Cardiac Implantable Electronic Devices Infection Assessment, Diagnosis and Management: A Review of the Literature
Abstract
:1. Introduction
2. Risk Factors for Cardiac Implantable Electronic Device Infections
3. Pathogenesis and Microbiology of Cardiac Implantable Electronic Device Infections
4. Pocket Infections
5. Cardiac Device-Related Infective Endocarditis and Bacteriemia
- A left-sided endocarditis in a CIED carrier: the therapeutic approach follows the current guidelines for valve endocarditis [33]. If surgery is required for left-sided endocarditis, an open-heart removal of the CIED is recommended regardless of the presence of acknowledged device involvement. If there is no indication for valve surgery, complete hardware extraction should be considered even if there is no evidence of associated device infection.
- An occult bacteraemia in a CIED carrier: in this case, there is not an alternative source of infection which resolves only after CIED extraction [34].
6. Diagnosis of CDRIE
7. Device Removal Versus Device Retention
8. Antimicrobial Therapy
9. Prevention of CDRIE
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Senning, Ä. Cardiac Pacing in Retrospect. Am. J. Surg. 1983, 145, 733–739. [Google Scholar] [CrossRef]
- Sandoe, J.A.T.; Barlow, G.; Chambers, J.B.; Gammage, M.; Guleri, A.; Howard, P.; Olson, E.; Perry, J.D.; Prendergast, B.D.; Spry, M.J.; et al. Guidelines for the Diagnosis, Prevention and Management of Implantable Cardiac Electronic Device Infection. Report of a Joint Working Party Project on Behalf of the British Society for Antimicrobial Chemotherapy (BSAC, Host Organization), British Heart Rh. J. Antimicrob. Chemother. 2015, 70, 325–359. [Google Scholar] [CrossRef]
- Blomströ M-Lundqvist, C.; Erba, P.A.; Burri, H.; Nielsen, J.C.; Bongiorni, M.G.; Poole, J. European Heart Rhythm Association (EHRA) International Consensus Document on How to Prevent, Diagnose, and Treat Cardiac Implantable Electronic Device Infections. Eur. Heart J. 2020, 12, 515–549. [Google Scholar]
- Sohail, M.R.; Henrikson, C.A.; Braid-Forbes, M.J.; Forbes, K.F.; Lerner, D.J. Mortality and Cost Associated with Cardiovascular Implantable Electronic Device Infections. Arch. Intern. Med. 2011, 171, 1821–1828. [Google Scholar] [CrossRef] [Green Version]
- Greenspon, A.J.; Patel, J.D.; Lau, E.; Ochoa, J.A.; Frisch, D.R.; Ho, R.T.; Pavri, B.B.; Kurtz, S.M. 16-Year Trends in the Infection Burden for Pacemakers and Implantable Cardioverter-Defibrillators in the United States: 1993 to 2008. J. Am. Coll. Cardiol. 2011, 58, 1001–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voigt, A.; Shalaby, A.; Saba, S. Continued Rise in Rates of Cardiovascular Implantable Electronic Device Infections in the United States: Temporal Trends and Causative Insights. PACE-Pacing Clin. Electrophysiol. 2010, 33, 414–419. [Google Scholar] [CrossRef]
- Korantzopoulos, P.; Sideris, S.; Dilaveris, P.; Gatzoulis, K.; Goudevenos, J.A. Infection Control in Implantation of Cardiac Implantable Electronic Devices: Current Evidence, Controversial Points, and Unresolved Issues. Europace 2016, 18, 473–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sohail, M.R.; Uslan, D.Z.; Khan, A.H.; Friedman, P.A.; Hayes, D.L.; Wilson, W.R.; Steckelberg, J.M.; Stoner, S.; Baddour, L.M. Management and Outcome of Permanent Pacemaker and Implantable Cardioverter-Defibrillator Infections. J. Am. Coll. Cardiol. 2007, 49, 1851–1859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landolina, M.; Gasparini, M.; Lunati, M.; Iacopino, S.; Boriani, G.; Bonanno, C.; Vado, A.; Proclemer, A.; Capucci, A.; Zucchiatti, C.; et al. Long-Term Complications Related to Biventricular Defibrillator Implantation: Rate of Surgical Revisions and Impact on Survival: Insights from the Italian Clinicalservice Database. Circulation 2011, 123, 2526–2535. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Grace, A.A.; Newell, S.A.; Stone, D.L.; Shapiro, L.M.; Schofield, P.M.; Petch, M.C. Early Complications After Dual Chamber Versus Single Chamber Pacemaker Implantation. Pacing Clin. Electrophysiol. 1994, 17, 2012–2015. [Google Scholar] [CrossRef]
- Sohail, M.R.; Uslan, D.Z.; Khan, A.H.; Friedman, P.A.; Hayes, D.L.; Wilson, W.R.; Steckelberg, J.M.; Stoner, S.M.; Baddour, L.M. Risk Factor Analysis of Permanent Pacemaker Infection. Clin. Infect. Dis. 2007, 45, 166–173. [Google Scholar] [CrossRef]
- Barbar, T.; Patel, R.; Thomas, G.; Cheung, J. Strategies to Prevent Cardiac Implantable Electronic Device Infection. J. Innov. Card. Rhythm Manag. 2020, 11, 3949. [Google Scholar] [CrossRef] [Green Version]
- Polyzos, K.A.; Konstantelias, A.A.; Falagas, M.E. Risk Factors for Cardiac Implantable Electronic Device Infection: A Systematic Review and Meta-Analysis. Europace 2015, 17, 767–777. [Google Scholar] [CrossRef]
- Catanchin, A.; Murdock, C.J.; Athan, E. Pacemaker Infections: A 10-Year Experience. Heart Lung Circ. 2007, 16, 434–439. [Google Scholar] [CrossRef]
- Sohail, M.R.; Henrikson, C.A.; Braid-Forbes, M.J.; Forbes, K.F.; Lerner, D.J. Comparison of Mortality in Women versus Men with Infections Involving Cardiovascular Implantable Electronic Device. Am. J. Cardiol. 2013, 112, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Johansen, J.B.; Jørgensen, O.D.; Møller, M.; Arnsbo, P.; Mortensen, P.T.; Nielsen, J.C. Infection after Pacemaker Implantation: Infection Rates and Risk Factors Associated with Infection in a Population-Based Cohort Study of 46299 Consecutive Patients. Eur. Heart J. 2011, 32, 991–998. [Google Scholar] [CrossRef]
- Leung, S.; Danik, S. Prevention, Diagnosis, and Treatment of Cardiac Implantable Electronic Device Infections. Curr. Cardiol. Rep. 2016, 18, 58. [Google Scholar] [CrossRef]
- Romeyer-Bouchard, C.; Da Costa, A.; Dauphinot, V.; Messier, M.; Bisch, L.; Samuel, B.; Lafond, P.; Ricci, P.; Isaaz, K. Prevalence and Risk Factors Related to Infections of Cardiac Resynchronization Therapy Devices. Eur. Heart J. 2010, 31, 203–210. [Google Scholar] [CrossRef] [Green Version]
- Sadeghi, H.; Alizadehdiz, A.; Fazelifar, A.; Emkanjoo, Z.; Haghjoo, M. New Insights into Predictors of Cardiac Implantable Electronic Device Infection. Texas Heart Inst. J. 2018, 45, 128–135. [Google Scholar] [CrossRef]
- Tarakji, K.G.; Krahn, A.D.; Poole, J.E.; Mittal, S.; Kennergren, C.; Biffi, M.; Korantzopoulos, P.; Dallaglio, P.D.; Lexcen, D.R.; Lande, J.D.; et al. Risk Factors for CIED Infection after Secondary Procedures: Insights from the WRAP-IT Trial. JACC Clin. Electrophysiol. 2022, 8, 101–111. [Google Scholar] [CrossRef]
- Uslan, D.Z.; Sohail, M.R.; St Sauver, J.L.; Friedman, P.A.; Hayes, D.L.; Stoner, S.M.; Wilson, W.R.; Steckelberg, J.M.; Baddour, L.M. Permanent Pacemaker and Implantable Cardioverter Defibrillator Infection: A Population-Based Study. Arch. Intern. Med. 2007, 167, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Welch, M.; Uslan, D.Z.; Greenspon, A.J.; Sohail, M.R.; Baddour, L.M.; Blank, E.; Carrillo, R.G.; Danik, S.B.; Del Rio, A.; Hellinger, W.; et al. Variability in Clinical Features of Early versus Late Cardiovascular Implantable Electronic Device Pocket Infections. PACE-Pacing Clin. Electrophysiol. 2014, 37, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Chua, J.D.; Wilkoff, B.L.; Lee, I.; Juratli, N.; Longworth, D.L.; Gordon, S.M. Diagnosis and Management of Infections Involving Implantable Electrophysiologic Cardiac Devices. Ann. Intern. Med. 2000, 133, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Del Río, A.; Anguera, I.; Miró, J.M.; Mont, L.; Fowler, V.G.; Azqueta, M.; Mestres, C.A. Surgical Treatment of Pacemaker and Defibrillator Lead Endocarditis: The Impact of Electrode Lead Extraction on Outcome. Chest 2003, 124, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Viganego, F.; O’Donoghue, S.; Eldadah, Z.; Shah, M.H.; Rastogi, M.; Mazel, J.A.; Platia, E.V. Effect of Early Diagnosis and Treatment with Percutaneous Lead Extraction on Survival in Patients with Cardiac Device Infections. Am. J. Cardiol. 2012, 109, 1466–1471. [Google Scholar] [CrossRef]
- Priori, S.G.; Blomstrom-Lundqvist, C.; Mazzanti, A.; Bloma, N.; Borggrefe, M.; Camm, J.; Elliott, P.M.; Fitzsimons, D.; Hatala, R.; Hindricks, G.; et al. 2015 ESC Guidelines for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the Europea. Eur. Heart J. 2015, 17, 1601–1687. [Google Scholar] [CrossRef] [Green Version]
- Del Pozo, J.L.; Patel, R. The Challenge of Treating Biofilm-Associated Bacterial Infections. Clin. Pharmacol. Ther. 2007, 82, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Jan, E.; Camou, F.; Texier-Maugein, J.; Whinnett, Z.; Caubet, O.; Ploux, S.; Pellegrin, J.L.; Ritter, P.; Metayer, P.L.; Roudaut, R.; et al. Microbiologic Characteristics and in Vitro Susceptibility to Antimicrobials in a Large Population of Patients with Cardiovascular Implantable Electronic Device Infection. J. Cardiovasc. Electrophysiol. 2012, 23, 375–381. [Google Scholar] [CrossRef]
- Palmisano, P.; Accogli, M.; Zaccaria, M.; Luzzi, G.; Nacci, F.; Anaclerio, M.; Favale, S. Rate, Causes, and Impact on Patient Outcome of Implantable Device Complications Requiring Surgical Revision: Large Population Survey from Two Centres in Italy. Europace 2013, 15, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.C.; Gerdes, J.C.; Varma, N. Infected Cardiac-Implantable Electronic Devices: Prevention, Diagnosis, and Treatment. Eur. Heart J. 2015, 36, 2484–2490. [Google Scholar] [CrossRef] [Green Version]
- Martinelli, M.; D’Orio Nishioka, S.A.; Varejão, T.; Uipe, D.; Pedrosa, A.A.A.; Costa, R.; Danik, S.B.; De Oliveira, J.C. Efficacy of Antibiotic Prophylaxis before the Implantation of Pacemakers and Cardioverter-Defibrillators: Results of a Large, Prospective, Randomized, Double-Blinded, Placebo-Controlled Trial. Circ. Arrhythmia Electrophysiol. 2009, 2, 29–34. [Google Scholar] [CrossRef]
- Li, J.S.; Sexton, D.J.; Mick, N.; Nettles, R.; Fowler, V.G.; Ryan, T.; Bashore, T.; Corey, G.R. Proposed Modifications to the Duke Criteria for the Diagnosis of Infective Endocarditis. Clin. Infect. Dis. 2000, 30, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. The Task Force for the Management of infective Endocarditis of the European Society of Cardiology (ESC) Endorsed by European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). 2015 ESC Guidelines for the management of infective endocarditis. Eur. Heart J. 2015, 36, 3075–3123. [Google Scholar] [CrossRef] [PubMed]
- Durante-Mangoni, E.; Mattucci, I.; Agrusta, F.; Tripodi, M.F.; Utili, R. Current Trends in the Management of Cardiac Implantable Electronic Device (CIED) Infections. Intern. Emerg. Med. 2013, 8, 465–476. [Google Scholar] [CrossRef]
- Gould, P.A.; Gula, L.J.; Yee, R.; Skanes, A.C.; Klein, G.J.; Krahn, A.D. Cardiovascular Implantable Electrophysiological Device-Related Infections: A Review. Curr. Opin. Cardiol. 2011, 26, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Massoure, P.L.; Reuter, S.; Lafitte, S.; Laborderie, J.; Bordachard, P.; Clementy, J.; Roudaut, R. Pacemaker Endocarditis: Clinical Features and Management of 60 Consecutive Cases. Pacing Clin. Electrophysiol. 2007, 30, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, M.; Okutucu, S.; Ascioglu, S.; Şahin, A.; Aksoy, H.; Deveci, O.S.; Kaya, E.B.; Aytemir, K.; Kabakci, G.; Tokgozoglu, L.; et al. Permanent Pacemaker and Implantable Cardioverter Defibrillator Infections: Seven Years of Diagnostic and Therapeutic Experience of a Single Center. Clin. Cardiol. 2010, 33, 406–411. [Google Scholar] [CrossRef]
- Leone, S.; Ravasio, V.; Durante-Mangoni, E.; Crapis, M.; Carosi, G.; Scotton, P.G.; Barzaghi, N.; Falcone, M.; Chinello, P.; Pasticci, M.B.; et al. Epidemiology, Characteristics, and Outcome of Infective Endocarditis in Italy: The Italian Study on Endocarditis. Infection 2012, 40, 527–535. [Google Scholar] [CrossRef]
- Sohail, M.R.; Palraj, B.R.; Khalid, S.; Uslan, D.Z.; Al-Saffar, F.; Friedman, P.A.; Hayes, D.L.; Lohse, C.M.; Wilson, W.R.; Steckelberg, J.M.; et al. Predicting Risk of Endovascular Device Infection in Patients with Staphylococcus Aureus Bacteremia (PREDICT-SAB). Circ. Arrhythmia Electrophysiol. 2015, 8, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Voet, J.G.; Vandekerckhove, Y.R.; Muyldermans, L.L.; Missault, L.H.; Matthys, L.J. Pacemaker Lead Infection: Report of Three Cases and Review of the Literature. Heart 1999, 81, 88–91. [Google Scholar] [CrossRef]
- Santangelo, L.; Russo, V.; Ammendola, E.; De Crescenzo, I.; Pagano, C.; Savarese, C.; Caruso, A.; Utili, R.; Calabrò, R. Superior Vena Cava Thrombosis after Intravascular AICD Lead Extraction: A Case Report. J. Vasc. Access 2006, 7, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Polewczyk, A.; Janion, M.; Kutarski, A. Cardiac Device Infections: Definition, Classification, Differential Diagnosis, and Management. Pol. Arch. Med. Wewn. 2016, 126, 275–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Downey, B.C.; Juselius, W.E.; Pandian, N.G.; Estes, N.A.M.; Link, M.S. Incidence and Significance of Pacemaker and Implantable Cardioverter-Defibrillator Lead Masses Discovered during Transesophageal Echocardiography. PACE-Pacing Clin. Electrophysiol. 2011, 34, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Kojodjojo, P.; John, R.M.; Epstein, L.M. Disseminated Malignancies Masquerading as Cardiovascular Implantable Electronic Devices Infections. Europace 2011, 13, 821–824. [Google Scholar] [CrossRef]
- DeSimone, D.C.; Sohail, M.R. Infection Management. Card. Electrophysiol. Clin. 2018, 10, 601–607. [Google Scholar] [CrossRef]
- Veloso, T.R.; Amiguet, M.; Rousson, V.; Giddey, M.; Vouillamoz, J.; Moreillon, P.; Entenza, J.M. Induction of Experimental Endocarditis by Continuous Low-Grade Bacteremia Mimicking Spontaneous Bacteremia in Humans. Infect. Immun. 2011, 79, 2006–2011. [Google Scholar] [CrossRef] [Green Version]
- Morgan, S.R. Original Articles. Except. Child 1984, 31, 74–79. [Google Scholar] [CrossRef]
- Uslan, D.Z.; Dowsley, T.F.; Sohail, M.R.; Hayes, D.L.; Friedman, P.A.; Wilson, W.R.; Steckelberg, J.M.; Baddour, L.M. Cardiovascular Implantable Electronic Device Infection in Patients with Staphylococcus Aureus Bacteremia. PACE-Pacing Clin. Electrophysiol. 2010, 33, 407–413. [Google Scholar] [CrossRef]
- Baddour, L.M.; Epstein, A.E.; Erickson, C.C.; Knight, B.P.; Levison, M.E.; Lockhart, P.B.; Masoudi, F.A.; Okum, E.J.; Wilson, W.R.; Beerman, L.B.; et al. Update on Cardiovascular Implantable Electronic Device Infections and Their Management: A Scientific Statement from the American Heart Association. Circulation 2010, 121, 458–477. [Google Scholar] [CrossRef] [Green Version]
- Lennerz, C.; Vrazic, H.; Haller, B.; Braun, S.; Petzold, T.; Ott, I.; Lennerz, A.; Michel, J.; Blažek, P.; Deisenhofer, I.; et al. Biomarker-Based Diagnosis of Pacemaker and Implantable Cardioverter Defibrillator Pocket Infections: A Prospective, Multicentre, Case-Control Evaluation. PLoS ONE 2017, 12, e0172384. [Google Scholar] [CrossRef]
- Arnold, C.J.; Chu, V.H. Cardiovascular Implantable Electronic Device Infections. Infect. Dis. Clin. N. Am. 2018, 32, 811–825. [Google Scholar] [CrossRef] [PubMed]
- Erba, P.A.; Sollini, M.; Conti, U.; Bandera, F.; Tascini, C.; De Tommasi, S.M.; Zucchelli, G.; Doria, R.; Menichetti, F.; Bongiorni, M.G.; et al. Radiolabeled WBC Scintigraphy in the Diagnostic Workup of Patients with Suspected Device-Related Infections. JACC Cardiovasc. Imaging 2013, 6, 1075–1086. [Google Scholar] [CrossRef] [PubMed]
- Ten Hove, D.; Slart, R.H.J.A.; Sinha, B.; Glaudemans, A.W.J.M.; Budde, R.P.J. 18 F-FDG PET/CT in Infective Endocarditis: Indications and Approaches for Standardization. Curr. Cardiol. Rep. 2021, 23, 130. [Google Scholar] [CrossRef] [PubMed]
- Juneau, D.; Golfam, M.; Hazra, S.; Zuckier, L.S.; Garas, S.; Redpath, C.; Bernick, J.; Leung, E.; Chih, S.; Wells, G.; et al. Positron Emission Tomography and Single-Photon Emission Computed Tomography Imaging in the Diagnosis of Cardiac Implantable Electronic Device Infection: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Imaging 2017, 10, e005772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cautela, J.; Alessandrini, S.; Cammilleri, S.; Giorgi, R.; Richet, H.; Casalta, J.P.; Habib, G.; Raoult, D.; Mundler, O.; Deharo, J.C. Diagnostic Yield of FDG Positron-Emission Tomography/Computed Tomography in Patients with CEID Infection: A Pilot Study. Europace 2013, 15, 252–257. [Google Scholar] [CrossRef] [Green Version]
- Sohail, M.R.; Uslan, D.Z.; Khan, A.H.; Friedman, P.A.; Hayes, D.L.; Wilson, W.R.; Steckelberg, J.M.; Jenkins, S.M.; Baddour, L.M. Infective Endocarditis Complicating Permanent Pacemaker and Implantable Cardioverter-Defibrillator Infection. Mayo Clin. Proc. 2008, 83, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Mulpuru, S.K.; Pretorius, V.G.; Birgersdotter-Green, U.M. Device Infections: Management and Indications for Lead Extraction. Circulation 2013, 128, 1031–1038. [Google Scholar] [CrossRef]
- Le, K.Y.; Sohail, M.R.; Friedman, P.A.; Uslan, D.Z.; Cha, S.S.; Hayes, D.L.; Wilson, W.R.; Steckelberg, J.M.; Baddour, L.M. Impact of Timing of Device Removal on Mortality in Patients with Cardiovascular Implantable Electronic Device Infections. Heart Rhythm 2011, 8, 1678–1685. [Google Scholar] [CrossRef]
- Huang, X.M.; Fu, H.X.; Zhong, L.; Cao, J.; Asirvatham, S.J.; Baddour, L.M.; Sohail, M.R.; Nkomo, V.T.; Nishimura, R.A.; Greason, K.L.; et al. Outcomes of Transvenous Lead Extraction for Cardiovascular Implantable Electronic Device Infections in Patients with Prosthetic Heart Valves. Circ. Arrhythmia Electrophysiol. 2016, 9, e004188. [Google Scholar] [CrossRef] [Green Version]
- Okada, A.; Tabata, H.; Shoda, M.; Shoin, W.; Kobayashi, H.; Okano, T.; Yoshie, K.; Kato, K.; Saigusa, T.; Ebisawa, S.; et al. Safe and Effective Transvenous Lead Extraction for Elderly Patients Utilizing Non-Laser and Laser Tools: A Single-Center Experience in Japan. Heart Vessels 2021, 36, 882–889. [Google Scholar] [CrossRef]
- Starck, C.T.; Gonzalez, E.; Al-Razzo, O.; Mazzone, P.; Delnoy, P.P.; Breitenstein, A.; Steffel, J.; Eulert-Grehn, J.; Lanmüller, P.; Melillo, F.; et al. Results of the Patient-Related Outcomes of Mechanical Lead Extraction Techniques (PROMET) Study: A Multicentre Retrospective Study on Advanced Mechanical Lead Extraction Techniques. Europace 2020, 22, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, F.M.; Schoenfeld, M.H.; Wilkoff, B.L.; Berul, C.I.; Birgersdotter-Green, U.M.; Carrillo, R.; Cha, Y.M.; Clancy, J.; Deharo, J.C.; Ellenbogen, K.A.; et al. 2017 HRS Expert Consensus Statement on Cardiovascular Implantable Electronic Device Lead Management and Extraction. Heart Rhythm 2017, 14, e503–e551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Athan, E.; Chu, V.H.; Tattevin, P.; Selton-Suty, C.; Jones, P.; Naber, C.; Miró, J.M.; Ninot, S.; Fernández-Hidalgo, N.; Durante-Mangoni, E.; et al. Clinical Characteristics and Outcome of Infective Endocarditis Involving Implantable Cardiac Devices. JAMA 2012, 307, 1727–1735. [Google Scholar] [CrossRef]
- Blomström-Lundqvist, C.; Traykov, V.; Erba, P.A.; Burri, H.; Nielsen, J.C.; Bongiorni, M.G.; Poole, J.; Boriani, G.; Costa, R.; Deharo, J.C.; et al. European Heart Rhythm Association (EHRA) International Consensus Document on How to Prevent, Diagnose, and Treat Cardiac Implantable Electronic Device Infections-Endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), The Latin American Heart Rhythm Society (LAHRS), International Society for Cardiovascular Infectious Diseases (ISCVID) and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2020, 41, 2012–2032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riaz, T.; Nienaber, J.J.C.; Baddour, L.M.; Walker, R.C.; Park, S.J.; Sohail, M.R. Cardiovascular Implantable Electronic Device Infections in Left Ventricular Assist Device Recipients. Pacing Clin. Electrophysiol. 2014, 37, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Grammes, J.A.; Schulze, C.M.; Al-Bataineh, M.; Yesenosky, G.A.; Saari, C.S.; Vrabel, M.J.; Horrow, J.; Chowdhury, M.; Fontaine, J.M.; Kutalek, S.P. Percutaneous Pacemaker and Implantable Cardioverter-Defibrillator Lead Extraction in 100 Patients with Intracardiac Vegetations Defined by Transesophageal Echocardiogram. J. Am. Coll. Cardiol. 2010, 55, 886–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez Baztarrica, G.; Gariglio, L.; Salvaggio, F.; Reolõn, E.; Blanco, N.; Mazzetti, H.; Villecco, S.; Botbol, A.; Porcile, R. Transvenous Extraction of Pacemaker Leads in Infective Endocarditis with Vegetations ≥20 Mm: Our Experience. Clin. Cardiol. 2012, 35, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Starck, C.T.; Schaerf, R.H.M.; Breitenstein, A.; Najibi, S.; Conrad, J.; Berendt, J.; Esmailian, F.; Eulert-Grehn, J.; Dreizler, T.; Falk, V. Transcatheter Aspiration of Large Pacemaker and Implantable Cardioverter-Defibrillator Lead Vegetations Facilitating Safe Transvenous Lead Extraction. Europace 2020, 22, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Schaerf, R.H.M.; Najibi, S.; Conrad, J. Percutaneous Vacuum-Assisted Thrombectomy Device Used for Removal of Large Vegetations on Infected Pacemaker and Defibrillator Leads as an Adjunct to Lead Extraction. J. Atr. Fibrillation 2016, 9, 1455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakajima, I.; Narui, R.; Tokutake, K.; Norton, C.A.; Stevenson, W.G.; Richardson, T.D.; Ellis, C.R.; Crossley, G.H.; Montgomery, J.A. Staphylococcus Bacteremia without Evidence of Cardiac Implantable Electronic Device Infection. Heart Rhythm 2021, 18, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.M.; Desimone, D.C.; Sohail, M.R.; Baddour, L.M.; Wilson, W.R.; Steckelberg, J.M.; Virk, A. Outcomes in Patients With Cardiovascular Implantable Electronic Device Infection Managed with Chronic Antibiotic Suppression. Clin. Infect. Dis. 2017, 64, 1516–1521. [Google Scholar] [CrossRef]
- El-Chami, M.F.; Al-Samadi, F.; Clementy, N.; Garweg, C.; Martinez-Sande, J.L.; Piccini, J.P.; Iacopino, S.; Lloyd, M.; Viñolas Prat, X.; Jacobsen, M.D.; et al. Updated Performance of the Micra Transcatheter Pacemaker in the Real-World Setting: A Comparison to the Investigational Study and a Transvenous Historical Control. Heart Rhythm 2018, 15, 1800–1807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garweg, C.; Vandenberk, B.; Jentjens, S.; Foulon, S.; Hermans, P.; Poels, P.; Haemers, P.; Ector, J.; Willems, R. Bacteraemia after Leadless Pacemaker Implantation. J. Cardiovasc. Electrophysiol. 2020, 31, 2440–2447. [Google Scholar] [CrossRef] [PubMed]
- Peacock, J.E.; Stafford, J.M.; Le, K.; Sohail, M.R.; Baddour, L.M.; Prutkin, J.M.; Danik, S.B.; Vikram, H.R.; Hernandez-Meneses, M.; Miró, J.M.; et al. Attempted Salvage of Infected Cardiovascular Implantable Electronic Devices: Are There Clinical Factors That Predict Success? Pacing Clin. Electrophysiol. 2018, 41, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Halawa, A.; Henry, P.D.; Sarubbi, F.A. Candida Endocarditis Associated with Cardiac Rhythm Management Devices: Review with Current Treatment Guidelines. Mycoses 2011, 54, e168–e174. [Google Scholar] [CrossRef]
- Krahn, A.D.; Longtin, Y.; Philippon, F.; Birnie, D.H.; Manlucu, J.; Angaran, P.; Rinne, C.; Coutu, B.; Low, R.A.; Essebag, V.; et al. Prevention of Arrhythmia Device Infection Trial: The PADIT Trial. J. Am. Coll. Cardiol. 2018, 72, 3098–3109. [Google Scholar] [CrossRef]
- Henrikson, C.A.; Sohail, M.R.; Acosta, H.; Johnson, E.E.; Rosenthal, L.; Pachulski, R.; Dan, D.; Paladino, W.; Khairallah, F.S.; Gleed, K.; et al. Antibacterial Envelope Is Associated with Low Infection Rates after Implantable Cardioverter-Defibrillator and Cardiac Resynchronization Therapy Device Replacement: Results of the Citadel and Centurion Studies. JACC Clin. Electrophysiol. 2017, 3, 1158–1167. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Wilkoff, B.L.; Kennergren, C.; Poole, J.E.; Corey, R.; Bracke, F.A.; Curnis, A.; Addo, K.; Martinez-Arraras, J.; Issa, Z.F.; et al. The World-Wide Randomized Antibiotic Envelope Infection Prevention (WRAP-IT) Trial: Long-Term Follow-Up. Heart Rhythm 2020, 17, 1115–1122. [Google Scholar] [CrossRef] [Green Version]
- Kewcharoen, J.; Kanitsoraphan, C.; Thangjui, S.; Leesutipornchai, T.; Saowapa, S.; Pokawattana, A.; Navaravong, L. Postimplantation Pocket Hematoma Increases Risk of Cardiac Implantable Electronic Device Infection: A Meta-Analysis. J. Arrhythmia 2021, 37, 635–644. [Google Scholar] [CrossRef]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on Cardiac Pacing and Cardiac Resynchronization TherapyDeveloped by the Task Force on Cardiac Pacing and Cardiac Resynchronization Therapy of the European Society of Cardiology (ESC) With the Special Contribution of the European Heart Rhythm Association (EHRA). Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef]
- Birnie, D.H.; Healey, J.S.; Wells, G.A.; Verma, A.; Tang, A.S.; Krahn, A.D.; Simpson, C.S.; Ayala-Paredes, F.; Coutu, B.; Leiria, T.L.L.; et al. Pacemaker or Defibrillator Surgery without Interruption of Anticoagulation. N. Engl. J. Med. 2013, 368, 2084–2093. [Google Scholar] [CrossRef] [PubMed]
- Birnie, D.H.; Healey, J.S.; Wells, G.A.; Ayala-Paredes, F.; Coutu, B.; Sumner, G.L.; Becker, G.; Verma, A.; Philippon, F.; Kalfon, E.; et al. Continued vs. Interrupted Direct Oral Anticoagulants at the Time of Device Surgery, in Patients with Moderate to High Risk of Arterial Thrombo-Embolic Events (BRUISE CONTROL-2). Eur. Heart J. 2018, 39, 3973–3979. [Google Scholar] [CrossRef] [PubMed]
- Steffel, J.; Collins, R.; Antz, M.; Cornu, P.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; Rowell, N.; et al. 2021 European Heart Rhythm Association Practical Guide on the Use of Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation. Europace 2021, 23, 1612–1676. [Google Scholar] [CrossRef]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Gui. J. Am. Coll. Cardiol. 2018, 72, 1677–1749. [Google Scholar] [CrossRef] [PubMed]
- Baddour, L.M.; Weiss, R.; Mark, G.E.; El-Chami, M.F.; Biffi, M.; Probst, V.; Lambiase, P.D.; Miller, M.A.; McClernon, T.; Hansen, L.K.; et al. Diagnosis and Management of Subcutaneous Implantable Cardioverter-Defibrillator Infections Based on Process Mapping. Pacing Clin. Electrophysiol. 2020, 43, 958–965. [Google Scholar] [CrossRef]
- El-Chami, M.F.; Bonner, M.; Holbrook, R.; Stromberg, K.; Mayotte, J.; Molan, A.; Sohail, M.R.; Epstein, L.M. Leadless Pacemakers Reduce Risk of Device-Related Infection: Review of the Potential Mechanisms. Heart Rhythm 2020, 17, 1393–1397. [Google Scholar] [CrossRef] [PubMed]
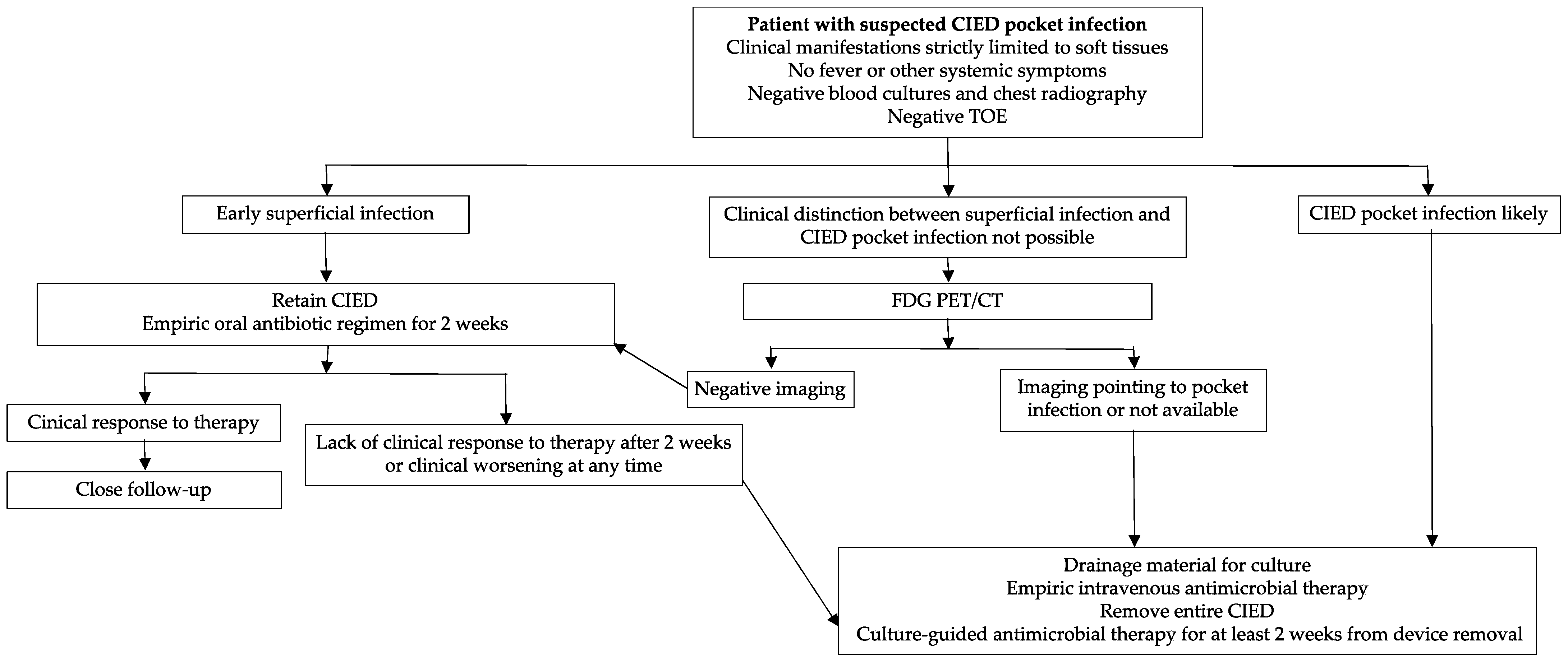



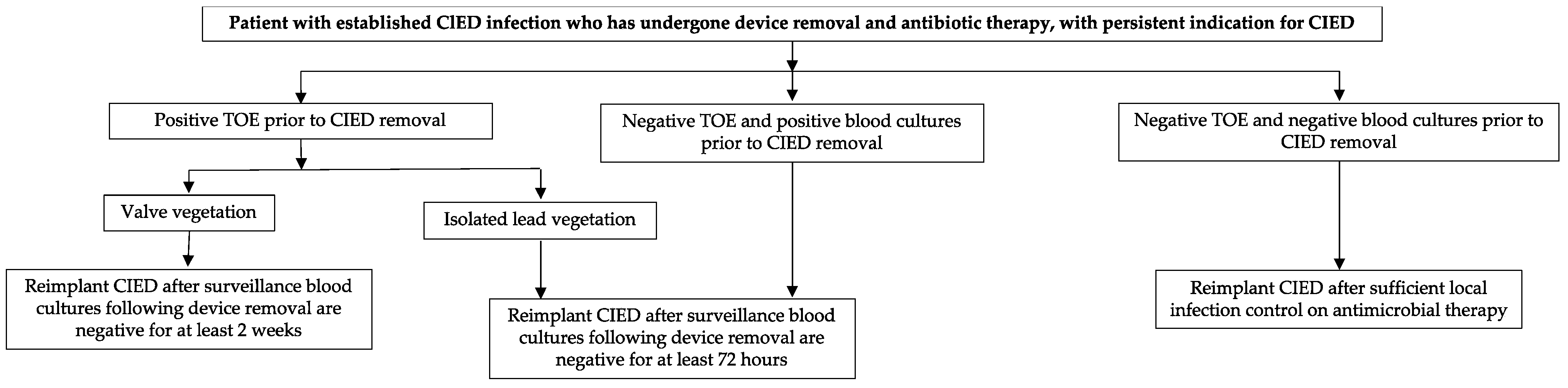
| Clinical Entity | Characteristics | Incidence | Time Period after Implant | Prognosis (+ Good Prognosis, − Bad Prognosis) | Management |
|---|---|---|---|---|---|
| Pocket Hematoma | Ecchymosis, mild effusion in the pocket and swelling. | 1–20% | Within 2 weeks (usually <48 h) | + | Compression bandage, removal of antithrombotic therapy, specific pocket compression vest. |
| Post-implantation inflammation | Erythema affecting the incision site, without purulent exudate, dehiscence, fluctuance or systemic signs of infection. | 1–10% | Within 30 days (usually <7 days) | + + | Close observation. Antibiotics not mandatory. |
| Superficial infection of surgical wound | A small, localised area of erythema and/or purulence associated with a suture defect. | 0.5–5% | Within 30 days (usually <14 days) | +/− | Removal of the suture and antimicrobial therapy, if indicated. |
| Uncomplicated pocket infection | From mild inflammation to deformation, fluctuance, adherence of pocket, purulent material drainage from incision sites, fistula formation, wound dehiscence and exposure of the generator or leads. | 0.5–2.2% | Whenever, traditionally within 1 year | − | Cardiac implantable electronic device removal/extraction associated with a specific antibiotic regimen. |
| Surgical Incision Infection | |
| Empirical Treatment | |
| Oral antibiotic covering StA: | flucloxacillin 1 gr (every 6–8 h) |
| If high MRSA prevalence: | trimethoprim-sulfamethoxazole, clyndamicin, doxyciclin, linezolid |
| Targeted after culture results | |
| Duration: 7–10 days | |
| Isolated Pocket Infection | |
| Empirical Treatment | |
| Intravenous treatment covering StA and multi-resistant CoNS | vancomycin 30–60 mg/kg/day i.v. in 2–3 doses daptomycin 8–10 mg/kg i.v. o.d.) |
| If systemic symptoms | |
| For additional Gram-negative coverage, combine with 3rd generation cephalosporin (or broader beta-lactam) or gentamicin | cephalosporins standard dose or gentamicin 5–7 mg/kg i.v. o.d. |
| Targeted after culture results | |
| If sensitive Staphylococci: | flucloxacillin 8 g/day i.v. in 4 doses or 1st generation cephalosporins (standard dose) |
| Targeted after culture results | |
| Duration post-extraction: 10–14 days | |
| Systemic Infections | |
| Without Vegetation on Leads or Valves ± Pocket Infection | |
| Empirical combination treatment covering multi-resistant Staphylococci and Gram-negative bacteria | vancomycin 30–60 mg/kg/day i.v. in 2–3 doses (alternative: daptomicin 8–10 mg/kg i.v. o.d.) plus 3rd generation cephalosporins standard dose i.v (or broader beta-lactam) or gentamicin 5–7 mg/kg i.v. o.d. |
| Targeted after culture results | |
| If sensitive Staphylococci | flucloxacillin 8 g/day i.v. in 4 doses 1st generation cephalosporin (standard dose) |
| Targeted after culture results | |
| Duration post-extraction: 4 weeks (2 weeks if negative blood cultures) | |
| CIED endocarditis with vegetation on leads and/or valves ± embolism | |
| Empirical treatment | vancomycin 30–60 mg/kg/day i.v. in 2–3 doses (alternative: daptomicin 8–10 mg/kg i.v. o.d.) plus 3rd generation cephalosporins standard dose i.v (or broader beta-lactam) or gentamicin 5–7 mg/kg i.v. o.d. |
| If prosthetic valve and staphylococcal infection | add rifampicin after 5–7 days, 900–1200 mg/day in two doses (orally or i.v.) |
| Adjust to culture results according to ESC Endocarditis Guidelines | |
Duration:
| |
| Bacteraemia in a CIED patient without signs of pocket infection or echocardiographic evidence of lead or valve involvement | |
| According to pathogen-specific treatment guidelines | |
| Attempted Salvage Therapy and Long-Term Suppressive Therapy | |
| I.v. antibiotics as in prosthetic valve endocarditis for 4–6 weeks | |
| Stop antibiotic therapy under close follow-up or continue individualised long-term suppressive oral therapy | |
| Strategy | Description |
|---|---|
| Antibiotic prophylaxis before CIED implantation | A first-generation cephalosporin. Vancomycin, teicoplanin and daptomicin in patients with contraindications to cephalosporins. |
| Antibiotic prophylaxis before other procedures | It is not recommended based on limited evidence |
| Antibacterial envelope | Reduces the rate of CDRIE |
| Hematoma pocket prevention | It is a risk factor for infection. Needle aspiration should be avoided. |
| Nasal swab | No studies for patients with CIED |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toriello, F.; Saviano, M.; Faggiano, A.; Gentile, D.; Provenzale, G.; Pollina, A.V.; Gherbesi, E.; Barbieri, L.; Carugo, S. Cardiac Implantable Electronic Devices Infection Assessment, Diagnosis and Management: A Review of the Literature. J. Clin. Med. 2022, 11, 5898. https://doi.org/10.3390/jcm11195898
Toriello F, Saviano M, Faggiano A, Gentile D, Provenzale G, Pollina AV, Gherbesi E, Barbieri L, Carugo S. Cardiac Implantable Electronic Devices Infection Assessment, Diagnosis and Management: A Review of the Literature. Journal of Clinical Medicine. 2022; 11(19):5898. https://doi.org/10.3390/jcm11195898
Chicago/Turabian StyleToriello, Filippo, Massimo Saviano, Andrea Faggiano, Domitilla Gentile, Giovanni Provenzale, Alberto Vincenzo Pollina, Elisa Gherbesi, Lucia Barbieri, and Stefano Carugo. 2022. "Cardiac Implantable Electronic Devices Infection Assessment, Diagnosis and Management: A Review of the Literature" Journal of Clinical Medicine 11, no. 19: 5898. https://doi.org/10.3390/jcm11195898






