Transcriptomic Analysis of Conserved Telomere Maintenance Component 1 (CTC1) and Its Association with Leukemia
Abstract
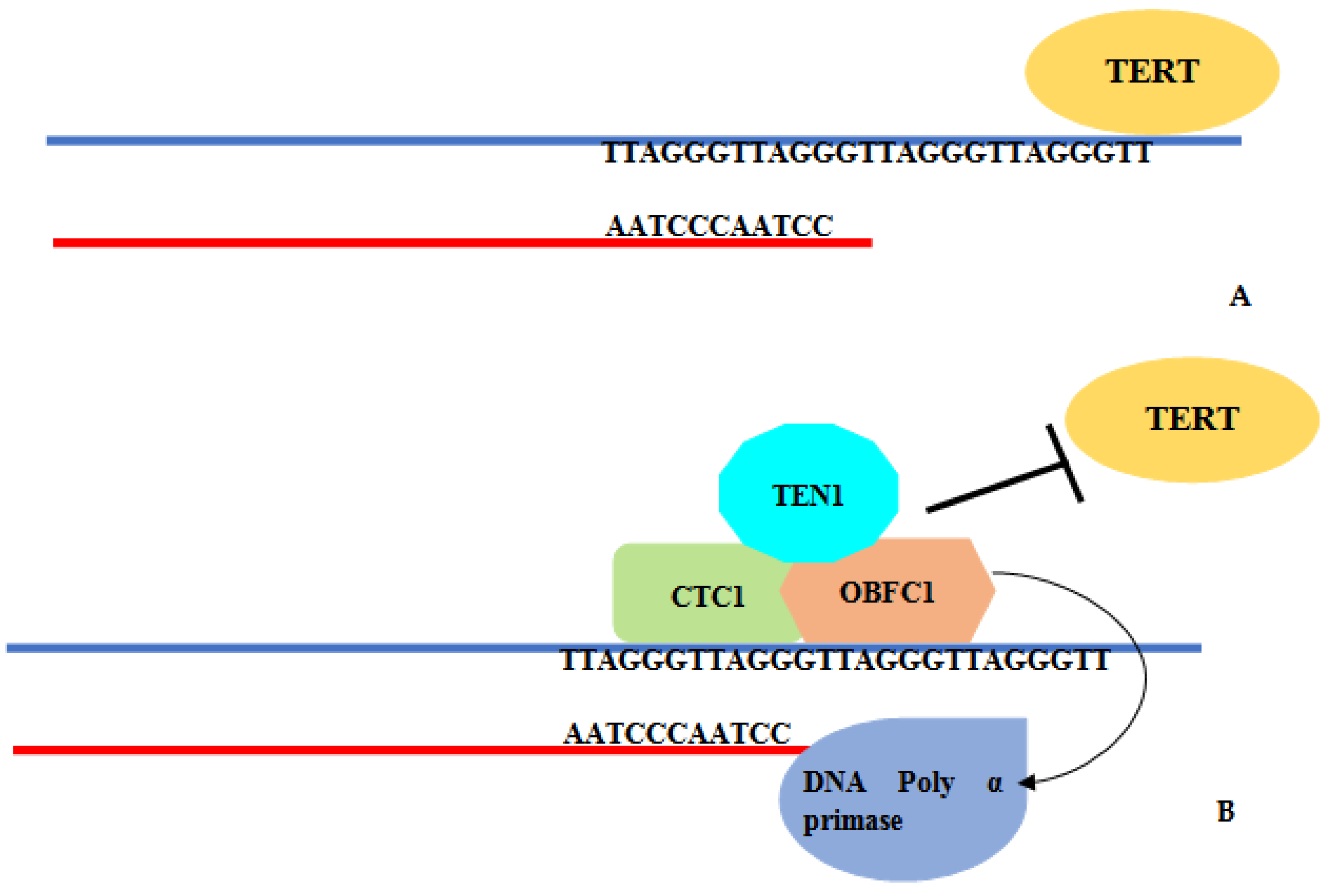
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. DNA and RNA Extraction
2.3. Telomere Length Measurement
2.4. Expression Analysis of CTC1, OBFC1, and TERT by qPCR
2.5. Statistical Analysis
3. Results
3.1. Cohort Description
3.2. Telomere Length Maintenance in ALL Cases
3.3. Elevated Expression of Telomere Modulating Genes (CTC1, OBFC1 and TERT) in ALL Cases

3.4. Telomere Modulating Genes Expression and ALL Immunophenotypes
3.5. Correlation among the Telomere Modulating Genes
3.6. Association of Genes Expression with ALL
3.7. Association of TEL with Leukemia
3.8. Association of Genes Expression with ALL Immunophenotypes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jebaraj, B.M.C.; Stilgenbauer, S. Telomere Dysfunction in Chronic Lymphocytic Leukemia. Front. Oncol. 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Innao, V.; Penna, G.; Gerace, D.; Allegra, A.G.; Musolino, C. Telomerase and telomere biology in hematological diseases: A new therapeutic target. Leuk. Res. 2017, 56, 60–74. [Google Scholar] [CrossRef] [PubMed]
- de Lange, T. Shelterin-mediated telomere protection. Annu. Rev. Genet. 2018, 52, 223–247. [Google Scholar] [CrossRef] [PubMed]
- Tardat, M.; Déjardin, J. Telomere chromatin establishment and its maintenance during mammalian development. Chromosoma 2018, 127, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Jafri, M.A.; Ansari, S.A.; Alqahtani, M.H.; Shay, J.W. Roles of telomeres and telomerase in cancer, and advances in telomerase-targeted therapies. Genome Med. 2016, 8, 69. [Google Scholar] [CrossRef]
- Ghimire, S.; Hill, C.V.; Sy, F.S.; Rodriguez, R. Decline in telomere length by age and effect modification by gender, allostatic load and comorbidities in National Health and Nutrition Examination Survey (1999–2002). PLoS ONE 2019, 14, e0221690. [Google Scholar] [CrossRef]
- Lin, J.; Cheon, J.; Brown, R.; Coccia, M.; Puterman, E.; Aschbacher, K.; Blackburn, E.H. Systematic and cell type-specific telomere length changes in subsets of lymphocytes. J. Immunol. Res. 2016, 2016, 5371050. [Google Scholar] [CrossRef]
- Ackermann, S.; Fischer, M. Telomere Maintenance in Pediatric Cancer. Int. J. Mol. Sci. 2019, 20, 5836. [Google Scholar] [CrossRef]
- Trybek, T.; Kowalik, A.; Góźdź, S.; Kowalska, A. Telomeres and telomerase in oncogenesis (Review). Oncol. Lett. 2020, 20, 1015–1027. [Google Scholar] [CrossRef]
- Nogueira, B.M.D.; Machado, C.B.; Montenegro, R.C.; De Moraes, M.E.A.; Moreira-Nunes, C.A. Telomere Length and Hematological Disorders: A Review. In Vivo 2020, 34, 3093–3101. [Google Scholar] [CrossRef]
- Li, Y.; Tergaonkar, V. Telomerase reactivation in cancers: Mechanisms that govern transcriptional activation of the wild-type vs. mutant TERT promoters. Transcription 2016, 7, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Tomita, K. How long does telomerase extend telomeres? Regulation of telomerase release and telomere length homeostasis. Curr. Genet. 2018, 64, 1177–1181. [Google Scholar] [CrossRef] [PubMed]
- Maciejowski, J.; De Lange, T. Telomeres in cancer: Tumour suppression and genome instability. Nat. Rev. Mol. Cell Biol. 2017, 18, 175–186. [Google Scholar] [CrossRef]
- Shastrula, P.K.; Rice, C.T.; Wang, Z.; Lieberman, P.M.; Skordalakes, E. Structural and functional analysis of an OB-fold in human Ctc1 implicated in telomere maintenance and bone marrow syndromes. Nucleic Acids Res. 2018, 46, 972–984. [Google Scholar] [CrossRef] [PubMed]
- Barazas, M.; Annunziato, S.; Pettitt, S.J.; de Krijger, I.; Ghezraoui, H.; Roobol, S.J.; Lutz, C.; Frankum, J.; Song, F.F.; Brough, R.; et al. The CST Complex Mediates End Protection at Double-Strand Breaks and Promotes PARP Inhibitor Sensitivity in BRCA1-Deficient Cells. Cell Rep. 2018, 23, 2107–2118. [Google Scholar] [CrossRef]
- Huang, C.; Dai, X.; Chai, W. Human Stn1 protects telomere integrity by promoting efficient lagging-strand synthesis at telomeres and mediating C-strand fill-in. Cell Res. 2012, 22, 1681–1695. [Google Scholar] [CrossRef]
- Rice, C.; Skordalakes, E. Structure and function of the telomeric CST complex. Comput. Struct. Biotechnol. J. 2016, 14, 161–167. [Google Scholar] [CrossRef]
- Stewart, J.A.; Wang, Y.; Ackerson, S.M.; Schuck, P.L. Emerging roles of CST in maintaining genome stability and human disease. Front. Biosci. 2018, 23, 1564–1586. [Google Scholar] [CrossRef]
- Hom, R.A.; Wuttke, D.S. Human CST Prefers G-Rich but Not Necessarily Telomeric Sequences. Biochemistry 2017, 56, 4210–4218. [Google Scholar] [CrossRef]
- Fan, H.-C.; Chang, F.-W.; Tsai, J.-D.; Lin, K.-M.; Chen, C.-M.; Lin, S.-Z.; Liu, C.-A.; Harn, H.-J. Telomeres and Cancer. Life 2021, 11, 1405. [Google Scholar] [CrossRef]
- Luo, Y.M.; Xia, N.X.; Yang, L.; Li, Z.; Yang, H.; Yu, H.J.; Liu, Y.; Lei, H.; Zhou, F.X.; Xie, C.H.; et al. CTC1 increases the radioresistance of human melanoma cells by inhibiting telomere shortening and apoptosis. Int. J. Mol. Med. 2014, 33, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Dang, Z.; Shen, Z.; Dai, H.; Bai, Y.; Li, B.; Shao, Y. Association of SNPs in the OBFC1 gene and laryngeal carcinoma in Chinese Han male population. Int. J. Clin. Oncol. 2019, 24, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Calado, R.T.; Young, N.S. Telomere diseases. N. Engl. J. Med. 2009, 361, 2353–2365. [Google Scholar] [CrossRef] [PubMed]
- Reddel, R.R. Telomere Maintenance Mechanisms in Cancer: Clinical Implications. Curr. Pharm. Des. 2014, 20, 6361–6374. [Google Scholar] [CrossRef]
- Wang, L.; Ma, T.; Liu, W.; Li, H.; Luo, Z.; Feng, X. Pan-Cancer Analyses Identify the CTC1-STN1-TEN1 Complex as a Protective Factor and Predictive Biomarker for Immune Checkpoint Blockade in Cancer. Front Genet. 2022, 13, 859617. [Google Scholar] [CrossRef]
- Desmeules, P.; Dufour, M.; Fernandes, M.J. A rapid flow cytometry assay for the assessment of calcium mobilization in human neutrophils in a small volume of lysed whole-blood. J. Immunol. Methods 2009, 340, 154–157. [Google Scholar] [CrossRef]
- Cawthon, R.M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002, 30, e47. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 25 July 2022).
- Paul, S.; Kantarjian, H.; Jabbour, E.J. Adult Acute Lymphoblastic Leukemia. Mayo Clin. Proc. 2016, 91, 1645–1666. [Google Scholar] [CrossRef]
- Maheswaran, R.; Morley, N. Incidence, socioeconomic deprivation, volume-outcome and survival in adult patients with acute lymphoblastic leukaemia in England. BMC Cancer 2018, 18, 25. [Google Scholar] [CrossRef]
- Tavasolian, F.; Abdollahi, E.; Vakili, M.; Amini, A. Relationship between ABO blood group and Acute Lymphoblastic Leukemia. Iran. J. Pediatr. Hematol. Oncol. 2014, 4, 1. [Google Scholar]
- Ghali, H.H.; Nayeef, A.M.; Hameed, A.H.; Fawzi, G.M. Relationship between ABO and Rh Blood Groups with Childhood Acute Lymphoblastic Leukemia. IOSR J. Res. Method Educ. IOSRJRME 2017, 7, 86–89. [Google Scholar] [CrossRef]
- Mushtaq, N.; Fadoo, Z.; Naqvi, A. Childhood acute lymphoblastic leukaemia: Experience from a single tertiary care facility of Pakistan. J. Pak. Med. Assoc. 2013, 63, 1399–1404. [Google Scholar] [PubMed]
- Terwilliger, T.; Abdul-Hay, M. Acute lymphoblastic leukemia: A comprehensive review and 2017 update. Blood Cancer J. 2017, 7, e577. [Google Scholar] [CrossRef] [PubMed]
- Safaei, A.; Shahryari, J.; Farzaneh, M.R.; Tabibi, N.; Hosseini, M. Cytogenetic Findings of Patients with Acute Lymphoblastic Leukemia in Fars Province. Iran. J. Med. Sci. 2013, 38, 301. [Google Scholar] [PubMed]
- Alonso, C.N.; Rossi, J.G.; Bernasconi, A.R.; Rampazzi, M.A.; Felice, M.S.; Rubio, P.L.; Eberle, S.E.; Medina, A.; Gallego, M.S.; Coccé, M.C. Cytogenetic and Molecular Findings in Children with Acute Lymphoblastic Leukemia: Experience of a Single Institution in Argentina. Mol. Syndr. 2015, 6, 193–203. [Google Scholar] [CrossRef]
- Reddy, P.; Shankar, R.; Koshy, T.; Radhakrishnan, V.; Ganesan, P.; Jayachandran, P.K.; Dhanushkodi, M.; Mehra, N.; Krupashankar, S.; Manasa, P.; et al. Evaluation of Cytogenetic Abnormalities in Patients with Acute Lymphoblastic Leukemia. Indian J. Hematol. Blood Transfus. 2019, 35, 640–648. [Google Scholar] [CrossRef]
- Carroll, A.J.; Shago, M.; Mikhail, F.M.; Raimondi, S.C.; Hirsch, B.A.; Loh, M.L.; Raetz, E.A.; Borowitz, M.J.; Wood, B.L.; Maloney, K.W.; et al. Masked hypodiploidy: Hypodiploid acute lymphoblastic leukemia (ALL) mimicking hyperdiploid ALL in children: A report from the Children’s Oncology Group. Cancer Genet. 2019, 238, 62–68. [Google Scholar] [CrossRef]
- Chaturvedi, A.; Shetty, D.; Ghogale, S.G.; Deshpande, N.; Badrinath, Y.; Chatterjee, G.; Girase, K.; Sriram, H.; Khanka, T.; Mishra, C.; et al. Detecting hypodiploidy with endoreduplication and masked hypodiploidy in B-cell acute lymphoblastic leukemia using multicolor flow cytometry. Cytom. Part B Clin. Cytom. 2022, 102, 199–208. [Google Scholar] [CrossRef]
- Okamoto, K.; Seimiya, H. Revisiting Telomere Shortening in Cancer. Cells 2019, 8, 107. [Google Scholar] [CrossRef]
- Vyas, C.M.; Ogata, S.; Reynolds, C.F.; Mischoulon, D.; Chang, G.; Cook, N.R.; Manson, J.E.; Crous-Bou, M.; De Vivo, I.; Okereke, O.I. Telomere length and its relationships with lifestyle and behavioural factors: Variations by sex and race/ethnicity. Age Ageing 2021, 50, 838–846. [Google Scholar] [CrossRef]
- Walsh, K.M.; Whitehead, T.P.; de Smith, A.J.; Smirnov, I.V.; Park, M.; Endicott, A.A.; Francis, S.S.; Codd, V.; Samani, N.J.; Metayer, C.; et al. Common genetic variants associated with telomere length confer risk for neuroblastoma and other childhood cancers. Carcinogenesis 2016, 37, 576–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Fang, M.; Sun, X.; Sun, J. Telomerase activity and telomere length in acute leukemia: Correlations with disease progression, subtypes and overall survival. Int. J. Lab. Hematol. 2010, 32, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, E.; Hashemi, M.; Naderi, M.; Bahari, G.; Safdari, V.; Taheri, M. Leukocyte Telomere Length Shortening, hTERT Genetic Polymorphisms and Risk of Childhood Acute Lymphoblastic Leukemia. Asian Pac. J. Cancer Prev. 2018, 19, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Lansdorp, P. Maintenance of telomere length in AML. Blood Adv. 2017, 1, 2467–2472. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Hsu, S.-J.; Kasbek, C.; Chaiken, M.; Price, C.M. CTC1-mediated C-strand fill-in is an essential step in telomere length maintenance. Nucleic Acids Res. 2017, 45, 4281–4293. [Google Scholar] [CrossRef]
- Zaug, A.J.; Lim, C.J.; Olson, C.L.; Carilli, M.T.; Goodrich, K.J.; Wuttke, D.S.; Cech, T.R. CST does not evict elongating telomerase but prevents initiation by ssDNA binding. Nucleic Acids Res. 2021, 49, 11653–11665. [Google Scholar] [CrossRef]
- Savage, S.A.; Bertuch, A.A. The genetics and clinical manifestations of telomere biology disorders. Genet. Med. 2010, 12, 753–764. [Google Scholar] [CrossRef]
- Karow, A.; Haubitz, M.; Leibundgut, E.O.; Helsen, I.; Preising, N.; Steiner, D.; Dantonello, T.; Ammann, R.; Roessler, J.; Kartal-Kaess, M.; et al. Targeting Telomere Biology in Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2021, 22, 6653. [Google Scholar] [CrossRef]
- Tabori, U.; Dome, J.S. Telomere Biology of Pediatric Cancer. Cancer Investig. 2007, 25, 197–208. [Google Scholar] [CrossRef]
- Angstadt, A.Y.; Thayanithy, V.; Subramanian, S.; Modiano, J.F.; Breen, M. A genome-wide approach to comparative oncology: High-resolution oligonucleotide aCGH of canine and human osteosarcoma pinpoints shared microaberrations. Cancer Genet. 2012, 205, 572–587. [Google Scholar] [CrossRef]
- Giaccherini, M.; Macauda, A.; Sgherza, N.; Sainz, J.; Gemignani, F.; Maldonado, J.M.S.; Jurado, M.; Tavano, F.; Mazur, G.; Jerez, A.; et al. Genetic polymorphisms associated with telomere length and risk of developing myeloproliferative neoplasms. Blood Cancer J. 2020, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Ojha, J.; Codd, V.; Nelson, C.P.; Samani, N.J.; Smirnov, I.V.; Madsen, N.R.; Hansen, H.M.; de Smith, A.J.; Bracci, P.M.; Wiencke, J.K.; et al. Genetic Variation Associated with Longer Telomere Length Increases Risk of Chronic Lymphocytic Leukemia. Cancer Epidemiol. Biomarkers Prev. 2016, 25, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, A.; Cantalupo, S.; Lasorsa, V.A.; Montella, A.; Cimmino, F.; Succoio, M.; Vermeulen, M.; Baltissen, M.P.; Esposito, M.; Avitabile, M.; et al. Functional annotation and investigation of the 10q24.33 melanoma risk locus identifies a common variant that influences transcriptional regulation of OBFC1. Hum. Mol. Genet. 2022, 31, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Stewart, J.; Price, C.M. Human CST abundance determines recovery from diverse forms of DNA damage and replication stress. Cell Cycle 2014, 13, 3488–3498. [Google Scholar] [CrossRef] [PubMed]
- Kibriya, M.G.; Raza, M.; Kamal, M.; Haq, Z.; Paul, R.; Mareczko, A.; Pierce, B.L.; Ahsan, H.; Jasmine, F. Relative Telomere Length Change in Colorectal Carcinoma and Its Association with Tumor Characteristics, Gene Expression and Microsatellite Instability. Cancers 2022, 14, 2250. [Google Scholar] [CrossRef]
- Çoğulu, O.; Kosova, B.; Karaca, E.; Gunduz, C.; Ozkinay, F.; Aksoylar, S.; Gülen, H.; Kantar, M.; Oniz, H.; Karapinar, D.; et al. Evaluation of Telomerase mRNA (hTERT) in Childhood Acute Leukemia. Leuk. Lymphoma 2004, 45, 2477–2480. [Google Scholar] [CrossRef]
- Rudant, J.; Orsi, L.; Bonaventure, A.; Goujon-Bellec, S.; Baruchel, A.; Petit, A.; Bertrand, Y.; Nelken, B.; Pasquet, M.; Michel, G.; et al. ARID5B, IKZF1 and Non-Genetic Factors in the Etiology of Childhood Acute Lymphoblastic Leukemia: The ESCALE Study. PLoS ONE 2015, 10, e0121348. [Google Scholar] [CrossRef]
- Pereira, F.A.C.; Mirra, A.P.; Latorre, M.D.R.D.D.O.; De Assunção, J.V. Fatores de risco ambientais e leucemia linfobla?stica aguda na infa?ncia. Rev. Cienc. Salud 2017, 15, 129–144. [Google Scholar] [CrossRef]
- Poncet, D.; Belleville, A.; de Roodenbeke, C.T.; de Climens, A.R.; Ben Simon, E.; Merle-Beral, H.; Callet-Bauchu, E.; Salles, G.; Sabatier, L.; Delic, J.; et al. Changes in the expression of telomere maintenance genes suggest global telomere dysfunction in B-chronic lymphocytic leukemia. Blood 2008, 111, 2388–2391. [Google Scholar] [CrossRef]
- Sallan, S.E. Myths and lessons from the adult/pediatric interface in acute lymphoblastic leukemia. Hematol. Am. Soc. Hematol. Educ. Program 2006, 2006, 128–132. [Google Scholar] [CrossRef]
- Chaudhury, S.; O’Connor, C.; Cañete, A.; Bittencourt-Silvestre, J.; Sarrou, E.; Prendergast, A.; Choi, J.; Johnston, P.; Wells, C.A.; Gibson, B.; et al. Age-specific biological and molecular profiling distinguishes peadiatric from adult acute myeloid leukemias. Nat. Commun. 2018, 9, 5280. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Primer Sequence (5′–3′) | ||
|---|---|---|
| TEL | F | GGTTTTTGAGGGTGAGGGTGAGGGTGAGGGTGAGGGT |
| R | TCCCGACTATCCCTATCCCTATCCCTATCCCTATCCCT | |
| β-globin | F | GCTTCTGACACAACTGTGTTCACTAGC |
| R | CACCAACT TCATCCACGTTCACC | |
| CTC1 | F | TGAGCTGGAAAGGAAACCGT |
| R | AGAAAGGCA GGACAATCGGA | |
| OBFC1 | F | TTTCACAGCTCAGCCCTAGA |
| R | AAGCTCT GCACTCTGTTCTC | |
| TERT | F | ATCAGACAGCACTTGAAGAGGGTG |
| R | CCCACGACGTAGTCCATGTTCAC | |
| Cases vs. Control | ||||||
|---|---|---|---|---|---|---|
| Pediatric | Adults | |||||
| Characteristics | Controls | Cases | p Value | Controls | Cases | p Value |
| Subjects (N) | 51 | 185 | - | 45 | 65 | - |
| Sex (Male) (%) | 16 (66.7%) (n = 24) * | 124 (67.0%) | 0.985 | 28 (80.0%) | 50 (78.0%) | 0.797 |
| Age (years) | 2.58 (3.06) (n = 24) * | 8.19 (4.67) | 0.001 | 28.9 (8.87) | 31.5 (9.11) | 0.182 |
| Blood groups (cases) and ALL immunophenotypes | ||||||
| Blood groups | Pediatric | Adults | p value | |||
| A | 21.0% | 26.4% | 0.688 | |||
| B | 47.2% | 34.0% | 0.399 | |||
| AB | 4.4% | 11.3% | 0.391 | |||
| O | 27.5% | 28.3% | 1.000 | |||
| B-cell ALL | 142 (77%) | 47 (72.3%) | 0.874 | |||
| T-cell ALL | 43 (23%) | 18 (27.7%) | 0.694 | |||
| Cytogenetic data of cases | ||||||
| Group | Immunophenotype | Cytogenetic abnormality | ||||
| Pediatric | B-cell ALL | Hyperdiploidy, Hyperdiploidy with other abnormalities (add X, 4,6,7) del 6q21q27 | ||||
| T-cell ALL | Mosaicism Hypodiploidy with add 1p36.6 Hypodiploidy with t(9;15)(p13;q11.2) | |||||
| Adults | B-cell ALL | Hyperdiploidy with other abnormality del 9p22p24, del 9p13p24, del 9q24.3 add 9p23, 10q24.3 t(1;19)(q25;p13.3),t(9;22)(q34;q11.2),4q12 gene translocation, FIP1L1-PDGFRA fusion. dup 1q25q44 | ||||
| T-cell ALL | del 9p21p24 | |||||
| Pediatric | Adults | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Association of Genes (CTC1, OBFC1, TERT) with ALL | ||||||||||||
| A | B | |||||||||||
| Model 1 (Univariate Analysis) | Model 2 (Multivariate Analysis) | Model 1 Univariate Analysis | Model 2 Multivariate Analysis | |||||||||
| Predictors | β-Estimate | SE | p Value | β-Estimate | SE | p Value | β-Estimate | SE | p Value | β-Estimate | SE | p Value |
| CTC1 | −0.045 | 0.009 | 3.40 × 10−6 | −0.013 | 0.007 | 0.040 | 0.071 | 0.022 | 0.002 | 0.053 | 0.023 | 0.025 |
| OBFC1 | −0.017 | 0.011 | 0.127 | −0.003 | 0.008 | 0.696 | 0.028 | 0.018 | 0.129 | 0.032 | 0.021 | 0.133 |
| TERT | −0.010 | 0.010 | 0.335 | −0.001 | 0.006 | 0.900 | 0.018 | 0.011 | 0.115 | 0.013 | 0.013 | 0.314 |
| Combined association of genes with ALL | ||||||||||||
| C | D | |||||||||||
| Model 3 Multivariate Analysis | Model 4 Multivariate Analysis | Model 3 Multivariate Analysis | Model 4 Multivariate Analysis | |||||||||
| CTC1 | −0.042 | 0.016 | 0.010 | −0.040 | 0.012 | 0.002 | 0.097 | 0.033 | 0.005 | 0.105 | 0.037 | 0.009 |
| OBFC1 | 0.002 | 0.020 | 0.907 | 0.027 | 0.014 | 0.056 | −0.036 | 0.029 | 0.225 | 0.006 | 0.038 | 0.878 |
| TERT | 0.001 | 0.014 | 0.982 | 0.003 | 0.009 | 0.686 | 0.037 | 0.029 | 0.039 | 0.011 | 0.025 | 0.671 |
| Pediatric | Adults | |||||
|---|---|---|---|---|---|---|
| Association of TEL with ALL | ||||||
| A | D | |||||
| Model 5 Univariate Analysis | Model 5 Univariate Analysis | |||||
| Predictors | β-Estimate | SE | p Value | β-Estimate | SE | p Value |
| TEL | 0.112 | 0.108 | 0.305 | 0.576 | 0.511 | 0.267 |
| Combined Effect of Genes and TEL with ALL | ||||||
| B | E | |||||
| (Model 6) Multivariate Analysis * | (Model 6) Multivariate Analysis * | |||||
| TEL | 0.471 | 0.541 | 0.394 | −1.044 | 2.319 | 0.683 |
| CTC1 | −0.026 | 0.037 | 0.480 | 0.193 | 0.212 | 0.429 |
| OBFC1 | −0.109 | 0.053 | 0.053 | −0.029 | 0.187 | 0.888 |
| TERT | 0.059 | 0.039 | 0.149 | 0.022 | 0.850 | 0.813 |
| C | F | |||||
| (Model 7) Multivariate Analysis ** | (Model 7) Multivariate Analysis ** | |||||
| TEL | 0.947 | 0.521 | 0.103 | −3.157 | 0.592 | 0.118 |
| CTC1 | 0.096 | 0.075 | 0.232 | 0.139 | 0.049 | 0.218 |
| OBFC1 | −0.011 | 0.058 | 0.085 | 0.081 | 0.044 | 0.321 |
| TERT | 0.018 | 0.035 | 0.627 | 0.001 | 0.019 | 0.975 |
| Pediatrics | Adults | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. Association of Genes with B-cell ALL | ||||||||||||
| Predictors | Univariate Analysis | Multivariate Analysis * | Univariate Analysis | Multivariate Analysis * | ||||||||
| β-Estimate | SE | p Value | β-Estimate | SE | p Value | β-Estimate | SE | p Value | β-Estimate | SE | p Value | |
| CTC1 | −0.057 | 0.017 | 0.002 | −0.028 | 0.019 | 0.148 | 0.056 | 0.043 | 0.208 | 0.118 | 0.059 | 0.059 |
| OBFC1 | −0.008 | 0.018 | 0.664 | 0.007 | 0.021 | 0.734 | −0.039 | 0.044 | 0.366 | −0.009 | 0.067 | 0.886 |
| TERT | 0.022 | 0.016 | 0.176 | 0.001 | 0.017 | 0.964 | −0.093 | 0.043 | 0.036 | −0.092 | 0.044 | 0.043 |
| B. Association of Genes with T-cell ALL | ||||||||||||
| CTC1 | −0.050 | 0.018 | 0.008 | −0.039 | 0.036 | 0.301 | −0.068 | 0.063 | 0.936 | 0.057 | 0.068 | 0.408 |
| OBFC1 | −0.017 | 0.017 | 0.321 | 0.032 | 0.035 | 0.372 | −0.014 | 0.039 | 0.721 | 0.003 | 0.068 | 0.996 |
| TERT | −0.019 | 0.016 | 0.233 | −0.047 | 0.022 | 0.054 | −0.085 | 0.042 | 0.053 | −0.093 | 0.047 | 0.057 |
| C. Combined Effect of Genes with B-cell ALL | ||||||||||||
| Pediatrics | Adults | |||||||||||
| Multivariate Analysis | Multivariate Analysis * | Multivariate Analysis | Multivariate Analysis * | |||||||||
| CTC1 | −0.057 | 0.023 | 0.016 | −0.080 | 0.028 | 0.008 | 0.060 | 0.065 | 0.375 | 0.119 | 0.157 | 0.481 |
| OBFC1 | −0.032 | 0.030 | 0.281 | 0.024 | 0.030 | 0.426 | −0.056 | 0.048 | 0.269 | −0.059 | 0.010 | 0.578 |
| * TERT | 0.047 | 0.023 | 0.040 | 0.027 | 0.021 | 0.205 | −0.095 | 0.067 | 0.188 | −0.050 | 0.130 | 0.720 |
| D. Combined Effect of Genes with T-cell ALL | ||||||||||||
| CTC1 | −0.039 | 0.022 | 0.082 | −0.057 | 0.026 | 0.040 | −0.024 | 0.064 | 0.721 | 0.100 | 0.182 | 0.609 |
| OBFC1 | −0.020 | 0.030 | 0.499 | 0.087 | 0.028 | 0.010 | 0.004 | 0.048 | 0.938 | −0.078 | 0.077 | 0.371 |
| TERT | −0.013 | 0.026 | 0.627 | −0.070 | 0.024 | 0.012 | −0.086 | 0.060 | 0.179 | −0.134 | 0.116 | 0.313 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zia, S.; Khan, N.; Tehreem, K.; Rehman, N.; Sami, R.; Baty, R.S.; Tayeb, F.J.; Almashjary, M.N.; Alsubhi, N.H.; Alrefaei, G.I.; et al. Transcriptomic Analysis of Conserved Telomere Maintenance Component 1 (CTC1) and Its Association with Leukemia. J. Clin. Med. 2022, 11, 5780. https://doi.org/10.3390/jcm11195780
Zia S, Khan N, Tehreem K, Rehman N, Sami R, Baty RS, Tayeb FJ, Almashjary MN, Alsubhi NH, Alrefaei GI, et al. Transcriptomic Analysis of Conserved Telomere Maintenance Component 1 (CTC1) and Its Association with Leukemia. Journal of Clinical Medicine. 2022; 11(19):5780. https://doi.org/10.3390/jcm11195780
Chicago/Turabian StyleZia, Saadiya, Netasha Khan, Komal Tehreem, Nazia Rehman, Rokayya Sami, Roua S. Baty, Faris J. Tayeb, Majed N. Almashjary, Nouf H. Alsubhi, Ghadeer I. Alrefaei, and et al. 2022. "Transcriptomic Analysis of Conserved Telomere Maintenance Component 1 (CTC1) and Its Association with Leukemia" Journal of Clinical Medicine 11, no. 19: 5780. https://doi.org/10.3390/jcm11195780
APA StyleZia, S., Khan, N., Tehreem, K., Rehman, N., Sami, R., Baty, R. S., Tayeb, F. J., Almashjary, M. N., Alsubhi, N. H., Alrefaei, G. I., & Shahid, R. (2022). Transcriptomic Analysis of Conserved Telomere Maintenance Component 1 (CTC1) and Its Association with Leukemia. Journal of Clinical Medicine, 11(19), 5780. https://doi.org/10.3390/jcm11195780






