Relationship between Menopausal Hormone Therapy and Oral Cancer: A Cohort Study Based on the Health Insurance Database in South Korea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Database
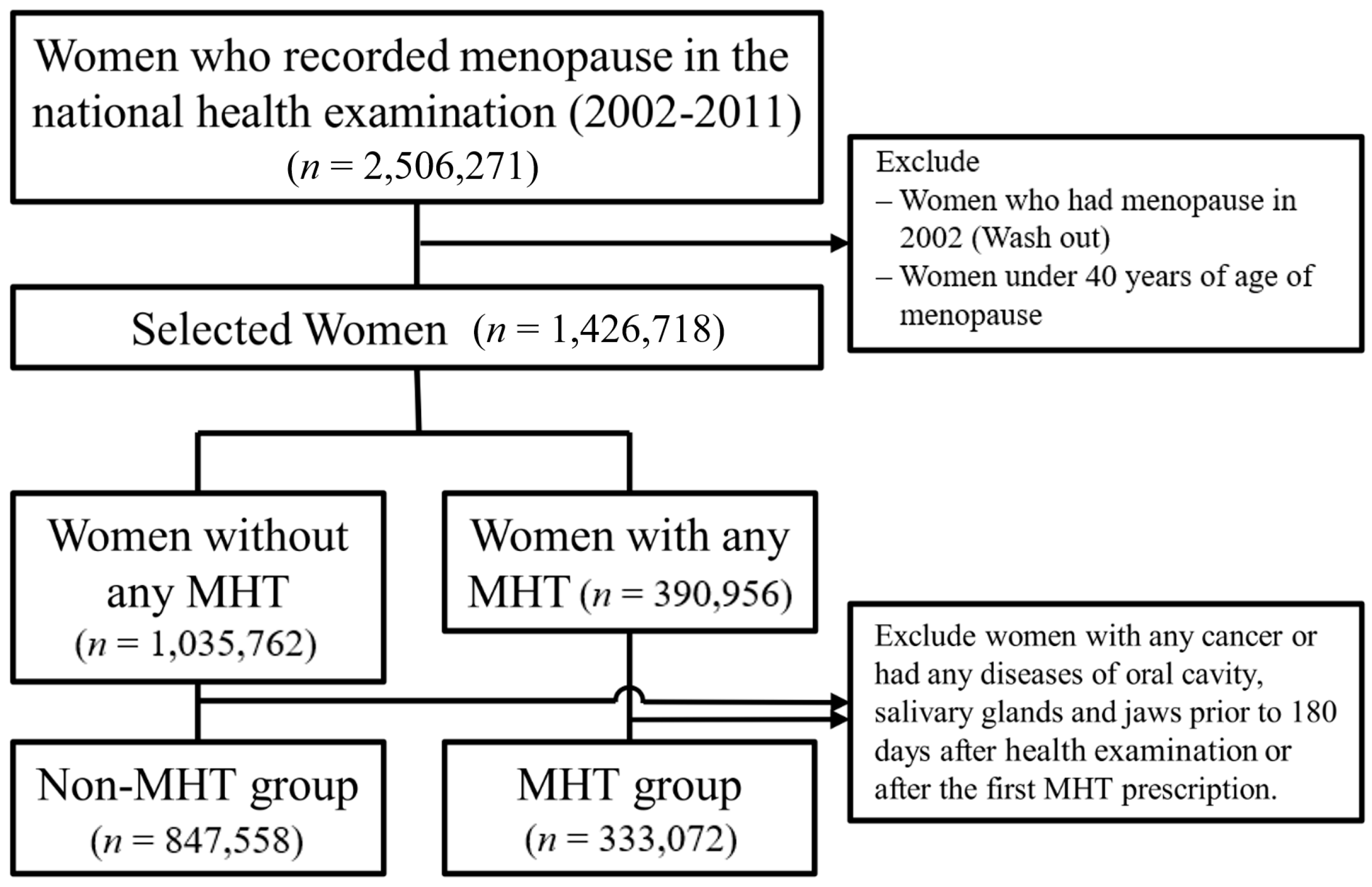
2.2. Selection of Participants
- (i)
- A participant confirmed as menopausal in 2002, to consider wash out;
- (ii)
- If a participant had one or more cancer-related diagnosis code (any C code) within 180 days after inclusion;
- (iii)
- If a participant had one or more disease of the oral cavity (K0, K1) within 180 days after inclusion.
2.3. Outcome
2.4. Variables
2.5. Statistical Analysis
2.6. Ethics
3. Result
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, M.; Nanavati, R.; Modi, T.G.; Dobariya, C. Oral cancer: Etiology and risk factors: A review. J. Cancer Res. Ther. 2016, 12, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Krolls, S.O.; Hoffman, S. Squamous cell carcinoma of the oral soft tissues: A statistical analysis of 14,253 cases by age, sex, and race of patients. J. Am. Dent. Assoc. 1976, 92, 571–574. [Google Scholar] [CrossRef] [PubMed]
- Blot, W.J.; McLaughlin, J.K.; Winn, D.M.; Austin, D.F.; Greenberg, R.S.; Preston-Martin, S.; Bernstein, L.; Schoenberg, J.B.; Stemhagen, A.; Fraumeni, J.F., Jr. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988, 48, 3282–3287. [Google Scholar] [PubMed]
- Farshadpour, F.; Hordijk, G.J.; Koole, R.; Slootweg, P.J. Non-smoking and non-drinking patients with head and neck squamous cell carcinoma: A distinct population. Oral Dis. 2007, 13, 239–243. [Google Scholar] [CrossRef]
- Wiseman, S.M.; Swede, H.; Stoler, D.L.; Anderson, G.R.; Rigual, N.R.; Hicks, W.L., Jr.; Douglas, W.G.; Tan, D.; Loree, T.R. Squamous cell carcinoma of the head and neck in nonsmokers and nondrinkers: An analysis of clinicopathologic characteristics and treatment outcomes. Ann. Surg. Oncol. 2003, 10, 551–557. [Google Scholar] [CrossRef]
- DeAngelis, A.; Breik, O.; Koo, K.; Iseli, T.; Nastri, A.; Fua, T.; Rischin, D.; McCullough, M.; Wiesenfeld, D. Non-smoking, non-drinking elderly females, a 5 year follow-up of a clinically distinct cohort of oral squamous cell carcinoma patients. Oral Oncol. 2018, 86, 113–120. [Google Scholar] [CrossRef]
- Koo, K.; Barrowman, R.; McCullough, M.; Iseli, T.; Wiesenfeld, D. Non-smoking non-drinking elderly females: A clinically distinct subgroup of oral squamous cell carcinoma patients. Int. J. Oral Maxillofac. Surg. 2013, 42, 929–933. [Google Scholar] [CrossRef]
- Rich, A.M.; Radden, B.G. Squamous cell carcinoma of the oral mucosa: A review of 244 cases in Australia. J. Oral Pathol. 1984, 13, 459–471. [Google Scholar] [CrossRef]
- Suba, Z. Gender-related hormonal risk factors for oral cancer. Pathol. Oncol. Res. 2007, 13, 195–202. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, C.E.; Field, J.K.; Marcus, M.W. Age at menopause and hormone replacement therapy as risk factors for head and neck and oesophageal cancer (Review). Oncol. Rep. 2017, 38, 1915–1922. [Google Scholar] [CrossRef] [Green Version]
- Langevin, S.M.; Grandis, J.R.; Taioli, E. Female hormonal and reproductive factors and head and neck squamous cell carcinoma risk. Cancer Lett. 2011, 310, 216–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freedman, N.D.; Lacey, J.V., Jr.; Hollenbeck, A.R.; Leitzmann, M.F.; Schatzkin, A.; Abnet, C.C. The association of menstrual and reproductive factors with upper gastrointestinal tract cancers in the NIH-AARP cohort. Cancer 2010, 116, 1572–1581. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M.; Biegner, T.; Teriete, P.; Hoefert, S.; Krimmel, M.; Munz, A.; Reinert, S. Estrogen and progesterone hormone receptor expression in oral cavity cancer. Med. Oral Patol. Oral Cir. Bucal. 2016, 21, e554–e558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukits, J.; Remenár, E.; Rásó, E.; Ladányi, A.; Kásler, M.; Tímár, J. Molecular identification, expression and prognostic role of estrogen- and progesterone receptors in head and neck cancer. Int. J. Oncol. 2007, 30, 155–160. [Google Scholar] [CrossRef] [Green Version]
- Egloff, A.M.; Rothstein, M.E.; Seethala, R.; Siegfried, J.M.; Grandis, J.R.; Stabile, L.P. Cross-talk between estrogen receptor and epidermal growth factor receptor in head and neck squamous cell carcinoma. Clin. Cancer Res. 2009, 15, 6529–6540. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.L.; Hsu, Y.-L.; Wu, T.-F.; Huang, C.-M.; Liou, L.-Y.; Chiu, Y.-W.; Hsiao, Y.-H.; Luo, F.-J.; Yuan, T.-C. Regulation of estrogen receptor α function in oral squamous cell carcinoma cells by FAK signaling. Endocr. Relat. Cancer 2014, 21, 555–565. [Google Scholar] [CrossRef]
- Ishida, H.; Wada, K.; Masuda, T.; Okura, M.; Kohama, K.; Sano, Y.; Nakajima, A.; Kogo, M.; Kamisaki, Y. Critical role of estrogen receptor on anoikis and invasion of squamous cell carcinoma. Cancer Sci. 2007, 98, 636–643. [Google Scholar] [CrossRef]
- Corrao, G.; Zambon, A.; Conti, V.; Nicotra, F.; La Vecchia, C.; Fornari, C.; Cesana, G.; Contiero, P.; Tagliabue, G.; Nappi, R.E.; et al. Menopause hormone replacement therapy and cancer risk: An Italian record linkage investigation. Ann. Oncol. 2008, 19, 150–155. [Google Scholar] [CrossRef]
- Kim, L.; Kim, J.A.; Kim, S. A guide for the utilization of health insurance review and assessment service national patient samples. Epidemiol. Health 2014, 36, e2014008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization; Regional Office for the Western Pacific. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment; Health Communications Australia: Sydney, Australia, 2000. [Google Scholar]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.-M.; Sundararajan, V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez, E.; Gallus, S.; Bosetti, C.; Franceschi, S.; Negri, E.; La Vecchia, C. Hormone replacement therapy and cancer risk: A systematic analysis from a network of case-control studies. Int. J. Cancer 2003, 105, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Lee, J.-H.; Kim, Y.K.; Myoung, H.; Yun, P.-Y. The role of tamoxifen in combination with cisplatin on oral squamous cell carcinoma cell lines. Cancer Lett. 2007, 245, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Akyu Takei, R.; Tomihara, K.; Yamazaki, M.; Moniruzzaman, R.; Heshiki, W.; Sekido, K.; Tachinami, H.; Sakurai, K.; Yonesi, A.; Imaue, S.; et al. Protumor role of estrogen receptor expression in oral squamous cell carcinoma cells. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 132, 549–565. [Google Scholar] [CrossRef] [PubMed]
- Gallus, S.; Bosetti, C.; Franceschi, S.; Levi, F.; Negri, E.; La Vecchia, C. Laryngeal cancer in women: Tobacco, alcohol, nutritional, and hormonal factors. Cancer Epidemiol. Biomarkers Prev. 2003, 12, 514–517. [Google Scholar] [PubMed]
- Bosetti, C.; Negri, E.; Franceschi, S.; Conti, E.; Levi, F.; Tomei, F.; La Vecchiaet, C. Risk factors for oral and pharyngeal cancer in women: A study from Italy and Switzerland. Br. J. Cancer 2000, 82, 204–207. [Google Scholar] [CrossRef]
- The 2017 hormone therapy position statement of The North American Menopause Society. Menopause 2017, 24, 728–753. [CrossRef]
- Cummings, S.R.; Ettinger, B.; Delmas, P.D.; Kenemans, P.; Stathopoulos, V.; Verweij, P.; Mol-Arts, M.; Kloosterboer, L.; Mosca, L.; Christiansen, C.; et al. The effects of tibolone in older postmenopausal women. N. Engl. J. Med. 2008, 359, 697–708. [Google Scholar] [CrossRef]

| Non-MHT | Tibolone | Combined Estrogen Plus Progestin by Manufacturer | Oral Estrogen | Combined Estrogen Plus Progestin by Physician | Topical Estrogen | Total | |
|---|---|---|---|---|---|---|---|
| Number of women | 847,558 | 167,674 | 111,494 | 46,278 | 5776 | 1850 | 1,180,630 |
| Median age (years) | 57 (52–64) | 53 (50–57) | 52 (50–56) | 52 (49–57) | 54 (51–59) | 53 (50–57) | 56 (52–62) |
| Age at inclusion (years) | |||||||
| 40–49 | 76,449 (9) | 29,682 (17.7) | 25,628 (23) | 12,251 (26.5) | 1017 (17.6) | 397 (21.5) | 145,424 (12.3) |
| 50–59 | 422,920 (49.9) | 110,026 (65.6) | 74,109 (66.5) | 26,006 (56.2) | 3449 (59.7) | 1131 (61.1) | 637,641 (54) |
| 60–69 | 249,788 (33.3) | 25,030 (15.2) | 10,908 (9.9) | 6661 (14.8) | 1154 (20.5) | 294 (16.1) | 293,835 (27.3) |
| ≥70 | 98,401 (11.6) | 2936 (1.8) | 849 (0.8) | 1360 (2.9) | 156 (2.7) | 28 (1.5) | 103,730 (8.8) |
| Median BMI (kg/m2) | 24 (22.1–26.1) | 23.5 (21.8–25.4) | 23.1 (21.5–25) | 23.7 (22–25.7) | 23.3 (21.6–25.2) | 23.7 (22.1–25.7) | 23.8 (21.9–25.9) |
| BMI (kg/m2) | |||||||
| <18.5 | 15,309 (1.8) | 2831 (1.7) | 2172 (2) | 653 (1.4) | 111 (1.9) | 36 (2) | 21,112 (1.8) |
| 18.5–22.9 | 284,993 (34.3) | 67,199 (40.5) | 50,000 (45.2) | 16,989 (37) | 2395 (41.8) | 678 (36.9) | 422,254 (36.3) |
| 23–24.9 | 220,558 (26.5) | 46,239 (27.8) | 29,811 (26.9) | 12,817 (27.9) | 1609 (28.1) | 493 (26.9) | 311,527 (26.8) |
| 25–29.9 | 274,470 (33) | 45,588 (27.5) | 26,512 (23.9) | 13,864 (30.2) | 1484 (25.9) | 575 (31.3) | 362,493 (31.2) |
| ≥30 | 36,225 (4.4) | 4180 (2.5) | 2215 (2) | 1560 (3.4) | 126 (2.2) | 53 (2.9) | 44,359 (3.8) |
| SES | |||||||
| Mid-high SES | 813,432 (96) | 161,857 (96.5) | 108,792 (97.6) | 45,008 (97.3) | 5632 (97.5) | 1800 (97.3) | 1,136,521 (96.3) |
| Low SES | 34,126 (4) | 5817 (3.5) | 2702 (2.4) | 1270 (2.7) | 144 (2.5) | 50 (2.7) | 44,109 (3.7) |
| Region | |||||||
| Urban area | 258,929 (30.6) | 53,369 (31.8) | 38,421 (34.5) | 14,944 (32.3) | 2992 (51.8) | 856 (46.3) | 369,511 (31.3) |
| Rural area | 588,629 (69.4) | 114,305 (68.2) | 73,073 (65.5) | 31,334 (67.7) | 2784 (48.2) | 994 (53.7) | 811,119 (68.7) |
| CCI | |||||||
| 0 | 559,530 (66) | 114,818 (68.5) | 79,524 (71.3) | 32,404 (70) | 4017 (69.5) | 1219 (65.9) | 791,512 (67) |
| 1 | 162,498 (19.2) | 32,158 (19.2) | 19,768 (17.7) | 8312 (18) | 1063 (18.4) | 340 (18.4) | 224,139 (19) |
| ≥2 | 125,530 (14.8) | 20,698 (12.3) | 12,202 (10.9) | 5562 (12) | 696 (12) | 291 (15.7) | 164,979 (14) |
| Parity (years) | |||||||
| 0 or no response | 147,119 (17.4) | 27,049 (16.1) | 14,604 (13.1) | 9955 (21.5) | 1185 (20.5) | 415 (22.4) | 200,327 (17) |
| 1 | 50,951 (6) | 14,569 (8.7) | 11,578 (10.4) | 3582 (7.7) | 433 (7.5) | 150 (8.1) | 81,263 (6.9) |
| 2 | 543,362 (73.3) | 110,609 (72.7) | 76,976 (74.6) | 28,103 (67.5) | 3565 (68.8) | 1102 (66.1) | 763,717 (73.1) |
| ≥3 | 106,126 (12.5) | 15,447 (9.2) | 8336 (7.5) | 4638 (10) | 593 (10.3) | 183 (9.9) | 135,323 (11.5) |
| Age at menarche (years) | |||||||
| <13 | 141,163 (16.7) | 24,863 (14.9) | 16,246 (14.7) | 8611 (18.9) | 1095 (19.1) | 356 (19.5) | 192,334 (16.4) |
| ≥13 | 702,034 (83.3) | 141,448 (85.1) | 94,584 (85.3) | 37,051 (81.1) | 4631 (80.9) | 1471 (80.5) | 981,219 (83.6) |
| Age at menopause (years) | |||||||
| 40–44 | 105,727 (12.5) | 20,387 (12.2) | 12,780 (11.5) | 10,088 (21.8) | 754 (13.1) | 372 (20.1) | 150,108 (12.7) |
| 45–49 | 244,173 (28.8) | 54,782 (32.7) | 37,370 (33.5) | 16,454 (35.6) | 1867 (32.3) | 668 (36.1) | 355,314 (30.1) |
| 50–54 | 424,057 (54.8) | 79,815 (51.5) | 53,930 (51.8) | 17,500 (39.7) | 2704 (50.8) | 699 (40.2) | 578,705 (53.4) |
| ≥55 | 73,601 (8.7) | 12,690 (7.6) | 7414 (6.6) | 2236 (4.8) | 451 (7.8) | 111 (6) | 96,503 (8.2) |
| Smoking | |||||||
| Never | 773,580 (96.3) | 151,051 (93.6) | 100,720 (93.3) | 42,217 (94.9) | 5314 (95.5) | 1705 (96.4) | 1,074,587 (95.6) |
| Past | 8338 (1) | 2818 (1.7) | 1996 (1.8) | 613 (1.4) | 74 (1.3) | 24 (1.4) | 13,863 (1.2) |
| Current | 21,499 (2.7) | 7482 (4.6) | 5206 (4.8) | 1664 (3.7) | 174 (3.1) | 39 (2.2) | 36,064 (3.2) |
| Alcohol (per week) | |||||||
| None | 684,433 (84.8) | 125,500 (77.2) | 82,043 (75.6) | 35,634 (79.4) | 4627 (82.5) | 1470 (81.9) | 933,707 (82.6) |
| ~2/week | 104,804 (13) | 31,424 (19.3) | 22,663 (20.9) | 8054 (17.9) | 859 (15.3) | 289 (16.1) | 168,093 (14.9) |
| 3–6/week | 12,893 (1.6) | 4240 (2.6) | 3088 (2.9) | 851 (1.9) | 86 (1.5) | 28 (1.6) | 21,186 (1.9) |
| Daily | 4641 (0.6) | 1340 (0.8) | 794 (0.7) | 340 (0.8) | 36 (0.6) | 7 (0.4) | 7158 (0.6) |
| Physical exercise (per week) | |||||||
| None | 522,432 (64.7) | 95,838 (59) | 64,610 (59.5) | 26,535 (59.2) | 3153 (56.3) | 940 (52.7) | 713,508 (63.1) |
| 1–2 | 135,837 (16.8) | 31,225 (19.2) | 21,254 (19.6) | 8717 (19.4) | 1111 (19.8) | 387 (21.7) | 198,531 (17.6) |
| 3–4 | 76,024 (9.4) | 18,907 (11.6) | 12,844 (11.8) | 5016 (11.2) | 744 (13.3) | 262 (14.7) | 113,797 (10.1) |
| 5–6 | 25,227 (3.1) | 6174 (3.8) | 4215 (3.9) | 1604 (3.6) | 223 (4) | 72 (4) | 37,515 (3.3) |
| Daily | 48,487 (6) | 10,261 (6.3) | 5654 (5.2) | 2962 (6.6) | 373 (6.7) | 123 (6.9) | 67,860 (6) |
| Period from menopause to inclusion (years) | |||||||
| <5 | 340,592 (40.2) | 97,498 (58.1) | 75,723 (67.9) | 24,366 (52.7) | 2977 (51.5) | 937 (50.6) | 542,093 (45.9) |
| 5–9 | 182,521 (21.5) | 38,929 (23.2) | 22,305 (20) | 11,632 (25.1) | 1413 (24.5) | 499 (27) | 257,299 (21.8) |
| ≥10 | 324,445 (38.3) | 31,247 (18.6) | 13,466 (12.1) | 10,280 (22.2) | 1386 (24) | 414 (22.4) | 381,238 (32.3) |
| MHT Characteristics | Tibolone | Combined Estrogen Plus Progestin by Manufacturer | Oral Estrogen | Combined Estrogen Plus Progestin by Physician | Topical Estrogen | Total MHT |
|---|---|---|---|---|---|---|
| Median duration (months) | 25 (11–59) | 25 (11–58) | 15 (9–40) | 16 (9–36) | 13 (8–25) | 23 (11–56) |
| Duration (years) | ||||||
| <5 | 126,162 (75.2) | 84,509 (75.8) | 38,224 (82.6) | 5026 (87) | 1764 (95.4) | 255,685 (76.8) |
| 5–9.9 | 30,171 (18) | 20,519 (18.4) | 5513 (11.9) | 575 (10) | 82 (4.4) | 56,860 (17.1) |
| ≥10 | 11,341 (6.8) | 6466 (5.8) | 2541 (5.5) | 175 (3) | 4 (0.2) | 20,527 (6.2) |
| Duration of previous other MHT (years) | 0 | |||||
| <5 | 163,170 (97.3) | 109,728 (98.4) | 45,619 (98.6) | 4829 (83.6) | 1830 (98.9) | 325,176 (97.6) |
| 5–9.9 | 4015 (2.4) | 1621 (1.5) | 586 (1.3) | 691 (12) | 19 (1) | 6932 (2.1) |
| ≥10 | 489 (0.3) | 145 (0.1) | 73 (0.2) | 256 (4.4) | 1 (0.1) | 964 (0.3) |
| Last dosage of Tibolone (per day) | ||||||
| 1.25 mg | 1602 (1) | |||||
| 2.5 mg | 165,898 (99) | |||||
| over 5 mg | 156 (0.1) | |||||
| Prescribed specialty | ||||||
| Gynecology | 56,418 (33.6) | 50,897 (45.6) | 18,863 (40.8) | 1343 (23.3) | 452 (24.4) | 127,973 (38.4) |
| Non-gynecology | 111,256 (66.4) | 60,597 (54.4) | 27,415 (59.2) | 4433 (76.7) | 1398 (75.6) | 205,099 (61.6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuk, J.-S.; Kim, B.Y. Relationship between Menopausal Hormone Therapy and Oral Cancer: A Cohort Study Based on the Health Insurance Database in South Korea. J. Clin. Med. 2022, 11, 5848. https://doi.org/10.3390/jcm11195848
Yuk J-S, Kim BY. Relationship between Menopausal Hormone Therapy and Oral Cancer: A Cohort Study Based on the Health Insurance Database in South Korea. Journal of Clinical Medicine. 2022; 11(19):5848. https://doi.org/10.3390/jcm11195848
Chicago/Turabian StyleYuk, Jin-Sung, and Bo Young Kim. 2022. "Relationship between Menopausal Hormone Therapy and Oral Cancer: A Cohort Study Based on the Health Insurance Database in South Korea" Journal of Clinical Medicine 11, no. 19: 5848. https://doi.org/10.3390/jcm11195848





