Prevalence and Risk Factor Analysis of Post-Intensive Care Syndrome in Patients with COVID-19 Requiring Mechanical Ventilation: A Multicenter Prospective Observational Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
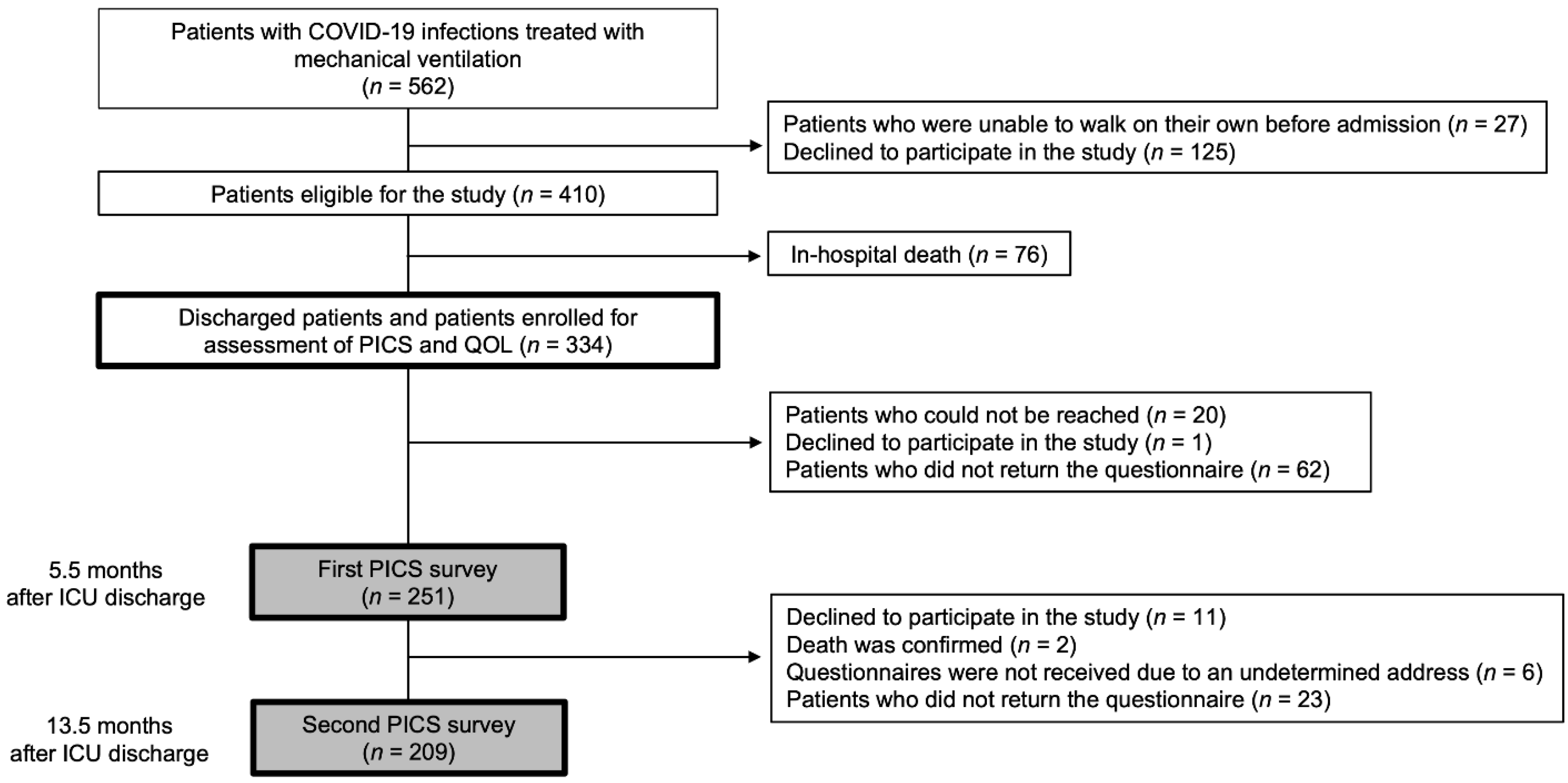
2.2. Study Population and Eligibility Criteria
2.3. Procedures
2.4. Variables and Measurements
2.5. Outcomes
2.6. Statistical Analysis
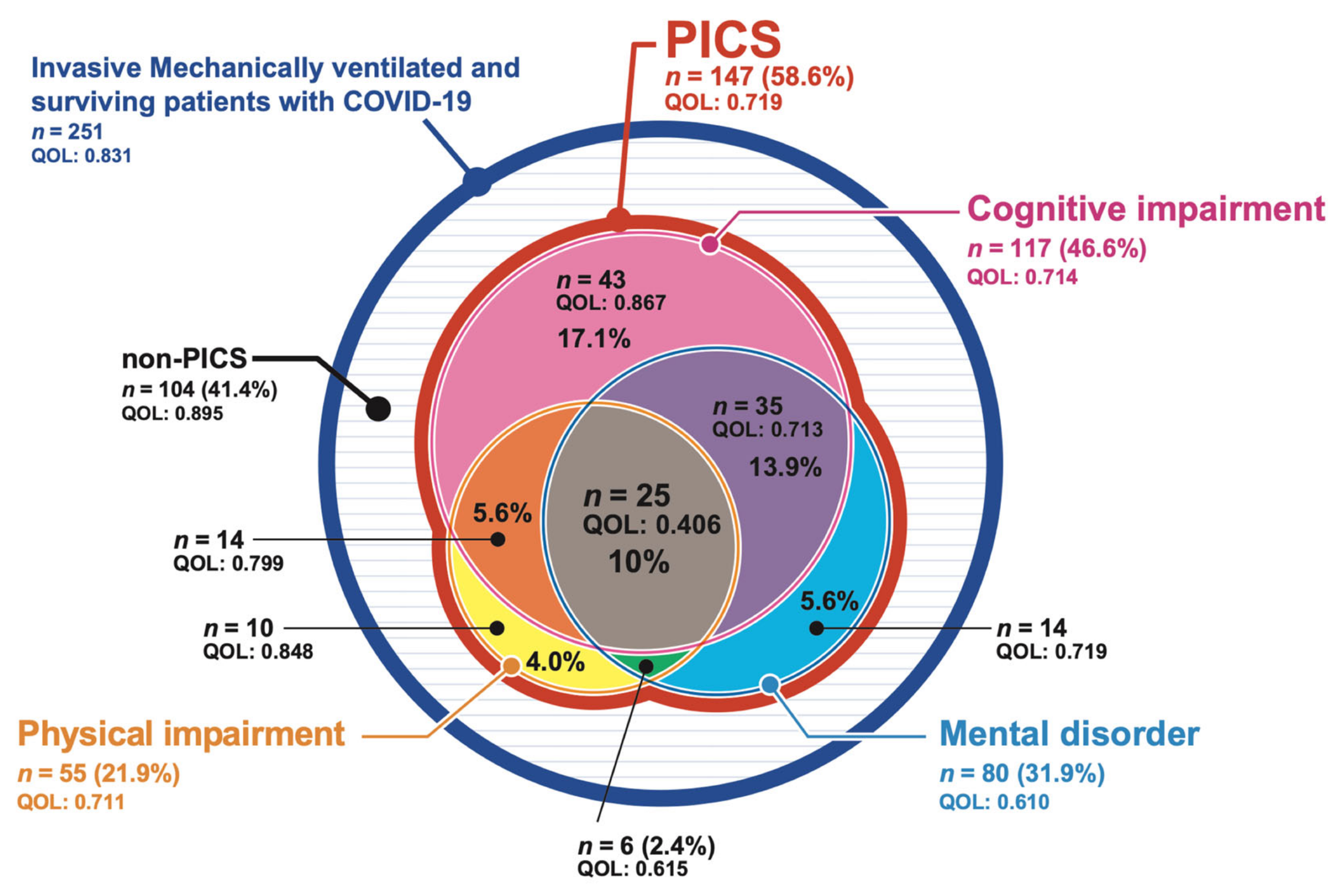
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| BI | Barthel Index |
| BMI | Body mass index |
| CRISIS | Cross ICU Searchable Information System |
| HADS | Hospital Anxiety and Depression Scale |
| ICU | Intensive care unit |
| PICS | Post-intensive care syndrome |
| QOL | Quality of life |
| SMQ | Short-Memory Questionnaire |
| SOFA | Sequential organ failure assessment |
References
- Needham, D.M.; Davidson, J.; Cohen, H.; Hopkins, R.O.; Weinert, C.; Wunsch, H.; Zawistowski, C.; Bemis-Dougherty, A.; Berney, S.C.; Bienvenu, O.J.; et al. Improving long-term outcomes after discharge from intensive care unit: Report from a stakeholders’ conference. Crit. Care Med. 2012, 40, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Myers, E.A.; Smith, D.A.; Allen, S.R.; Kaplan, L.J. Post-ICU syndrome: Rescuing the undiagnosed. JAAPA 2016, 29, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, M.E.; Shull, W.H.; Biester, R.C.; Taichman, D.B.; Lynch, S.; Demissie, E.; Hansen-Flaschen, J.; Christie, J.D. Cognitive, mood and quality of life impairments in a select population of ARDS survivors. Respirology 2009, 14, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, R.O.; Weaver, L.K.; Collingridge, D.; Parkinson, R.B.; Chan, K.J.; Orme, J.F., Jr. Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2005, 171, 340–347. [Google Scholar] [CrossRef]
- Harvey, M.A.; Davidson, J.E. Postintensive care syndrome: Right care, right now…and later. Crit. Care Med. 2016, 44, 381–385. [Google Scholar] [CrossRef]
- Kaukonen, K.M.; Bailey, M.; Suzuki, S.; Pilcher, D.; Bellomo, R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA 2014, 311, 1308–1316. [Google Scholar] [CrossRef]
- Yende, S.; Austin, S.; Rhodes, A.; Finfer, S.; Opal, S.; Thompson, T.; Bozza, F.A.; LaRosa, S.P.; Ranieri, V.M.; Angus, D.C. Long-term quality of life among survivors of severe sepsis: Analyses of two international trials. Crit. Care Med. 2016, 44, 1461–1467. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; the Northwell COVID-19 Research Consortium; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the coronavirus Disease 2019 (COVID-19) outbreak in China: Summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Al-Aly, Z.; Xie, Y.; Bowe, B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 2021, 594, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Groff, D.; Sun, A.; Ssentongo, A.E.; Ba, D.M.; Parsons, N.; Poudel, G.R.; Lekoubou, A.; Oh, J.S.; Ericson, J.E.; Ssentongo, P.; et al. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: A systematic review. JAMA Netw. Open 2021, 4, e2128568. [Google Scholar] [CrossRef] [PubMed]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, A.F.; Minguet, P.; Colson, C.; Kellens, I.; Chaabane, S.; Delanaye, P.; Cavalier, E.; Chase, J.G.; Lambermont, B.; Misset, B. Post-intensive care syndrome after a critical COVID-19: Cohort study from a Belgian follow-up clinic. Ann. Intensive Care 2021, 11, 118. [Google Scholar] [CrossRef]
- Martillo, M.A.; Dangayach, N.S.; Tabacof, L.; Spielman, L.A.; Dams-O’Connor, K.; Chan, C.C.; Kohli-Seth, R.; Cortes, M.; Escalon, M.X. Postintensive care syndrome in survivors of critical illness related to coronavirus Disease 2019: Cohort study from a New York City Critical Care Recovery Clinic. Crit. Care Med. 2021, 49, 1427–1438. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Writing Committee for the COMEBAC Study Group; Morin, L.; Savale, L.; Pham, T.; Colle, R.; Figueiredo, S.; Harrois, A.; Gasnier, M.; Lecoq, A.L.; Meyrignac, O.; et al. Four-Month Clinical Status of a Cohort of Patients After Hospitalization for COVID-19. JAMA 2021, 325, 1525–1534. [Google Scholar] [CrossRef]
- Bellan, M.; Soddu, D.; Balbo, P.E.; Baricich, A.; Zeppegno, P.; Avanzi, G.C.; Baldon, G.; Bartolomei, G.; Battaglia, M.; Battistini, S.; et al. Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge. JAMA Netw. Open 2021, 4, e2036142. [Google Scholar] [CrossRef]
- Janiri, D.; Carfì, A.; Kotzalidis, G.D.; Bernabei, R.; Landi, F.; Sani, G.; Gemelli Against COVID-19 Post-Acute Care Study Group. Posttraumatic stress disorder in patients after severe COVID-19 infection. JAMA Psychiatry 2021, 78, 567–569. [Google Scholar] [CrossRef]
- Mongodi, S.; Salve, G.; Tavazzi, G.; Politi, P.; Mojoli, F.; COVID-19 Post-ICU team; COVID-19 Pavia Crisis Unit. High prevalence of acute stress disorder and persisting symptoms in ICU survivors after COVID-19. Intensive Care Med. 2021, 47, 616–618. [Google Scholar] [CrossRef]
- Japan ECMOnet for COVID-19; Shime, N. Save the ICU and save lives during the COVID-19 pandemic. J. Intensive Care 2020, 8, 40. [Google Scholar] [PubMed]
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The Barthel index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar] [PubMed]
- Katz, S.; Ford, A.B.; Moskowitz, R.W.; Jackson, B.A.; Jaffe, M.W. Studies of illness in the aged. The index of ADL: A standardized measure of biological and psychosocial function. JAMA 1963, 185, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Jorm, A.F. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): A review. Int. Psychogeriatr. 2004, 16, 275–293. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Herdman, M.; Gudex, C.; Lloyd, A.; Janssen, M.; Kind, P.; Parkin, D.; Bonsel, G.; Badia, X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual. Life Res. 2011, 20, 1727–1736. [Google Scholar] [CrossRef]
- Shah, S.; Vanclay, F.; Cooper, B. Improving the sensitivity of the Barthel index for stroke rehabilitation. J. Clin. Epidemiol. 1989, 42, 703–709. [Google Scholar] [CrossRef]
- Koss, E.; Patterson, M.B.; Ownby, R.; Stuckey, J.C.; Whitehouse, P.J. Memory evaluation in Alzheimer’s disease. Caregivers’ appraisals and objective testing. Arch. Neurol. 1993, 50, 92–97. [Google Scholar] [CrossRef]
- Nikayin, S.; Rabiee, A.; Hashem, M.D.; Huang, M.; Bienvenu, O.J.; Turnbull, A.E.; Needham, D.M. Anxiety symptoms in survivors of critical illness: A systematic review and meta-analysis. Gen. Hosp. Psychiatry 2016, 43, 23–29. [Google Scholar] [CrossRef]
- Rabiee, A.; Nikayin, S.; Hashem, M.D.; Huang, M.; Dinglas, V.D.; Bienvenu, O.J.; Turnbull, A.E.; Needham, D.M. Depressive symptoms after critical illness: A systematic review and meta-analysis. Crit. Care Med. 2016, 44, 1744–1753. [Google Scholar] [CrossRef]
- Davydow, D.S.; Hough, C.L.; Langa, K.M.; Iwashyna, T.J. Presepsis depressive symptoms are associated with incidentcognitive impairment in survivors of severe sepsis: A prospective cohort study of older Americans. J. Am. Geriatr. Soc. 2012, 60, 2290–2296. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Li, Z.; Jiang, L.; Wang, Y.; Xi, X. Risk factors for intensive care unit-acquired weakness: A systematic review and meta-analysis. Acta Neurol. Scand. 2018, 138, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Eastment, M.C.; Berry, K.; Locke, E.; Green, P.; O’Hare, A.; Crothers, K.; Dominitz, J.A.; Fan, V.S.; Shah, J.A.; Ioannou, G.N. BMI and outcomes of SARS-CoV-2 among US veterans. Obesity 2021, 29, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, A.; Nam, H.; Yeh, C.; Lee, J.; Liebovitz, D.; Achenbach, C. Is BMI higher in younger patients with COVID-19? Association between BMI and COVID-19 hospitalization by age. Obesity 2020, 28, 1811–1814. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, M.E.; Still, M.; Anderson, B.J.; Bienvenu, O.J.; Brodsky, M.B.; Brummel, N.; Butcher, B.; Clay, A.S.; Felt, H.; Ferrante, L.E.; et al. Society of Critical Care Medicine’s international consensus conference on prediction and identification of long-term impairments after critical illness. Crit. Care Med. 2020, 48, 1670–1679. [Google Scholar] [CrossRef]
- Pandharipande, P.P.; Girard, T.D.; Jackson, J.C.; Morandi, A.; Thompson, J.L.; Pun, B.T.; Brummel, N.E.; Hughes, C.G.; Vasilevskis, E.E.; Shintani, A.K.; et al. Long-term cognitive impairment after critical illness. N. Engl. J. Med. 2013, 369, 1306–1316. [Google Scholar] [CrossRef]
- Marsh, L.C.; Leach, R.M.; Blane, J.; Daly, K.; Barrett, N.A.; Slack, A.; Kopelman, M.D. Long-term cognitive and psychiatric outcomes of acute respiratory distress syndrome managed with Extracorporeal Membrane Oxygenation. Respir. Med. 2021, 183, 106419. [Google Scholar] [CrossRef]
- De Jonghe, B.; Sharshar, T.; Lefaucheur, J.P.; Authier, F.J.; Durand-Zaleski, I.; Boussarsar, M.; Cerf, C.; Renaud, E.; Mesrati, F.; Carlet, J.; et al. Paresis acquired in the intensive care unit: A prospective multicenter study. JAMA 2002, 288, 2859–2867. [Google Scholar] [CrossRef]
- Le, M.Q.; Rosales, R.; Shapiro, L.T.; Huang, L.Y. The down side of prone positioning: The case of a coronavirus 2019 survivor. Am. J. Phys. Med. Rehabilit. 2020, 99, 870–872. [Google Scholar] [CrossRef]
- Fuke, R.; Hifumi, T.; Kondo, Y.; Hatakeyama, J.; Takei, T.; Yamakawa, K.; Inoue, S.; Nishida, O. Early rehabilitation to prevent postintensive care syndrome in patients with critical illness: A systematic review and meta-analysis. BMJ Open 2018, 8, e019998. [Google Scholar] [CrossRef] [Green Version]
- Desai, S.V.; Law, T.J.; Needham, D.M. Long-term complications of critical care. Crit. Care Med. 2011, 39, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Davydow, D.S.; Gifford, J.M.; Desai, S.V.; Needham, D.M.; Bienvenu, O.J. Posttraumatic stress disorder in general intensive care unit survivors: A systematic review. Gen. Hosp. Psychiatry 2008, 30, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Marra, A.; Pandharipande, P.P.; Girard, T.D.; Patel, M.B.; Hughes, C.G.; Jackson, J.C.; Thompson, J.L.; Chandrasekhar, R.; Ely, E.W.; Brummel, N.E. Co-occurrence of post-intensive care syndrome problems among 406 survivors of critical illness. Crit. Care Med. 2018, 46, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, D.; Fujitani, S.; Morimoto, T.; Dote, H.; Takita, M.; Takaba, A.; Hino, M.; Nakamura, M.; Irie, H.; Adachi, T.; et al. Prevalence of post-intensive care syndrome among Japanese intensive care unit patients: A prospective, multicenter, observational J-PICS study. Crit. Care 2021, 25, 69. [Google Scholar] [CrossRef]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef]
- Al-Ramadan, A.; Rabab’h, O.; Shah, J.; Gharaibeh, A. Acute and post-acute neurological complications of COVID-19. Neurol. Int. 2021, 13, 102–119. [Google Scholar] [CrossRef] [PubMed]
- Ellul, M.A.; Benjamin, L.; Singh, B.; Lant, S.; Michael, B.D.; Easton, A.; Kneen, R.; Defres, S.; Sejvar, J.; Solomon, T. Neurological associations of COVID-19. Lancet Neurol. 2020, 19, 767–783. [Google Scholar] [CrossRef]
- Troyer, E.A.; Kohn, J.N.; Hong, S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav. Immun. 2020, 87, 34–39. [Google Scholar] [CrossRef]
- Wilcox, M.E.; Shankar-Hari, M.; McAuley, D.F. Delirium in COVID-19: Can we make the unknowns knowns? Intensive Care Med. 2021, 47, 1144–1147. [Google Scholar] [CrossRef]
- Ahmad, I.; Rathore, F.A. Neurological manifestations and complications of COVID-19: A literature review. J. Clin. Neurosci. 2020, 77, 8–12. [Google Scholar] [CrossRef]
- Liu, K.; Nakamura, K.; Katsukawa, H.; Nydahl, P.; Ely, E.W.; Kudchadkar, S.R.; Takahashi, K.; Elhadi, M.; Gurjar, M.; Leong, B.K.; et al. Implementation of the ABCDEF bundle for critically ill ICU patients during the COVID-19 pandemic: A multi-national 1-day point prevalence study. Front. Med. 2021, 8, 735860. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Nakamura, K.; Katsukawa, H.; Elhadi, M.; Nydahl, P.; Ely, E.W.; Kudchadkar, S.R.; Takahashi, K.; Inoue, S.; Lefor, A.K.; et al. ABCDEF bundle and supportive ICU practices for patients with coronavirus disease 2019 infection: An international point prevalence study. Crit. Care Explor. 2021, 3, e0353. [Google Scholar] [CrossRef] [PubMed]
- Pun, B.T.; Balas, M.C.; Barnes-Daly, M.A.; Thompson, J.L.; Aldrich, J.M.; Barr, J.; Byrum, D.; Carson, S.S.; Devlin, J.W.; Engel, H.J.; et al. Caring for critically ill patients with the ABCDEF bundle: Results of the ICU liberation collaborative in over 15,000 adults. Crit. Care Med. 2019, 47, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Gentile, L.F.; Cuenca, A.G.; Efron, P.A.; Ang, D.; Bihorac, A.; McKinley, B.A.; Moldawer, L.L.; Moore, F.A. Persistent inflammation and immunosuppression: A common syndrome and new horizon for surgical intensive care. J. Trauma Acute Care Surg. 2012, 72, 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Mira, J.C.; Gentile, L.F.; Mathias, B.J.; Efron, P.A.; Brakenridge, S.C.; Mohr, A.M.; Moore, F.A.; Moldawer, L.L. Sepsis pathophysiology, chronic critical illness, and persistent inflammation-immunosuppression and catabolism syndrome. Crit. Care Med. 2017, 45, 253–262. [Google Scholar] [CrossRef]
- Nakamura, K.; Ogura, K.; Nakano, H.; Naraba, H.; Takahashi, Y.; Sonoo, T.; Hashimoto, H.; Morimura, N. C-reactive protein clustering to clarify persistent inflammation, immunosuppression and catabolism syndrome. Intensive Care Med. 2020, 46, 437–443. [Google Scholar] [CrossRef]
- Efron, P.A.; Mohr, A.M.; Bihorac, A.; Horiguchi, H.; Hollen, M.K.; Segal, M.S.; Baker, H.V.; Leeuwenburgh, C.; Moldawer, L.L.; Moore, F.A.; et al. Persistent inflammation, immunosuppression, and catabolism and the development of chronic critical illness after surgery. Surgery 2018, 164, 178–184. [Google Scholar] [CrossRef]
- Chelluri, L.; Im, K.A.; Belle, S.H.; Schulz, R.; Rotondi, A.J.; Donahoe, M.P.; Sirio, C.A.; Mendelsohn, A.B.; Pinsky, M.R. Long-term mortality and quality of life after prolonged mechanical ventilation. Crit. Care Med. 2004, 32, 61–69. [Google Scholar] [CrossRef]
- Huang, M.; Parker, A.M.; Bienvenu, O.J.; Dinglas, V.D.; Colantuoni, E.; Hopkins, R.O.; Needham, D.M.; National Institutes of Health, National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network. Psychiatric symptoms in acute respiratory distress syndrome survivors: A 1-year national multicenter study. Crit. Care Med. 2016, 44, 954–965. [Google Scholar] [CrossRef]
- Patel, M.B.; Jackson, J.C.; Morandi, A.; Girard, T.D.; Hughes, C.G.; Thompson, J.L.; Kiehl, A.L.; Elstad, M.R.; Wasserstein, M.L.; Goodman, R.B.; et al. Incidence and risk factors for Intensive Care Unit-related post-traumatic stress disorder in veterans and civilians. Am. J. Respir. Crit. Care Med. 2016, 193, 1373–1381. [Google Scholar] [CrossRef] [Green Version]
- Hopkins, R.O.; Key, C.W.; Suchyta, M.R.; Weaver, L.K.; Orme, J.F., Jr. Risk factors for depression and anxiety in survivors of acute respiratory distress syndrome. Gen. Hosp. Psychiatry 2010, 32, 147–155. [Google Scholar] [CrossRef]
- Xie, H.; Huang, X.; Zhang, Q.; Wei, Y.; Zeng, X.; Chang, F.; Wu, S. The prevalence of and factors associated with anxiety and depression among working-age adults in Mainland China at the early remission stage of the coronavirus 2019 pandemic. Front. Psychol. 2022, 13, 839852. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, Y.; Huang, Y.M.; Wang, M.; Ling, W.; Sui, Y.; Zhao, H.L. Obesity in patients with COVID-19: A systematic review and meta-analysis. Metabolism 2020, 113, 154378. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Mahawar, K.; Xia, Z.; Yang, W.; El-Hasani, S. Obesity and mortality of COVID-19. Meta-analysis. Obes. Res. Clin. Pract. 2020, 14, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Manolis, A.S.; Manolis, A.A.; Manolis, T.A.; Apostolaki, N.E.; Melita, H. COVID-19 infection and body weight: A deleterious liaison in a J-curve relationship. Obes. Res. Clin. Pract. 2021, 15, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Safar Zadeh, E.; Lungu, A.O.; Cochran, E.K.; Brown, R.J.; Ghany, M.G.; Heller, T.; Kleiner, D.E.; Gorden, P. The liver diseases of lipodystrophy: The long-term effect of leptin treatment. J. Hepatol. 2013, 59, 131–137. [Google Scholar] [CrossRef]
- Bagshaw, S.M.; Stelfox, H.T.; Johnson, J.A.; McDermid, R.C.; Rolfson, D.B.; Tsuyuki, R.T.; Ibrahim, Q.; Majumdar, S.R. Long-term association between frailty and health-related quality of life among survivors of critical illness: A prospective multicenter cohort study. Crit. Care Med. 2015, 43, 973–982. [Google Scholar] [CrossRef]
- Eeles, E.M.; White, S.V.; O’Mahony, S.M.; Bayer, A.J.; Hubbard, R.E. The impact of frailty and delirium on mortality in older inpatients. Age Ageing 2012, 41, 412–416. [Google Scholar] [CrossRef] [Green Version]




| First PICS Survey (5.5 ± 3.1 Months after ICU Discharge) | |||
|---|---|---|---|
| PICS (n = 147) | Non-PICS (n = 104) | p Value | |
| Age, yr, median (IQR) | 68 (60, 75) | 65 (56.3, 72) | 0.010 |
| Male, n (%) | 114 (77.6) | 86 (82.7) | 0.32 |
| BMI, kg/m2, median (IQR) | 24.7 (22.0, 28.4) | 25.9 (23.3, 29.0) | 0.040 |
| SOFA score on the day of ventilation start, median (IQR) | 5 (4, 7) | 5 (4, 7) | 0.73 |
| Clinical frailty scale before hospitalization, median (IQR) | 2 (1, 3) | 2 (1, 2) | 0.27 |
| Delirium, n (%) | 36 (24.5) | 14 (13.5) | 0.030 |
| Duration of delirium within 1 week of ICU admission, day, median (IQR) | 2.5 (1, 5) | 2 (1, 3) | 0.30 |
| Duration of invasive mechanical ventilation, day, median (IQR) | 10 (6, 17) | 8 (6, 13) | 0.0030 |
| Length of ICU stay, day, median (IQR) | 13 (8, 21) | 10 (8, 17) | 0.010 |
| Length of hospital stay, day, median (IQR) | 26 (15, 51) | 20 (10, 32) | 0.0030 |
| Comorbidity, n (%) | |||
| Hypertension | 62 (42.2) | 52 (50) | 0.22 |
| Diabetes | 49 (33.3) | 31 (29.8) | 0.56 |
| Cardiac disease | 13 (8.8) | 13 (12.5) | 0.35 |
| Chronic kidney disease | 3 (2.0) | 4 (3.9) | 0.32 |
| Autoimmune diseases | 7 (4.8) | 2 (1.9) | 0.20 |
| Malignant tumors | 7 (4.8) | 7 (6.7) | 0.50 |
| COPD | 12 (8.2) | 9 (8.7) | 0.89 |
| Immunodeficiency | 5 (3.4) | 3 (2.9) | 0.56 |
| Treatment received during hospital stay | |||
| Reintubation, n (%) | 9 (6.1) | 1 (1.0) | 0.040 |
| ECMO, n (%) | 17 (11.6) | 16 (15.4) | 0.38 |
| Duration of ECMO, day, median (IQR) | 14 (9, 18) | 10.5 (9, 17) | 0.79 |
| Tracheostomy, n (%) | 35 (23.8) | 16 (15.4) | 0.10 |
| Corticosteroid, n (%) | 118 (80.3) | 76 (73.1) | 0.18 |
| Maximum prednisolone dose, mg/day, median (IQR) | 44 (30, 100) | 40 (0, 75) | 0.030 |
| Continuous neuromuscular blocking agent, n (%) | 63 (42.9) | 49 (47.1) | 0.50 |
| Prone position, n (%) | 82 (55.8) | 50 (48.1) | 0.23 |
| Continuous renal replacement therapy, n (%) | 12 (8.2) | 7 (6.7) | 0.67 |
| Rehabilitation program, n (%) | 83 (56.5) | 57 (54.8) | 0.80 |
| Time from ICU admission to rehabilitation program initiation, day, median (IQR) | 5 (2, 20) | 4 (2.5, 12) | 0.37 |
| Second PICS Survey (13.5 ± 3.2 Months after ICU Discharge) | |||
|---|---|---|---|
| PICS (n = 127) | Non-PICS (n = 82) | p Value | |
| Age, yr, median (IQR) | 68 (60, 75) | 66 (56, 73.3) | 0.12 |
| Male, n (%) | 98 (77.2) | 69 (84.1) | 0.22 |
| BMI, kg/m2, median (IQR) | 24.7 (22, 27.8) | 25.9 (23.1, 29.1) | 0.049 |
| SOFA score on the day of ventilation start, median (IQR) | 5 (4, 7) | 4.5 (3, 7) | 0.26 |
| Clinical frailty scale before hospitalization, median (IQR) | 2 (1, 3) | 1 (1, 2) | 0.26 |
| Delirium, n (%) | 25 (19.7) | 13 (15.9) | 0.48 |
| Duration of delirium within 1 week of ICU admission, day, median (IQR) | 2 (1, 4) | 2 (2, 4) | 0.58 |
| Duration of invasive mechanical ventilation, day, median (IQR) | 9 (6, 17) | 9 (6, 14) | 0.53 |
| Length of ICU stay, day, median (IQR) | 11 (8, 21) | 11 (8, 17) | 0.39 |
| Length of hospital stay, day, median (IQR) | 23 (14, 43) | 20 (11, 35) | 0.13 |
| Comorbidity, n (%) | |||
| Hypertension | 56 (44.1) | 40 (48.8) | 0.51 |
| Diabetes | 32 (25.2) | 29 (35.4) | 0.11 |
| Cardiac disease | 9 (7.1) | 9 (11) | 0.33 |
| Chronic kidney disease | 4 (3.1) | 2 (2.4) | 0.56 |
| Autoimmune diseases | 5 (3.9) | 2 (2.4) | 0.44 |
| Malignant tumors | 6 (4.7) | 4 (4.9) | 0.60 |
| COPD | 10 (7.9) | 7 (8.5) | 0.86 |
| Immunodeficiency | 4 (3.1) | 1 (1.2) | 0.35 |
| Treatment received during hospital stay | |||
| Reintubation, n (%) | 7 (5.5) | 2 (2.4) | 0.24 |
| ECMO, n (%) | 12 (9.4) | 13 (15.9) | 0.16 |
| Duration of ECMO, day, median (IQR) | 14 (8.3, 23.3) | 10 (9, 14.5) | 0.57 |
| Tracheostomy, n (%) | 24 (18.9) | 15 (18.3) | 0.91 |
| Corticosteroid, n (%) | 102 (80.3) | 62 (75.6) | 0.42 |
| Maximum prednisolone dose, mg/day, median (IQR) | 44 (30, 100) | 42.6 (15, 82.5) | 0.20 |
| Continuous neuromuscular blocking agent, n (%) | 54 (42.5) | 39 (47.6) | 0.47 |
| Prone position, n (%) | 70 (55.1) | 43 (52.4) | 0.70 |
| Continuous renal replacement therapy, n (%) | 13 (10.2) | 5 (6.1) | 0.30 |
| Rehabilitation program, n (%) | 72 (56.7) | 42 (51.2) | 0.44 |
| Time from ICU admission to rehabilitation program initiation, day, median (IQR) | 6 (2, 16) | 4 (2, 19.3) | 0.60 |
| First PICS Survey Assessment of PICS (n = 251) | Second PICS Survey Assessment of PICS (n = 209) | |
|---|---|---|
| Dyspnea, n (%) | 118 (47.0) | 96 (45.9) |
| Walking difficulty, n (%) | 89 (35.7) | 54 (25.8) |
| Weight loss, n (%) | 154 (61.4) | 48 (23.0) |
| Memory impairment, n (%) | 74 (29.7) | 66 (31.6) |
| Executive dysfunction, n (%) | 120 (47.8) | 93 (44.5) |
| Depression, n (%) | 103 (41.0) | 81 (38.8) |
| Anxiety, n (%) | 144 (57.4) | 107 (51.2) |
| Sleeping disorder, n (%) | 113 (45.0) | 92 (44.0) |
| Visual analog scale, median (IQR) | ||
| Physical condition (on a scale of 1 to 10) | 7.3 (5.5, 8.5) | 7.4 (6.2, 8.7) |
| Cognitive function (on a scale of 1 to 10) | 9 (7.4, 9.9) | 8.6 (7.1, 9.9) |
| Mental health (on a scale of 1 to 10) | 8.3 (6.0, 9.5) | 8 (6.5, 9.4) |
| Barthel Index, median (IQR) | 100 (95, 100) | 100 (95, 100) |
| Short-Memory Questionnaire, median (IQR) | 40 (34.8, 44) | 39 (34, 43) |
| HADS score, median (IQR) | 8 (3.8, 14) | 7 (3, 14) |
| HADS-Anxiety score | 4 (1, 7) | 3 (1, 7) |
| HADS-Depression score | 4 (1, 7) | 4 (1, 7) |
| EQ-5D-5L, median (IQR) | 0.831 (0.710, 1) | 0.844 (0.759, 1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hatakeyama, J.; Inoue, S.; Liu, K.; Yamakawa, K.; Nishida, T.; Ohshimo, S.; Hashimoto, S.; Kanda, N.; Maruyama, S.; Ogata, Y.; et al. Prevalence and Risk Factor Analysis of Post-Intensive Care Syndrome in Patients with COVID-19 Requiring Mechanical Ventilation: A Multicenter Prospective Observational Study. J. Clin. Med. 2022, 11, 5758. https://doi.org/10.3390/jcm11195758
Hatakeyama J, Inoue S, Liu K, Yamakawa K, Nishida T, Ohshimo S, Hashimoto S, Kanda N, Maruyama S, Ogata Y, et al. Prevalence and Risk Factor Analysis of Post-Intensive Care Syndrome in Patients with COVID-19 Requiring Mechanical Ventilation: A Multicenter Prospective Observational Study. Journal of Clinical Medicine. 2022; 11(19):5758. https://doi.org/10.3390/jcm11195758
Chicago/Turabian StyleHatakeyama, Junji, Shigeaki Inoue, Keibun Liu, Kazuma Yamakawa, Takeshi Nishida, Shinichiro Ohshimo, Satoru Hashimoto, Naoki Kanda, Shuhei Maruyama, Yoshitaka Ogata, and et al. 2022. "Prevalence and Risk Factor Analysis of Post-Intensive Care Syndrome in Patients with COVID-19 Requiring Mechanical Ventilation: A Multicenter Prospective Observational Study" Journal of Clinical Medicine 11, no. 19: 5758. https://doi.org/10.3390/jcm11195758







