zhao, X.; Wang, Y.; Wu, D.; Wang, Y. Stroke care development in Mainland China: Past, present and future. Int. J. Stroke 2008, 3, 288–289. [Google Scholar] [CrossRef]
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Peters, S.A.; Huxley, R.R.; Woodward, M. Diabetes as a risk factor for stroke in women compared with men: A systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet 2014, 383, 1973–1980. [Google Scholar] [CrossRef]
- Sarwar, N.; Gao, P.; Seshasai, S.R.; Gobin, R.; Kaptoge, S.; di Angelantonio, E.; Ingelsson, E.; Lawlor, D.A.; Selvin, E.; Stampfer, M.; et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Saver, J.L.; Hong, K.S.; Song, S.; Chang, K.H.; Ovbiagele, B. Effect of pre-diabetes on future risk of stroke: Meta-analysis. BMJ 2012, 344, e3564. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of medical care in diabetes—2010. Diabetes Care 2010, 33 (Suppl. 1), S11–S61. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Z.; Zhang, M.; Zhu, Y.; Zhao, Z.; Huang, Z.; Li, C.; Zhou, M.; Farmer, A.J.; Tang, J.; et al. Independent effects of 2hPG, FPG and HbA1c on cardiovascular risk: Analysis of a nationally representative sample from China. Diabetes Res. Clin. Pract. 2021, 173, 108672. [Google Scholar] [CrossRef]
- Weykamp, C. HbA1c: A review of analytical and clinical aspects. Ann. Lab. Med. 2013, 33, 393–400. [Google Scholar] [CrossRef]
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef]
- Lin, L.; Wang, A.; He, Y.; Wang, W.; Gao, Z.; Tang, X.; Yan, L.; Wan, Q.; Luo, Z.; Qin, G.; et al. Effects of the hemoglobin glycation index on hyperglycemia diagnosis: Results from the REACTION study. Diabetes Res. Clin. Pract. 2021, 180, 109039. [Google Scholar] [CrossRef]
- Cohen, R.M.; Snieder, H.; Lindsell, C.J.; Beyan, H.; Hawa, M.I.; Blinko, S.; Edwards, R.; Spector, T.D.; Leslie, R.D. Evidence for independent heritability of the glycation gap (glycosylation gap) fraction of HbA1c in nondiabetic twins. Diabetes Care 2006, 29, 1739–1743. [Google Scholar] [CrossRef]
- Hempe, J.M.; Gomez, R.; McCarter, R.J., Jr.; Chalew, S.A. High and low hemoglobin glycation phenotypes in type 1 diabetes: A challenge for interpretation of glycemic control. J. Diabetes Complicat. 2002, 16, 313–320. [Google Scholar] [CrossRef]
- Ahn, C.H.; Min, S.H.; Lee, D.H.; Oh, T.J.; Kim, K.M.; Moon, J.H.; Choi, S.H.; Park, K.S.; Jang, H.C.; Ha, J.; et al. Hemoglobin Glycation Index Is Associated with Cardiovascular Diseases in People with Impaired Glucose Metabolism. J. Clin. Endocrinol. Metab. 2017, 102, 2905–2913. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Jing, J.; Wang, Y.; Liu, L.; Wang, Y.; He, Y. Association of hemoglobin glycation index with outcomes of acute ischemic stroke in type 2 diabetic patients. Neurol. Res. 2018, 40, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, Z.; Zhou, Y.; Yu, S.; Yang, H.; Sun, G.; Zheng, L.; Afzal, J.; Liu, Y.; Sun, Y. The effects of transitions in metabolic health and obesity status on incident cardiovascular disease: Insights from a general Chinese population. Eur. J. Prev. Cardiol. 2021, 28, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Shi, C.; Li, T.; Ouyang, N.; Guo, X.; Chen, Y.; Li, Z.; Zhou, Y.; Yang, H.; Yu, S.; et al. A nomogram integrating non-ECG factors with ECG to screen left ventricular hypertrophy among hypertensive patients from northern China. J. Hypertens. 2022, 40, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Pickering, T.G.; Hall, J.E.; Appel, L.J.; Falkner, B.E.; Graves, J.; Hill, M.N.; Jones, D.W.; Kurtz, T.; Sheps, S.G.; Roccella, E.J. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: A statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 2005, 111, 697–716. [Google Scholar] [CrossRef]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr.; et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA 2003, 289, 2560–2572. [Google Scholar] [CrossRef]
- Chamberlain, J.J.; Rhinehart, A.S.; Shaefer, C.F., Jr.; Neuman, A. Diagnosis and Management of Diabetes: Synopsis of the 2016 American Diabetes Association Standards of Medical Care in Diabetes. Ann. Intern. Med. 2016, 164, 542–552. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- McCarter, R.J.; Hempe, J.M.; Gomez, R.; Chalew, S.A. Biological variation in HbA1c predicts risk of retinopathy and nephropathy in type 1 diabetes. Diabetes Care 2004, 27, 1259–1264. [Google Scholar] [CrossRef]
- Xing, L.; Jing, L.; Tian, Y.; Liu, S.; Lin, M.; Du, Z.; Ren, G.; Sun, Q.; Shi, L.; Dai, D.; et al. High prevalence of stroke and uncontrolled associated risk factors are major public health challenges in rural northeast China: A population-based study. Int. J. Stroke 2020, 15, 399–411. [Google Scholar] [CrossRef]
- Lei, C.; Wu, B.; Liu, M.; Chen, Y. Association between hemoglobin A1C levels and clinical outcome in ischemic stroke patients with or without diabetes. J. Clin. Neurosci. 2015, 22, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, M.; Carlberg, B.; Eliasson, M. The disparity in long-term survival after a first stroke in patients with and without diabetes persists: The Northern Sweden MONICA study. Cerebrovasc. Dis. 2012, 34, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; He, J.; Li, M.; Tang, X.; Hu, R.; Shi, L.; Su, Q.; Peng, K.; Xu, M.; Xu, Y.; et al. Predictive Value of Fasting Glucose, Postload Glucose, and Hemoglobin A(1c) on Risk of Diabetes and Complications in Chinese Adults. Diabetes Care 2019, 42, 1539–1548. [Google Scholar] [CrossRef] [PubMed]
- Hägg, S.; Thorn, L.M.; Forsblom, C.M.; Gordin, D.; Saraheimo, M.; Tolonen, N.; Wadén, J.; Liebkind, R.; Putaala, J.; Tatlisumak, T.; et al. Different risk factor profiles for ischemic and hemorrhagic stroke in type 1 diabetes mellitus. Stroke 2014, 45, 2558–2562. [Google Scholar] [CrossRef] [PubMed]
- Rawshani, A.; Rawshani, A.; Franzén, S.; Sattar, N.; Eliasson, B.; Svensson, A.M.; Zethelius, B.; Miftaraj, M.; McGuire, D.K.; Rosengren, A.; et al. Risk Factors, Mortality, and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2018, 379, 633–644. [Google Scholar] [CrossRef]
- Fiorentino, T.V.; Marini, M.A.; Succurro, E.; Sciacqua, A.; Andreozzi, F.; Perticone, F.; Sesti, G. Elevated hemoglobin glycation index identify non-diabetic individuals at increased risk of kidney dysfunction. Oncotarget 2017, 8, 79576–79586. [Google Scholar] [CrossRef]
- Fiorentino, T.V.; Marini, M.A.; Succurro, E.; Andreozzi, F.; Sciacqua, A.; Hribal, M.L.; Perticone, F.; Sesti, G. Association between hemoglobin glycation index and hepatic steatosis in non-diabetic individuals. Diabetes Res. Clin. Pract. 2017, 134, 53–61. [Google Scholar] [CrossRef]
- Hempe, J.M.; Yang, S.; Liu, S.; Hsia, D.S. Standardizing the haemoglobin glycation index. Endocrinol. Diabetes Metab. 2021, 4, e00299. [Google Scholar] [CrossRef]
- Van Steen, S.C.; Schrieks, I.C.; Hoekstra, J.B.; Lincoff, A.M.; Tardif, J.C.; Mellbin, L.G.; Rydén, L.; Grobbee, D.E.; DeVries, J.H. The haemoglobin glycation index as predictor of diabetes-related complications in the AleCardio trial. Eur. J. Prev. Cardiol. 2017, 24, 858–866. [Google Scholar] [CrossRef]
- Van Steen, S.C.; Woodward, M.; Chalmers, J.; Li, Q.; Marre, M.; Cooper, M.E.; Hamet, P.; Mancia, G.; Colagiuri, S.; Williams, B.; et al. Haemoglobin glycation index and risk for diabetes-related complications in the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial. Diabetologia 2018, 61, 780–789. [Google Scholar] [CrossRef]
- Klein, K.R.; Franek, E.; Marso, S.; Pieber, T.R.; Pratley, R.E.; Gowda, A.; Kvist, K.; Buse, J.B. Hemoglobin glycation index, calculated from a single fasting glucose value, as a prediction tool for severe hypoglycemia and major adverse cardiovascular events in DEVOTE. BMJ Open Diabetes Res. Care 2021, 9, e002339. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Jeong, J.S.; Yun, J.S.; Kwon, H.S.; Baek, K.H.; Song, K.H.; Ahn, Y.B.; Ko, S.H. Hemoglobin glycation index predicts cardiovascular disease in people with type 2 diabetes mellitus: A 10-year longitudinal cohort study. J. Diabetes Complicat. 2018, 32, 906–910. [Google Scholar] [CrossRef] [PubMed]
- Hsia, D.S.; Rasouli, N.; Pittas, A.G.; Lary, C.W.; Peters, A.; Lewis, M.R.; Kashyap, S.R.; Johnson, K.C.; LeBlanc, E.S.; Phillips, L.S.; et al. Implications of the Hemoglobin Glycation Index on the Diagnosis of Prediabetes and Diabetes. J. Clin. Endocrinol. Metab. 2020, 105, e130–e138. [Google Scholar] [CrossRef] [PubMed]
- Hempe, J.M.; Liu, S.; Myers, L.; McCarter, R.J.; Buse, J.B.; Fonseca, V. The hemoglobin glycation index identifies subpopulations with harms or benefits from intensive treatment in the ACCORD trial. Diabetes Care 2015, 38, 1067–1074. [Google Scholar] [CrossRef]
| Variable | Normal Population | DM Patients | p Value | ||
|---|---|---|---|---|---|
| Low HGI n = 1276 | High HGI n = 1344 | Low HGI n = 131 | High HGI n = 183 | ||
| Age (years) | 54.4 ± 10.3 | 56.0 ± 9.9 | 57.9 ± 9.3 | 58.1 ± 8.5 | <0.001 |
| Male | 639 (50.1) | 556 (41.4) | 74 (56.5) | 68 (37.2) | <0.001 |
| Smoking | 465 (36.4) | 483 (35.9) | 58 (44.3) | 57 (31.1) | 0.122 |
| Drinking | 322 (25.2) | 202 (15.0) | 43 (32.8) | 23 (12.6) | <0.001 |
| History of CVD | 189 (14.8) | 219 (16.3) | 38 (29.0) | 52 (28.4) | <0.001 |
| Hypertension | 516 (40.4) | 544 (40.5) | 85 (64.9) | 118 (64.5) | <0.001 |
| BMI (kg/m2) | 24.06 ± 3.51 | 24.30 ± 3.84 | 25.77 ± 3.56 | 25.63 ± 3.68 | <0.001 |
| SBP (mmHg) | 136.28 ± 21.39 | 135.77 ± 22.28 | 148.15 ± 24.34 | 147.81 ± 23.67 | <0.001 |
| DBP (mmHg) | 81.33 ± 11.22 | 80.40 ± 11.74 | 84.01 ± 13.20 | 83.98 ± 11.90 | <0.001 |
| FPG (mmol/L) | 5.61 ± 0.53 | 5.42 ± 0.52 | 9.46 ± 2.92 | 9.08 ± 2.65 | <0.001 |
| TC (mmol/L) | 5.05 ± 1.02 | 5.12 ± 1.04 | 5.27 ± 1.20 | 5.45 ± 1.13 | <0.001 |
| TG (mmol/L) | 1.74 ± 1.66 | 1.67 ± 1.44 | 2.73 ± 2.49 | 2.56 ± 2.32 | <0.001 |
| HDL-C (mmol/L) | 1.32 ± 0.29 | 1.31 ± 0.29 | 1.26 ± 0.29 | 1.26 ± 0.26 | 0.004 |
| LDL-C (mmol/L) | 2.61 ± 0.62 | 2.71 ± 0.68 | 2.67 ± 0.64 | 2.85 ± 0.70 | <0.001 |
| eGFR (mL/min/1.73 m2) | 89.35 ± 13.34 | 87.34 ± 12.57 | 86.18 ± 16.03 | 85.78 ± 13.12 | <0.001 |
| HbA1c (%) | 4.73 ± 0.49 | 5.59 ± 0.38 | 6.11 ± 1.06 | 7.67 ± 1.67 | <0.001 |
| HGI | −0.50 ± 0.38 | 0.43 ± 0.32 | −0.81 ± 0.86 | 0.92 ± 0.87 | <0.001 |
| Variable | Incidence of Stroke | |||
|---|---|---|---|---|
| Crude HRs | Adjusted HRs | |||
| HR (95% CI) | p | HR (95% CI) | p | |
| Continuous | ||||
| per SD of HGI | 1.284 (1.047–1.574) | 0.022 | 1.279 (1.063–1.539) | 0.009 |
| Categorical | ||||
| HGI < 0 | reference | - | reference | - |
| HGI ≥ 0 | 1.977 (1.221–3.205) | 0.020 | 1.780 (1.120–2.830) | 0.015 |
| DM | High HGI | Events/Participants | Incidence of Stroke | |||
|---|---|---|---|---|---|---|
| Crude HRs | Adjusted HRs | |||||
| HR (95% CI) | p | HR (95% CI) | p | |||
| − | − | 21/1276 | Reference | - | Reference | - |
| + | − | 3/131 | 1.601 (0.478–5.368) | 0.446 | 1.138 (0.337–3.847) | 0.835 |
| − | + | 47/1344 | 1.966 (1.173–3.295) | 0.039 | 1.701 (0.337–3.847) | 0.036 |
| + | + | 8/183 | 2.566 (1.128–5.836) | 0.025 | 2.655 (1.251–5.636) | 0.011 |
| Incident Stroke | IDI | NRI |
|---|---|---|
| Conventional model | - | - |
| Conventional model+ HGI | 0.012 (0.007–0.015) | 0.036 (0.0198–0.0522) |
| p value | <0.001 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Li, Q.; Guo, X.; Zhou, Y.; Li, Z.; Yang, H.; Yu, S.; Sun, Y.; Zhang, X. The Value of Hemoglobin Glycation Index–Diabetes Mellitus System in Evaluating and Predicting Incident Stroke in the Chinese Population. J. Clin. Med. 2022, 11, 5814. https://doi.org/10.3390/jcm11195814
Wang P, Li Q, Guo X, Zhou Y, Li Z, Yang H, Yu S, Sun Y, Zhang X. The Value of Hemoglobin Glycation Index–Diabetes Mellitus System in Evaluating and Predicting Incident Stroke in the Chinese Population. Journal of Clinical Medicine. 2022; 11(19):5814. https://doi.org/10.3390/jcm11195814
Chicago/Turabian StyleWang, Pengbo, Qiyu Li, Xiaofan Guo, Ying Zhou, Zhao Li, Hongmei Yang, Shasha Yu, Yingxian Sun, and Xingang Zhang. 2022. "The Value of Hemoglobin Glycation Index–Diabetes Mellitus System in Evaluating and Predicting Incident Stroke in the Chinese Population" Journal of Clinical Medicine 11, no. 19: 5814. https://doi.org/10.3390/jcm11195814
APA StyleWang, P., Li, Q., Guo, X., Zhou, Y., Li, Z., Yang, H., Yu, S., Sun, Y., & Zhang, X. (2022). The Value of Hemoglobin Glycation Index–Diabetes Mellitus System in Evaluating and Predicting Incident Stroke in the Chinese Population. Journal of Clinical Medicine, 11(19), 5814. https://doi.org/10.3390/jcm11195814






