Association of Glycosylated Hemoglobin Level and Cancer-Related Mortality in Patients without Diabetes
Abstract
1. Introduction
2. Materials and Methods
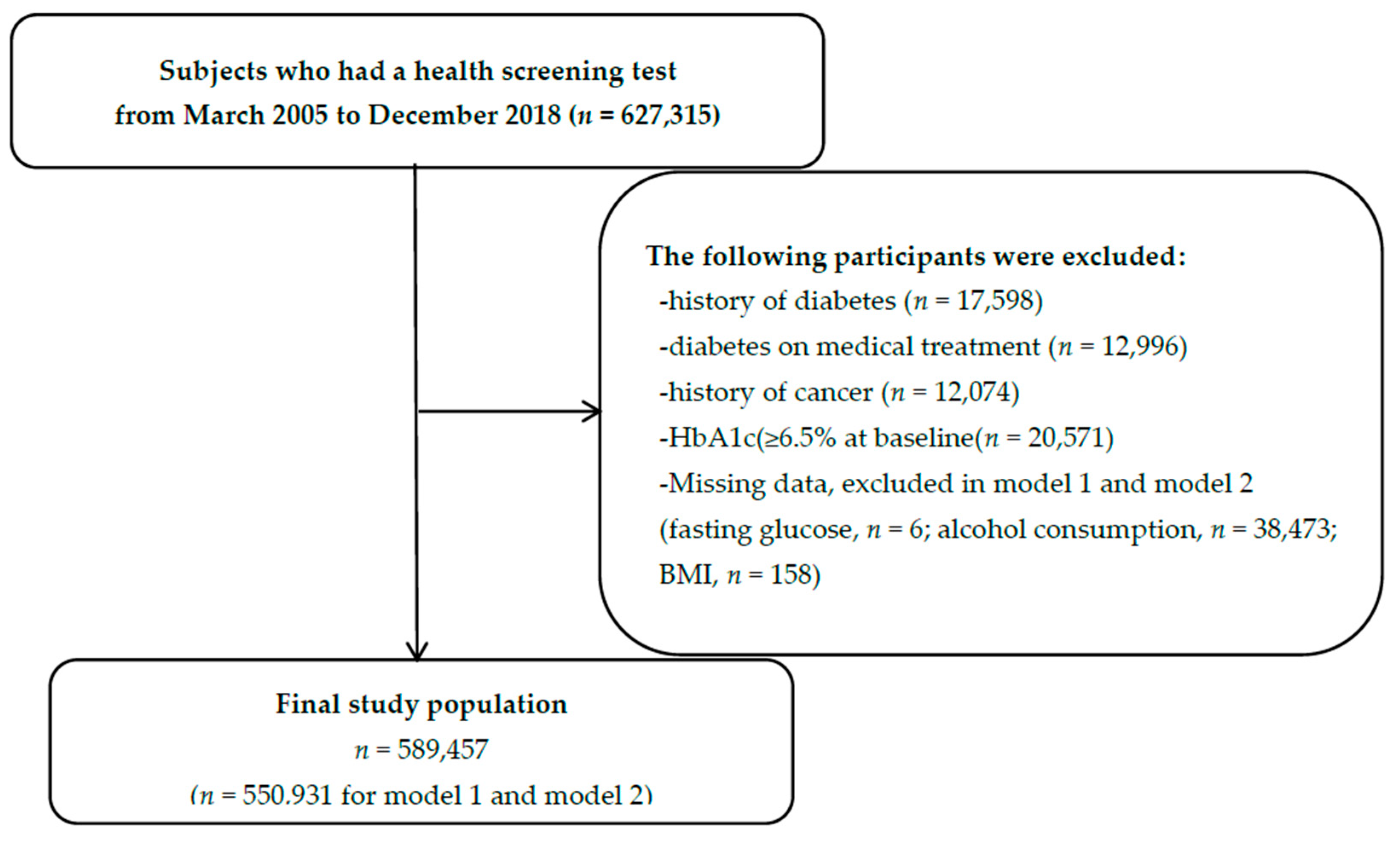
2.1. Study Population and Study Period
2.2. Laboratory Assays
2.3. Cancer Outcomes
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Cohort Description
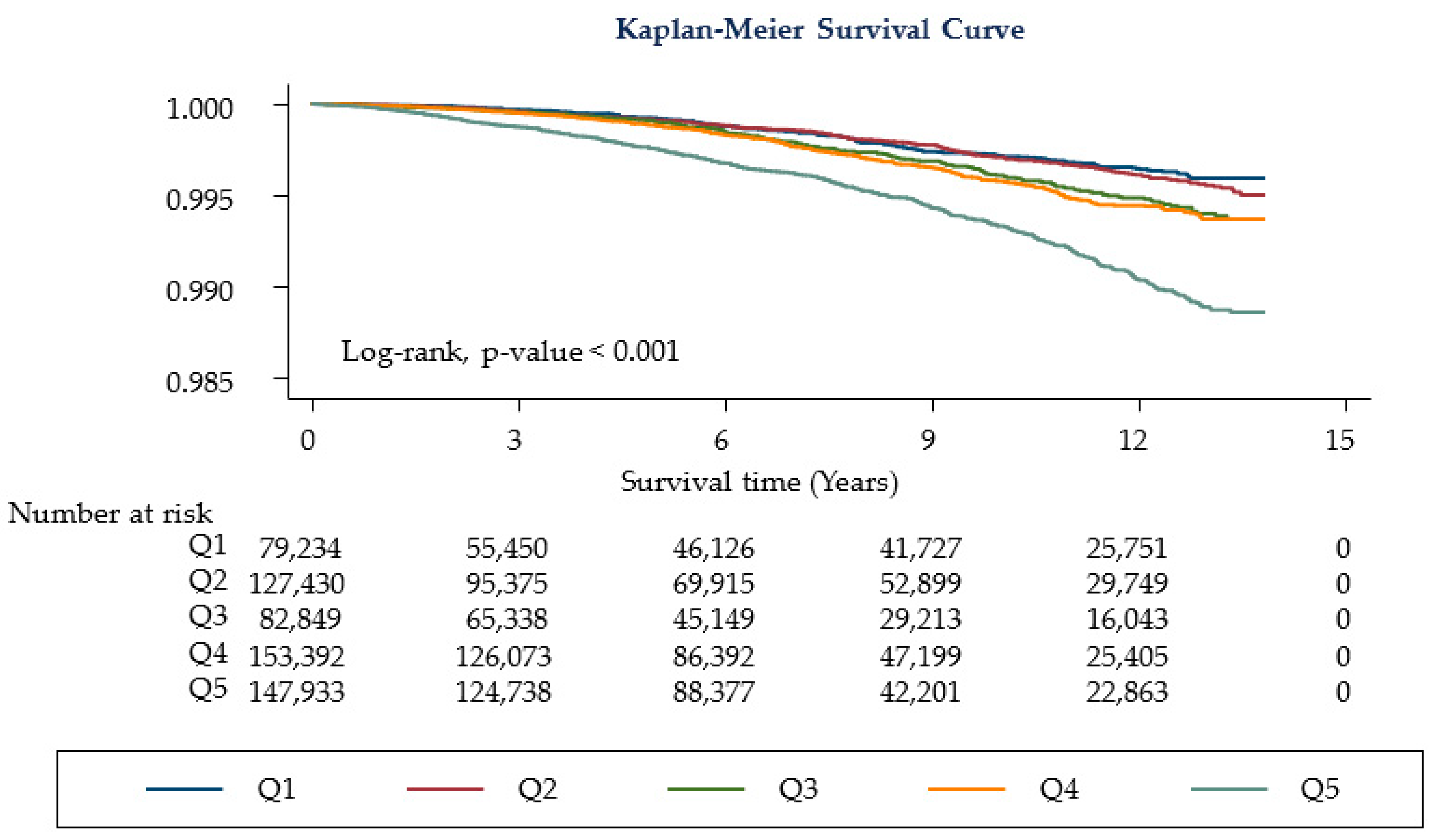
3.2. Cancer Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Upadhyay, J.; Polyzos, S.A.; Perakakis, N.; Thakkar, B.; Paschou, S.A.; Katsiki, N.; Underwood, P.; Park, K.H.; Seufert, J.; Kang, E.S.; et al. Pharmacotherapy of type 2 diabetes: An update. Metabolism 2018, 78, 13–42. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.-W.; Won, Y.-J.; Hong, S.; Kong, H.-J.; Lee, E.S. Prediction of Cancer Incidence and Mortality in Korea, 2020. Cancer Res. Treat. 2020, 52, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.M.; Cusi, K. Prediabetes: A Worldwide Epidemic. Endocrinol. Metab. Clin. N. Am. 2016, 45, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.-H.; Son, J.W.; Kang, S.; Kim, W.J.; Kim, H.-S.; Kim, H.S.; Seo, M.; Shin, H.-J.; Lee, S.-S.; Jeong, S.J. Diabetes Fact Sheets in Korea, 2020: An Appraisal of Current Status. Diabetes Metab. J. 2021, 45, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Selvin, E. Hemoglobin A1c—Using Epidemiology to Guide Medical Practice: Kelly West Award Lecture 2020. Diabetes Care 2021, 1, dci210035. [Google Scholar] [CrossRef] [PubMed]
- Consensus Committee. Consensus Statement on the Worldwide Standardization of the Hemoglobin A1C Measurement: The American Diabetes Association, European Association for the Study of Diabetes, International Federation of Clinical Chemistry and Laboratory Medicine, and the International Diabetes Federation. Diabetes Care 2007, 30, 2399–2400. [Google Scholar] [CrossRef]
- Welsh, K.J.; Kirkman, M.S.; Sacks, D.B. Role of Glycated Proteins in the Diagnosis and Management of Diabetes: Research Gaps and Future Directions. Diabetes Care 2016, 39, 1299–1306. [Google Scholar] [CrossRef]
- International Expert Committee. International Expert Committee Report on the Role of the A1C Assay in the Diagnosis of Diabetes. Diabetes Care 2009, 32, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E.; Harlan, D.M.; Archer, M.C.; Bergenstal, R.M.; Gapstur, S.M.; Habel, L.A.; Pollak, M.; Regensteiner, J.G.; Yee, D. Diabetes and cancer: A consensus report. CA Cancer J. Clin. 2010, 60, 207–221. [Google Scholar] [CrossRef] [PubMed]
- De Beer, J.; Liebenberg, L. Does cancer risk increase with HbA 1c, independent of diabetes? Br. J. Cancer 2014, 110, 2361–2368. [Google Scholar] [CrossRef] [PubMed]
- Goto, A.; Noda, M.; Sawada, N.; Kato, M.; Hidaka, A.; Mizoue, T.; Shimazu, T.; Yamaji, T.; Iwasaki, M.; Sasazuki, S. High hemoglobin A1c levels within the non-diabetic range are associated with the risk of all cancers. Int. J. Cancer 2016, 138, 1741–1753. [Google Scholar] [CrossRef] [PubMed]
- Rentsch, C.T.; Farmer, R.E.; Eastwood, S.V.; Mathur, R.; Garfield, V.; Farmaki, A.-E.; Bhaskaran, K.; Chaturvedi, N.; Smeeth, L. Risk of 16 cancers across the full glycemic spectrum: A population-based cohort study using the UK Biobank. BMJ Open Diabetes Res. Care 2020, 8, e001600. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; He, J.; Li, M.; Tang, X.; Hu, R.; Shi, L.; Su, Q.; Peng, K.; Xu, M.; Xu, Y.; et al. Predictive Value of Fasting Glucose, Postload Glucose, and Hemoglobin A1c on Risk of Diabetes and Complications in Chinese Adults. Diabetes Care 2019, 42, 1539–1548. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.A.; Spechler, S.J.; Huerta, S.; Dredar, S.; Little, B.B.; Cryer, B. Elevated HbA1c Is an Independent Predictor of Aggressive Clinical Behavior in Patients with Colorectal Cancer: A Case-Control Study. Dig. Dis. Sci. 2008, 53, 2486–2494. [Google Scholar] [CrossRef] [PubMed]
- De Bruijn, K.M.J.; Arends, L.R.; Hansen, B.E.; Leeflang, S.; Ruiter, R.; van Eijck, C.H.J. Systematic review and meta-analysis of the association between diabetes mellitus and incidence and mortality in breast and colorectal cancer. Br. J. Surg. 2013, 100, 1421–1429. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, H.; Chen, P.; Yang, J.; Lin, S.; Liu, T.; Chen, S.; Lu, S.; Chen, J.; Chen, W.; et al. An Elevated HbA1c Level Is Associated With Short-Term Adverse Outcomes in Patients With Gastrointestinal Cancer and Type 2 Diabetes Mellitus. J. Clin. Med. Res. 2017, 9, 303–309. [Google Scholar] [CrossRef][Green Version]
- Bonagiri, P.R.; Shubrook, J.H. Review of Associations Between Type 2 Diabetes and Cancer. Clin. Diabetes 2020, 38, 256–265. [Google Scholar] [CrossRef]
- Bancks, M.P.; Odegaard, A.O.; Pankow, J.S.; Koh, W.P.; Yuan, J.M.; Gross, M.D.; Pereira, M.A. Glycated hemoglobin and all-cause and cause-specific mortality in Singaporean Chinese without diagnosed diabetes: The Singapore Chinese Health Study. Diabetes Care 2014, 37, 3180–3187. [Google Scholar] [CrossRef][Green Version]
- Islam, Z.; Akter, S.; Inoue, Y.; Hu, H.; Kuwahara, K.; Nakagawa, T.; Honda, T.; Yamamoto, S.; Okazaki, H.; Miyamoto, T.; et al. Prediabetes, Diabetes, and the Risk of All-Cause and Cause-Specific Mortality in a Japanese Working Population: Japan Epidemiology Collaboration on Occupational Health Study. Diabetes Care 2021, 44, 757–764. [Google Scholar] [CrossRef]
- Hope, C.; Robertshaw, A.; Cheung, K.; Idris, I.; English, E. Relationship between HbA1c and cancer in people with or without diabetes: A systematic review. Diabet. Med. 2016, 33, 1013–1025. [Google Scholar] [CrossRef]
- Choe, Y.J.; Choe, S.A.; Cho, S.I. Trends in Infectious Disease Mortality, South Korea, 1983–2015. Emerg. Infect. Dis. 2018, 24, 320–327. [Google Scholar] [CrossRef] [PubMed]
- KIM, Y.J.; SHIM, J.-S.; CHOI, C.-B.; BAE, S.-C. Mortality and Incidence of Malignancy in Korean Patients with Rheumatoid Arthritis. J. Rheumatol. 2012, 39, 226–232. [Google Scholar] [CrossRef]
- Kim, N.H.; Chang, Y.; Lee, S.R.; Ryu, S.; Kim, H.J. Glycemic Status, Insulin Resistance, and Risk of Pancreatic Cancer Mortality in Individuals With and Without Diabetes. Off. J. Am. Coll. Gastroenterol. ACG 2020, 115, 1840–1848. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J. An Overview of Current Physical Activity Recommendations in Primary Care. Korean J. Fam. Med. 2019, 40, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Ryan, H.; Trosclair, A.; Gfroerer, J. Adult current smoking: Differences in definitions and prevalence estimates—NHIS and NSDUH, 2008. J. Environ. Public Health 2012, 2012, 918368. [Google Scholar] [CrossRef] [PubMed]
- Baur, D.M.; Klotsche, J.; Hamnvik, O.P.; Sievers, C.; Pieper, L.; Wittchen, H.U.; Stalla, G.K.; Schmid, R.M.; Kales, S.N.; Mantzoros, C.S. Type 2 diabetes mellitus and medications for type 2 diabetes mellitus are associated with risk for and mortality from cancer in a German primary care cohort. Metabolism 2011, 60, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, X.; Zhang, X.; Yang, Y.; Feng, Z.; Liu, X. Association of serum hemoglobin A1c, C-peptide and insulin-like growth factor-1 levels with the occurrence and development of lung cancer. Mol. Clin. Oncol. 2014, 2, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Saydah, S.H.; Platz, E.A.; Rifai, N.; Pollak, M.N.; Brancati, F.L.; Helzlsouer, K.J. Association of markers of insulin and glucose control with subsequent colorectal cancer risk. Cancer Epidemiol. Prev. Biomark. 2003, 12, 412–418. [Google Scholar]
- Platz, E.A.; Hankinson, S.E.; Rifai, N.; Colditz, G.A.; Speizer, F.E.; Giovannucci, E. Glycosylated hemoglobin and risk of colorectal cancer and adenoma (United States). Cancer Causes Control 1999, 10, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, S.; Rohrmann, S.; Jenab, M.; Biessy, C.; Sieri, S.; Palli, D.; Tumino, R.; Mattiello, A.; Vineis, P.; Nieters, A. Glycosylated hemoglobin and risk of colorectal cancer in men and women, the European prospective investigation into cancer and nutrition. Cancer Epidemiol. Prev. Biomark. 2008, 17, 3108–3115. [Google Scholar] [CrossRef] [PubMed]
- Joshu, C.E.; Prizment, A.E.; Dluzniewski, P.J.; Menke, A.; Folsom, A.R.; Coresh, J.; Yeh, H.C.; Brancati, F.L.; Platz, E.A.; Selvin, E. Glycated hemoglobin and cancer incidence and mortality in the Atherosclerosis in Communities (ARIC) Study, 1990–2006. Int. J. Cancer 2012, 131, 1667–1677. [Google Scholar] [CrossRef] [PubMed]
- Silbernagel, G.; Grammer, T.B.; Winkelmann, B.R.; Boehm, B.O.; März, W. Glycated Hemoglobin Predicts All-Cause, Cardiovascular, and Cancer Mortality in People Without a History of Diabetes Undergoing Coronary Angiography. Diabetes Care 2011, 34, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Ramdass, V.; Caskey, E.; Sklarz, T.; Ajmeri, S.; Patel, V.; Balogun, A.; Pomary, V.; Hall, J.; Qari, O.; Tripathi, R. Association Between Obesity and Cancer Mortality: An Internal Medicine Outpatient Clinic Perspective. J. Clin. Med. Res. 2021, 13, 377. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.H.; Qiao, Q.; Zethelius, B.; Pyörälä, K.; Söderberg, S.; Pajak, A.; Stehouwer, C.D.A.; Heine, R.J.; Jousilahti, P.; Ruotolo, G.; et al. Diabetes, prediabetes and cancer mortality. Diabetologia 2010, 53, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Plácido, J.; Ferreira, J.V.; de Oliveira, F.; Sant’Anna, P.; Monteiro-Junior, R.S.; Laks, J.; Deslandes, A.C. Association among 2-min step test, functional level and diagnosis of dementia. Dement. Neuropsychol. 2019, 13, 97–103. [Google Scholar] [CrossRef]
- Park, J.H.; Hong, J.Y.; Park, Y.S.; Kang, G.; Han, K.; Park, J.O. Association of prediabetes, diabetes, and diabetes duration with biliary tract cancer risk: A nationwide cohort study. Metabolism 2021, 123, 154848. [Google Scholar] [CrossRef]
- Yoon, Y.S.; Keum, N.; Zhang, X.; Cho, E.; Giovannucci, E.L. Hyperinsulinemia, insulin resistance and colorectal adenomas: A meta-analysis. Metabolism 2015, 64, 1324–1333. [Google Scholar] [CrossRef]
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism 2019, 92, 121–135. [Google Scholar] [CrossRef]
- Hardikar, P.S.; Joshi, S.M.; Bhat, D.S.; Raut, D.A.; Katre, P.A.; Lubree, H.G.; Jere, A.; Pandit, A.N.; Fall, C.H.D.; Yajnik, C.S. Spuriously High Prevalence of Prediabetes Diagnosed by HbA1c in Young Indians Partly Explained by Hematological Factors and Iron Deficiency Anemia. Diabetes Care 2012, 35, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Cui, A.; Ding, D.; Li, Y. Regulation of Hepatic Metabolism and Cell Growth by the ATF/CREB Family of Transcription Factors. Diabetes 2021, 70, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Dewidar, B.; Kahl, S.; Pafili, K.; Roden, M. Metabolic liver disease in diabetes—From mechanisms to clinical trials. Metabolism 2020, 111s, 154299. [Google Scholar] [CrossRef]
- Mossenta, M.; Busato, D.; Dal Bo, M.; Toffoli, G. Glucose Metabolism and Oxidative Stress in Hepatocellular Carcinoma: Role and Possible Implications in Novel Therapeutic Strategies. Cancers 2020, 12, 1668. [Google Scholar] [CrossRef]
- Cheung, K.S.; Chan, E.W.; Chen, L.; Seto, W.K.; Wong, I.C.K.; Leung, W.K. Diabetes Increases Risk of Gastric Cancer After Helicobacter pylori Eradication: A Territory-Wide Study With Propensity Score Analysis. Diabetes Care 2019, 42, 1769–1775. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Tao, M.; Zhao, L.; Zhang, X. The association between diabetes/hyperglycemia and the prognosis of cervical cancer patients: A systematic review and meta-analysis. Medicine 2017, 96, e7981. [Google Scholar] [CrossRef]
- Jousheghany, F.; Phelps, J.; Crook, T.; Hakkak, R. Relationship between level of HbA1C and breast cancer. BBA Clin. 2016, 6, 45–48. [Google Scholar] [CrossRef][Green Version]
- Marrone, M.T.; Selvin, E.; Barber, J.R.; Platz, E.A.; Joshu, C.E. Hyperglycemia, Classified with Multiple Biomarkers Simultaneously in Men without Diabetes, and Risk of Fatal Prostate Cancer. Cancer Prev. Res. 2019, 12, 103–112. [Google Scholar] [CrossRef]
- Zhang, Y.-B.; Pan, X.-F.; Chen, J.; Cao, A.; Zhang, Y.-G.; Xia, L.; Wang, J.; Li, H.; Liu, G.; Pan, A. Combined lifestyle factors, incident cancer, and cancer mortality: A systematic review and meta-analysis of prospective cohort studies. Br. J. Cancer 2020, 122, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Taylor, J.A. Genome-wide age-related DNA methylation changes in blood and other tissues relate to histone modification, expression and cancer. Carcinogenesis 2014, 35, 356–364. [Google Scholar] [CrossRef] [PubMed]
| Missing Data | Overall | HbA1c Category | p Value for Trend | |||||
|---|---|---|---|---|---|---|---|---|
| Q1 (3–5.1) | Q2 (5.2–5.3) | Q3 (5.4–5.4) | Q4 (5.5–5.6) | Q5 (5.7–6.4) | ||||
| Characteristics 1 | n = 79,234 | n = 127,430 | n = 82,849 | n = 153,392 | n = 146,552 | |||
| Age (years) | 39.25 ± 10.31 | 36 ± 7.86 | 36.79 ± 8.52 | 37.72 ± 9.23 | 39.27 ± 10.1 | 44.01 ± 11.88 | <0.001 | |
| Male, n (%) | 305,526 (51.83) | 41,582 (52.48) | 66,528 (52.21) | 43,642 (52.68) | 80,440 (52.44) | 73,334 (50.04) | <0.001 | |
| BMI (kg/m2) | 158 | 23.25 ± 3.3 | 22.53 ± 2.94 | 22.77 ± 3.06 | 22.99 ± 3.17 | 23.28 ± 3.28 | 24.18 ± 3.55 | <0.001 |
| Fasting glucose (mg/dL) | 6 | 93.05 ± 9.05 | 89.68 ± 7.69 | 90.84 ± 7.69 | 91.85 ± 7.9 | 93.04 ± 8.15 | 97.46 ± 10.52 | <0.001 |
| Red blood cell count (106/mm3) | 14 | 4.75 ± 0.46 | 4.75 ± 0.48 | 4.75 ± 0.46 | 4.75 ± 0.46 | 4.75 ± 0.46 | 4.73 ± 0.45 | <0.001 |
| Alcohol amount grams (g/day) | 38,473 | 5 (1–14) | 6 (1–15) | 5 (1–14) | 5 (1–14) | 5 (1–14) | 4 (0–14) | 0.896 |
| Regular exercise | 85,659 (14.53) | 10,819 (13.65) | 18,302 (14.36) | 11,989 (14.47) | 22,380 (14.59) | 22,169 (15.13) | <0.001 | |
| Current smoker (%) | 122,128 (20.72) | 17,007 (21.46) | 26,560 (20.84) | 17,345 (20.94) | 31,790 (20.72) | 29,426 (20.08) | 0.066 | |
| ≥College graduate (%) | 344,242 (58.4) | 48,219 (60.86) | 77,357 (60.71) | 50,158 (60.54) | 91,186 (59.45) | 77,322 (52.76) | <0.001 | |
| HbA1c Category | Person-Years | Cancer Mortality | Incidence Rate (per 104 PY) | Age- and Sex Adjusted HR (95% CI) | Model 1 | Model 2 |
|---|---|---|---|---|---|---|
| Total period | 4,213,135.7 | 1,712 | 4.06 (3.88–4.26) | |||
| Q1 | 616,081.62 | 170 | 2.76 (2.37–3.21) | 1 (reference) | 1 (reference) | 1 (reference) |
| Q2 | 917,985.11 | 262 | 2.85 (2.53–3.22) | 1.03 (0.85–1.25) | 1.02 (0.84–1.25) | 1.02 (0.84–1.25) |
| Q3 | 581,155.08 | 210 | 3.61 (3.16–4.14) | 1.2 (0.98–1.46) | 1.22 (0.99–1.5) | 1.22 (0.99–1.5) |
| Q4 | 1,067,645.1 | 401 | 3.76 (3.41–4.14) | 1.07 (0.89–1.28) | 1.04 (0.86–1.25) | 1.04 (0.86–1.26) |
| Q5 | 1,030,268.8 | 669 | 6.49 (6.02–7) | 1.19 (1.00–1.41) | 1.23 (1.02–1.47) | 1.25 (1.04–1.50) |
| p value for trend | 0.043 | 0.021 | 0.013 | |||
| Excluding death within 1 year cases | 3,644,481.4 | 1634 | 4.48 (4.27–4.71) | |||
| Q1 | 541,105.56 | 164 | 3.03 (2.6–3.53) | 1 (reference) | 1 (reference) | 1 (reference) |
| Q2 | 796,118.44 | 255 | 3.2 (2.83–3.62) | 1.05 (0.86–1.27) | 1.03 (0.85–1.27) | 1.04 (0.85–1.27) |
| Q3 | 501,217.89 | 202 | 4.03 (3.51–4.63) | 1.21 (0.98–1.48) | 1.22 (0.99–1.51) | 1.23 (0.99–1.52) |
| Q4 | 918,578.41 | 384 | 4.18 (3.78–4.62) | 1.08 (0.9–1.3) | 1.04 (0.86–1.26) | 1.05 (0.86–1.27) |
| Q5 | 887,461.14 | 629 | 7.09 (6.55–7.66) | 1.19 (0.99–1.42) | 1.21 (1.01–1.46) | 1.23 (1.02–1.49) |
| p value for trend | 0.060 | 0.045 | 0.027 |
| HbA1c Category | Person-Years | Cancer Mortality | Incidence Rate (per 104 PY) | Age- and Sex Adjusted HR (95% CI) | Model 1 | Model 2 |
|---|---|---|---|---|---|---|
| Lung cancer | 4,213,135.7 | 372 | 0.88 (0.8–0.98) | |||
| Q1 | 616,081.62 | 27 | 0.44 (0.3–0.64) | 1 (reference) | 1 (reference) | 1 (reference) |
| Q2 | 917,985.11 | 47 | 0.51 (0.38–0.68) | 1.19 (0.74–1.91) | 1.17 (0.72–1.88) | 1.17 (0.72–1.88) |
| Q3 | 581,155.08 | 43 | 0.74 (0.55–0.998) | 1.52 (0.94–2.46) | 1.48 (0.91–2.41) | 1.48 (0.91–2.41) |
| Q4 | 1,067,645.1 | 72 | 0.67 (0.54–0.85) | 1.16 (0.74–1.81) | 1.11 (0.70–1.74) | 1.11 (0.70–1.75) |
| Q5 | 1,030,268.8 | 183 | 1.78 (1.54–2.05) | 1.88 (1.24–2.84) | 1.81 (1.19–2.77) | 1.84 (1.2–2.81) |
| p value for trend | 0.001 | 0.002 | 0.002 | |||
| Colorectal cancer | 4,213,135.7 | 118 | 0.28 (0.23–0.34) | |||
| Q1 | 616,081.62 | 5 | 0.08 (0.03–0.19) | 1 (reference) | 1 (reference) | 1 (reference) |
| Q2 | 917,985.11 | 20 | 0.22 (0.14–0.34) | 2.68 (1.01–7.16) | 2.46 (0.91–6.62) | 2.47 (0.91–6.65) |
| Q3 | 581,155.08 | 10 | 0.17 (0.09–0.32) | 1.98 (0.67–5.79) | 1.63 (0.53–4.99) | 1.64 (0.54–5.03) |
| Q4 | 1,067,645.1 | 29 | 0.27 (0.19–0.39) | 2.7 (1.04–7.01) | 2.18 (0.82–5.80) | 2.21 (0.83–5.88) |
| Q5 | 1,030,268.8 | 54 | 0.52 (0.4–0.68) | 3.34 (1.31–8.51) | 3.30 (1.28–8.50) | 3.48 (1.35–8.98) |
| p-value for trend | 0.018 | 0.017 | 0.011 | |||
| Liver cancer | 4,213,135.7 | 215 | 0.51 (0.45–0.58) | |||
| Q1 | 616,081.62 | 33 | 0.54 (0.38–0.75) | 1 (reference) | 1 (reference) | 1 (reference) |
| Q2 | 917,985.11 | 45 | 0.49 (0.37–0.66) | 0.91 (0.58–1.43) | 0.85 (0.53–1.36) | 0.85 (0.53–1.36) |
| Q3 | 581,155.08 | 30 | 0.52 (0.36–0.74) | 0.85 (0.52–1.39) | 0.78 (0.46–1.31) | 0.78 (0.46–1.32) |
| Q4 | 1,067,645.1 | 48 | 0.45 (0.34–0.60) | 0.62 (0.40–0.98) | 0.64 (0.40–1.02) | 0.64 (0.41–1.02) |
| Q5 | 1,030,268.8 | 59 | 0.57 (0.44–0.74) | 0.50 (0.32–0.78) | 0.48 (0.30–0.76) | 0.49 (0.31–0.78) |
| p-value for trend | <0.001 | 0.001 | 0.001 | |||
| Stomach cancer | 4,213,135.7 | 172 | 0.41 (0.35–0.47) | |||
| Q1 | 616,081.62 | 18 | 0.29 (0.18–0.46) | 1 (reference) | 1 (reference) | 1 (reference) |
| Q2 | 917,985.11 | 17 | 0.19 (0.12–0.30) | 0.63 (0.33–1.23) | 0.65 (0.33–1.28) | 0.65 (0.33–1.29) |
| Q3 | 581,155.08 | 27 | 0.46 (0.32–0.68) | 1.49 (0.82–2.70) | 1.62 (0.87–2.99) | 1.62 (0.88–3) |
| Q4 | 1,067,645.1 | 52 | 0.49 (0.37–0.64) | 1.39 (0.81–2.39) | 1.43 (0.81–2.52) | 1.44 (0.82–2.53) |
| Q5 | 1,030,268.8 | 58 | 0.56 (0.44–0.73) | 1.13 (0.65–1.95) | 1.34 (0.76–2.37) | 1.38 (0.78–2.44) |
| p-value for trend | 0.151 | 0.044 | 0.034 | |||
| Prostate cancer † | 4,213,135.7 | 25 | 0.06 (0.04–0.09) | |||
| Q1 | 616,081.62 | 0 | - | N/A | N/A | N/A |
| Q2 | 917,985.11 | 2 | 0.02 (0.01–0.09) | 1 (reference) | 1 (reference) | 1 (reference) |
| Q3 | 581,155.08 | 4 | 0.07 (0.03–0.18) | 2.56 (0.47–14.04) | 5.33 (0.59–47.82) | 5.38 (0.6–48.24) |
| Q4 | 1,067,645.1 | 8 | 0.07 (0.04–0.15) | 2.15 (0.45–10.26) | 4.42 (0.55–35.84) | 4.51 (0.56–36.56) |
| Q5 | 1,030,268.8 | 11 | 0.11 (0.06–0.19) | 1.81 (0.39–8.38) | 3.85 (0.48–30.76) | 4.21 (0.53–33.66) |
| p-value for trend | 0.160 | 0.087 | 0.064 | |||
| Breast cancer ‡ | 4,213,135.7 | 70 | 0.17 (0.13–0.21) | |||
| Q1 | 616,081.62 | 7 | 0.11 (0.05–0.24) | 1 (reference) | 1 (reference) | 1 (reference) |
| Q2 | 917,985.11 | 12 | 0.13 (0.07–0.23) | 1.05 (0.41–2.67) | 1.13 (0.42–3.06) | 1.13 (0.42–3.06) |
| Q3 | 581,155.08 | 10 | 0.17 (0.09–0.32) | 1.37 (0.52–3.62) | 1.29 (0.45–3.73) | 1.29 (0.45–3.73) |
| Q4 | 1,067,645.1 | 22 | 0.21 (0.14–0.31) | 1.56 (0.66–3.67) | 1.36 (0.53–3.5) | 1.36 (0.53–3.5) |
| Q5 | 1,030,268.8 | 19 | 0.18 (0.12–0.29) | 1.18 (0.48–2.89) | 1.33 (0.51–3.49) | 1.36 (0.52–3.57) |
| p value for trend | 0.498 | 0.498 | 0.465 | |||
| Cervical cancer ‡ | 4,213,135.7 | 16 | 0.04 (0.02–0.06) | |||
| Q1 | 616,081.62 | 3 | 0.05 (0.02–0.15) | 1 (reference) | 1 (reference) | 1 (reference) |
| Q2 | 917,985.11 | 3 | 0.03 (0.01–0.1) | 0.59 (0.12–2.93) | 0.91 (0.15–5.44) | 0.91 (0.15–5.44) |
| Q3 | 581,155.08 | 1 | 0.02 (0–0.12) | 0.30 (0.03–2.92) | 0.47 (0.04–5.17) | 0.47 (0.04–5.18) |
| Q4 | 1,067,645.1 | 2 | 0.02 (0–0.07) | 0.31 (0.05–1.88) | 0.47 (0.06–3.38) | 0.47 (0.07–3.39) |
| Q5 | 1,030,268.8 | 7 | 0.07 (0.03–0.14) | 0.99 (0.24–4.09) | 0.94 (0.17–5.28) | 0.96 (0.17–5.4) |
| p value for trend | 0.929 | 0.842 | 0.865 | |||
| Pancreatic cancer | 4,213,135.7 | 166 | 0.39 (0.34–0.46) | |||
| Q1 | 616,081.62 | 12 | 0.19 (0.11–0.34) | 1 (reference) | 1 (reference) | 1 (reference) |
| Q2 | 917,985.11 | 23 | 0.25 (0.17–0.38) | 1.33 (0.66–2.67) | 1.38 (0.67–2.85) | 1.37 (0.67–2.84) |
| Q3 | 581,155.08 | 21 | 0.36 (0.24–0.55) | 1.79 (0.88–3.65) | 1.86 (0.89–3.89) | 1.84 (0.88–3.86) |
| Q4 | 1,067,645.1 | 37 | 0.35 (0.25–0.48) | 1.51 (0.78–2.91) | 1.49 (0.75–2.96) | 1.47 (0.74–2.93) |
| Q5 | 1,030,268.8 | 73 | 0.71 (0.56–0.89) | 2.01 (1.07–3.77) | 2.06 (1.07–3.99) | 1.99 (1.02–3.86) |
| P for trend | 0.022 | 0.028 | 0.044 |
| HbA1c Category | p-Value for Interaction | |||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| Model 1 | ||||||
| Sex | 0.316 | |||||
| Male | 1 (reference) | 1.09 (0.86–1.38) | 1.24 (0.96–1.58) | 1 (0.8–1.26) | 1.23 (0.99–1.53) | |
| Female | 1 (reference) | 0.84 (0.58–1.23) | 1.11 (0.75–1.63) | 1.04 (0.73–1.46) | 1.18 (0.84–1.65) | |
| Regular exercise | 0.038 | |||||
| No | 1 (reference) | 1.07 (0.85–1.35) | 1.36 (1.07–1.73) | 1.17 (0.94–1.46) | 1.32 (1.07–1.63) | |
| Yes | 1 (reference) | 0.86 (0.58–1.27) | 0.8 (0.52–1.24) | 0.64 (0.43–0.95) | 0.96 (0.67–1.37) | |
| Smoking status | 0.096 | |||||
| Never/former smoker | 1 (reference) | 0.90 (0.71–1.15) | 1.18 (0.92–1.51) | 0.96 (0.77–1.21) | 1.13 (0.91–1.4) | |
| Current smoker | 1 (reference) | 1.33 (0.9–1.95) | 1.3 (0.85–1.97) | 1.12 (0.76–1.64) | 1.47 (1.02–2.11) | |
| Education level | 0.129 | |||||
| ≤High school | 1 (reference) | 0.68 (0.46–1.02) | 0.9 (0.61–1.35) | 0.78 (0.55–1.1) | 0.9 (0.65–1.25) | |
| ≥College graduate | 1 (reference) | 1.3 (0.91–1.86) | 1.61 (1.11–2.34) | 1.21 (0.85–1.73) | 1.37 (0.97–1.95) | |
| Obesity | 0.985 | |||||
| BMI < 25 | 1 (reference) | 1.03 (0.81–1.3) | 1.26 (0.98–1.61) | 1.06 (0.85–1.33) | 1.25 (1.005–1.56) | |
| BMI ≥ 25 | 1 (reference) | 1.003 (0.69–1.45) | 1.1 (0.74–1.62) | 0.95 (0.67–1.35) | 1.09 (0.78–1.51) | |
| Model 2 | ||||||
| Sex | 0.276 | |||||
| Male | 1 (reference) | 1.09 (0.86–1.38) | 1.24 (0.97–1.59) | 1.01 (0.8–1.26) | 1.26 (1.02–1.57) | |
| Female | 1 (reference) | 0.84 (0.58–1.23) | 1.11 (0.75–1.63) | 1.04 (0.73–1.46) | 1.18 (0.84–1.65) | |
| Regular exercise | 0.038 | |||||
| No | 1 (reference) | 1.07 (0.85–1.35) | 1.36 (1.07–1.73) | 1.18 (0.95–1.46) | 1.33 (1.08–1.65) | |
| Yes | 1 (reference) | 0.86 (0.58–1.27) | 0.8 (0.52–1.25) | 0.64 (0.43–0.96) | 0.99 (0.69–1.42) | |
| Smoking status | 0.092 | |||||
| Never/former smoker | 1 (reference) | 0.9 (0.71–1.15) | 1.18 (0.92–1.51) | 0.97 (0.77–1.21) | 1.15 (0.92–1.42) | |
| Current smoker | 1 (reference) | 1.33 (0.9–1.95) | 1.3 (0.85–1.98) | 1.12 (0.77–1.64) | 1.5 (1.05–2.16) | |
| Education level | 0.129 | |||||
| ≤High school | 1 (reference) | 0.69 (0.46–1.02) | 0.91 (0.61–1.36) | 0.78 (0.55–1.11) | 0.92 (0.67–1.28) | |
| ≥College graduate | 1 (reference) | 1.3 (0.91–1.87) | 1.62 (1.11–2.35) | 1.22 (0.86–1.73) | 1.39 (0.98–1.97) | |
| Obesity | 0.986 | |||||
| BMI < 25 | 1 (reference) | 1.03 (0.81–1.3) | 1.26 (0.98–1.61) | 1.06 (0.85–1.33) | 1.25 (1.005–1.56) | |
| BMI ≥ 25 | 1 (reference) | 1.01 (0.7–1.46) | 1.11 (0.75–1.64) | 0.97 (0.68–1.37) | 1.14 (0.82–1.58) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, T.K.; Lee, M.Y.; Lee, S.A.; Cheong, E.S.; Seo, M.H.; Sung, K.C. Association of Glycosylated Hemoglobin Level and Cancer-Related Mortality in Patients without Diabetes. J. Clin. Med. 2022, 11, 5933. https://doi.org/10.3390/jcm11195933
Yoo TK, Lee MY, Lee SA, Cheong ES, Seo MH, Sung KC. Association of Glycosylated Hemoglobin Level and Cancer-Related Mortality in Patients without Diabetes. Journal of Clinical Medicine. 2022; 11(19):5933. https://doi.org/10.3390/jcm11195933
Chicago/Turabian StyleYoo, Tae Kyung, Mi Yeon Lee, Sul A. Lee, Eun Sun Cheong, Mi Hae Seo, and Ki Chul Sung. 2022. "Association of Glycosylated Hemoglobin Level and Cancer-Related Mortality in Patients without Diabetes" Journal of Clinical Medicine 11, no. 19: 5933. https://doi.org/10.3390/jcm11195933
APA StyleYoo, T. K., Lee, M. Y., Lee, S. A., Cheong, E. S., Seo, M. H., & Sung, K. C. (2022). Association of Glycosylated Hemoglobin Level and Cancer-Related Mortality in Patients without Diabetes. Journal of Clinical Medicine, 11(19), 5933. https://doi.org/10.3390/jcm11195933





