The Relative Preservation of the Central Retinal Layers in Leber Hereditary Optic Neuropathy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Genetic Analysis
2.3. Ophthalmologic Examination and Workup
2.3.1. Electrophysiology
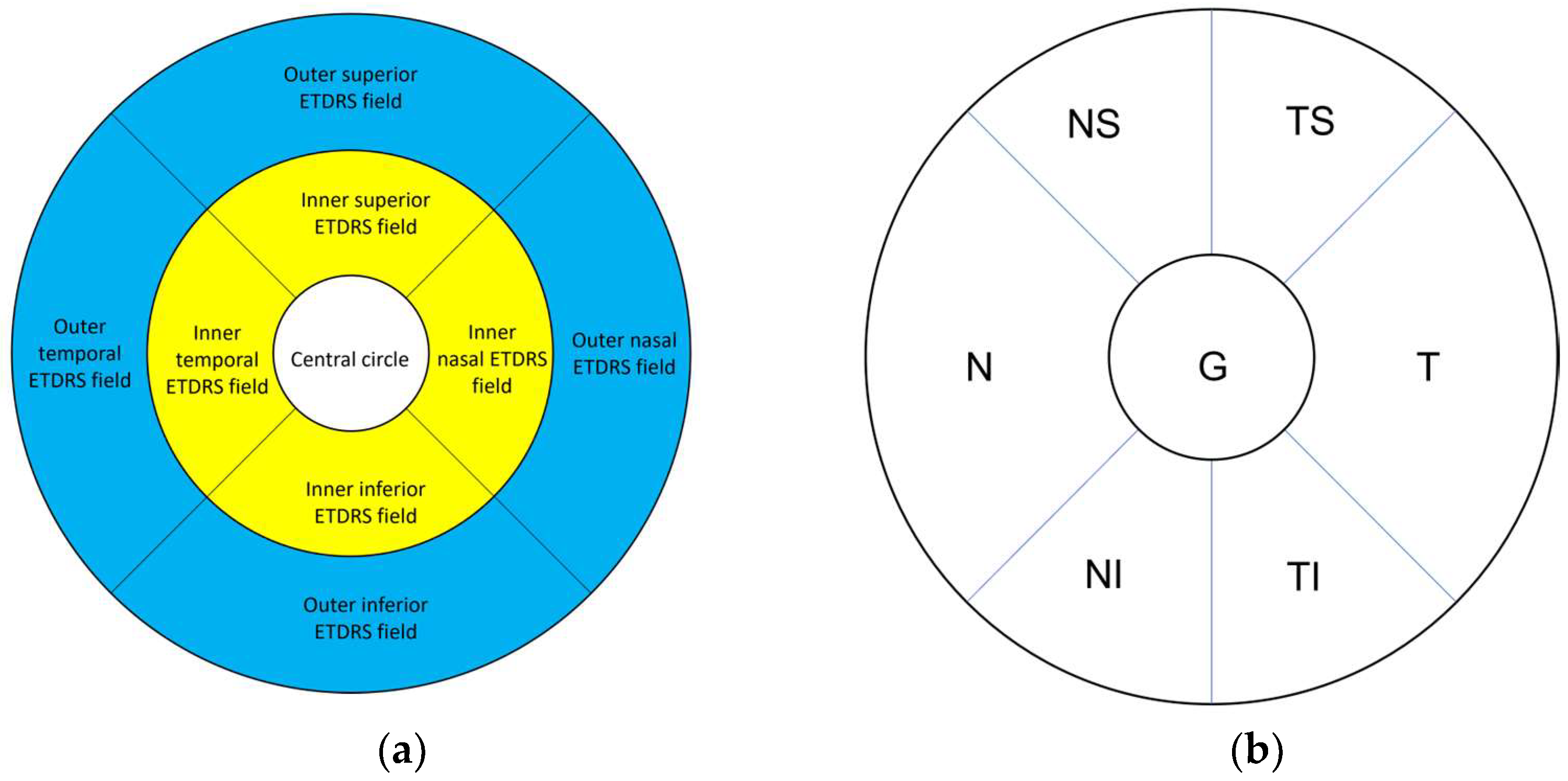
2.3.2. Optical Coherence Tomography and Retinal Segmentation Study
2.3.3. Peripapillary RNFL Thickness Study
2.4. Statistical Analysis
3. Results
3.1. Patient Demographic Data
3.2. Electrophysiology
3.3. Segmentation Analysis
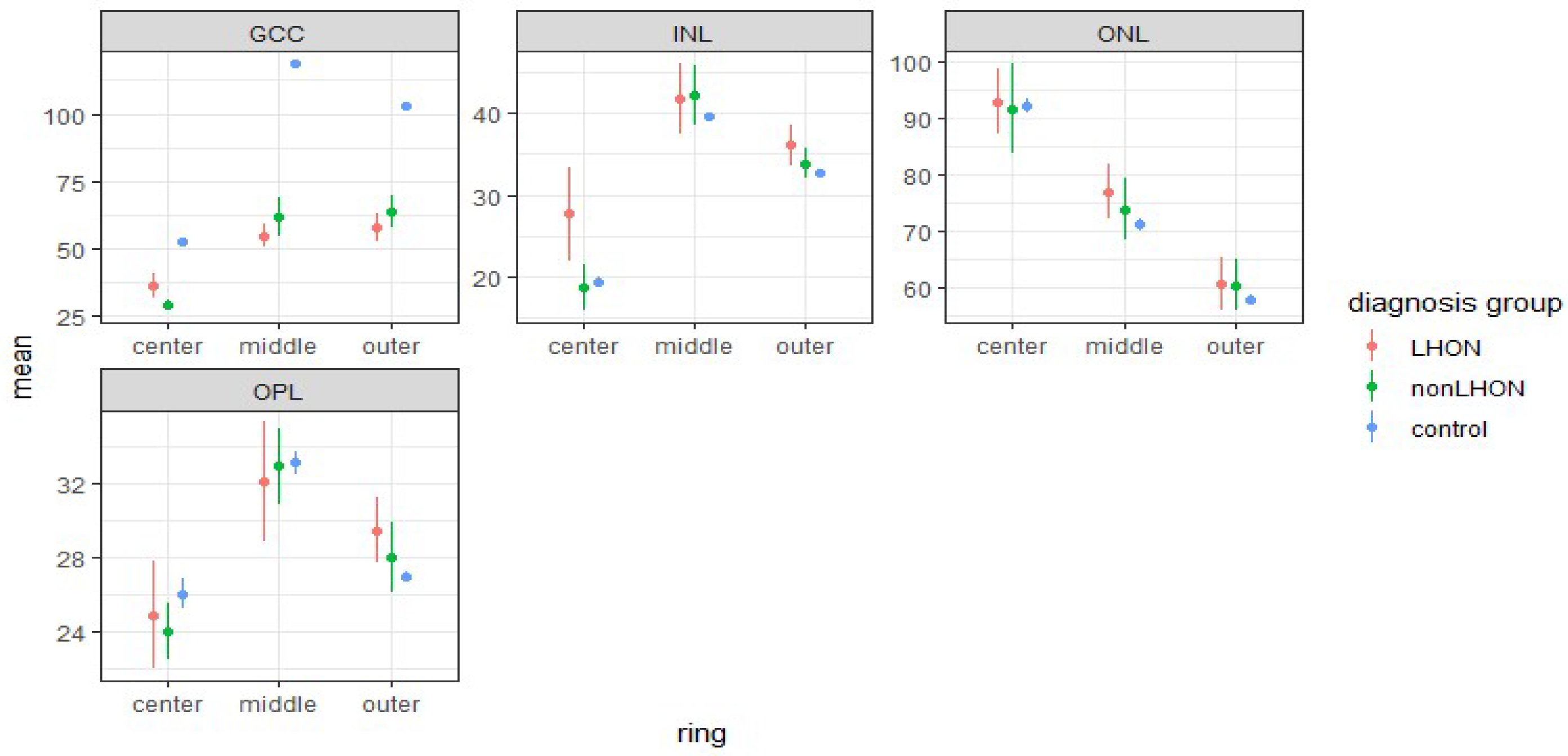
3.3.1. Comparison of Retinal Layers of LHON and nonLHON Group against Healthy Controls
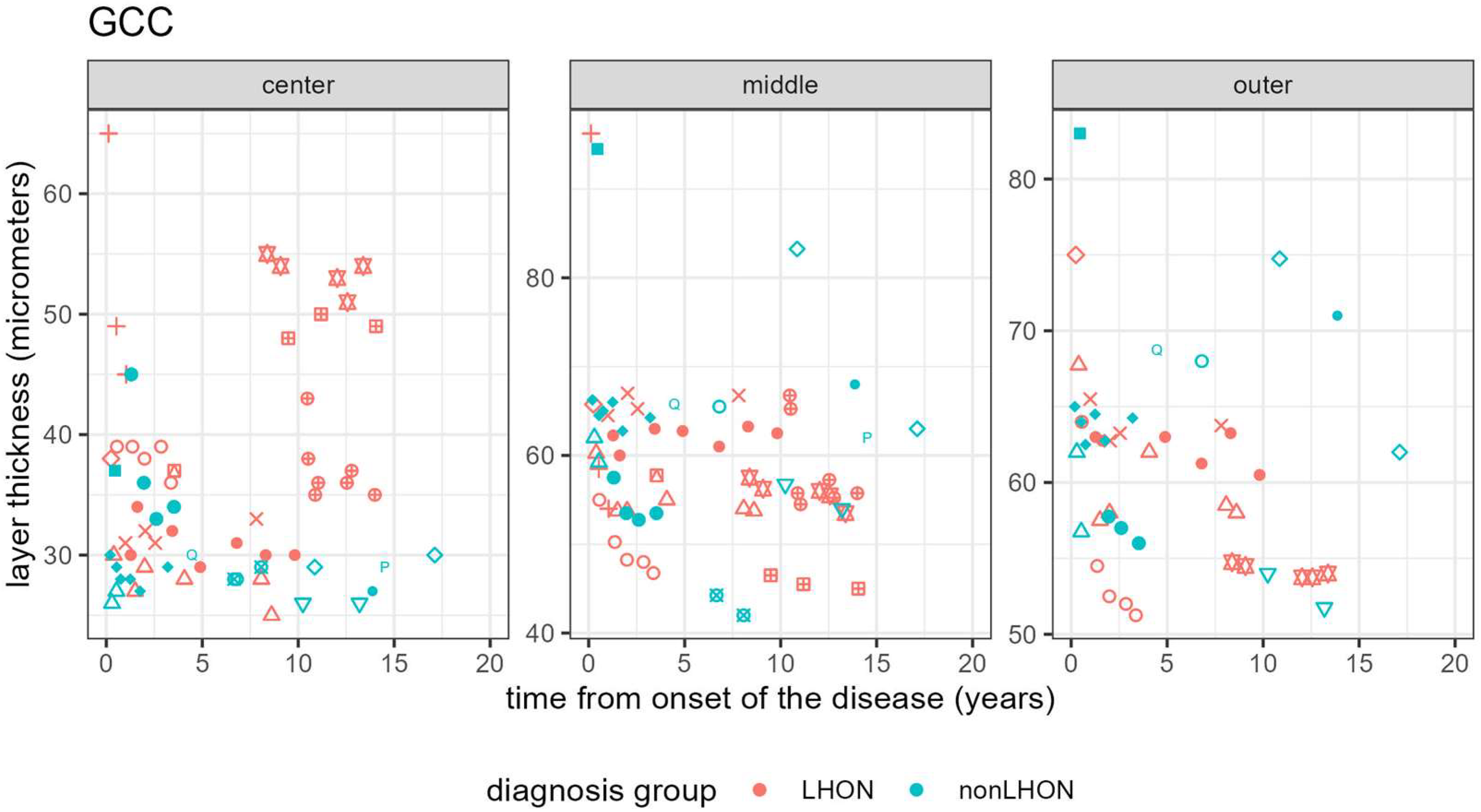
3.3.2. Longitudinal Analysis
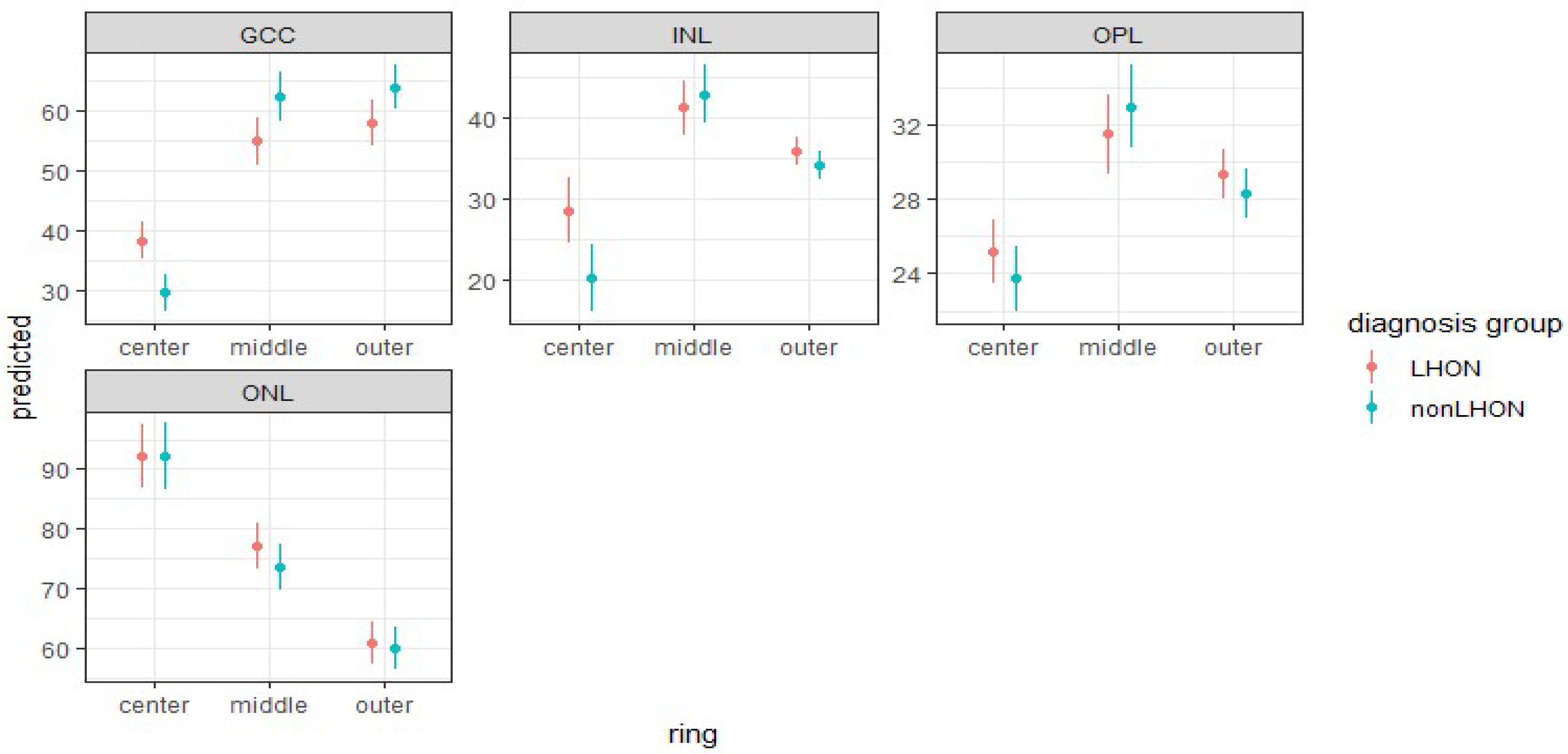
3.3.3. Comparison of Retinal Layers’ Thicknesses in ETDRS Rings between LHON and nonLHON Group
3.3.4. Retinal Layer Thickness Ratios between Middle and Outer ETDRS Rings, and Central Circle
3.3.5. Dominant Optic Atrophy Patients
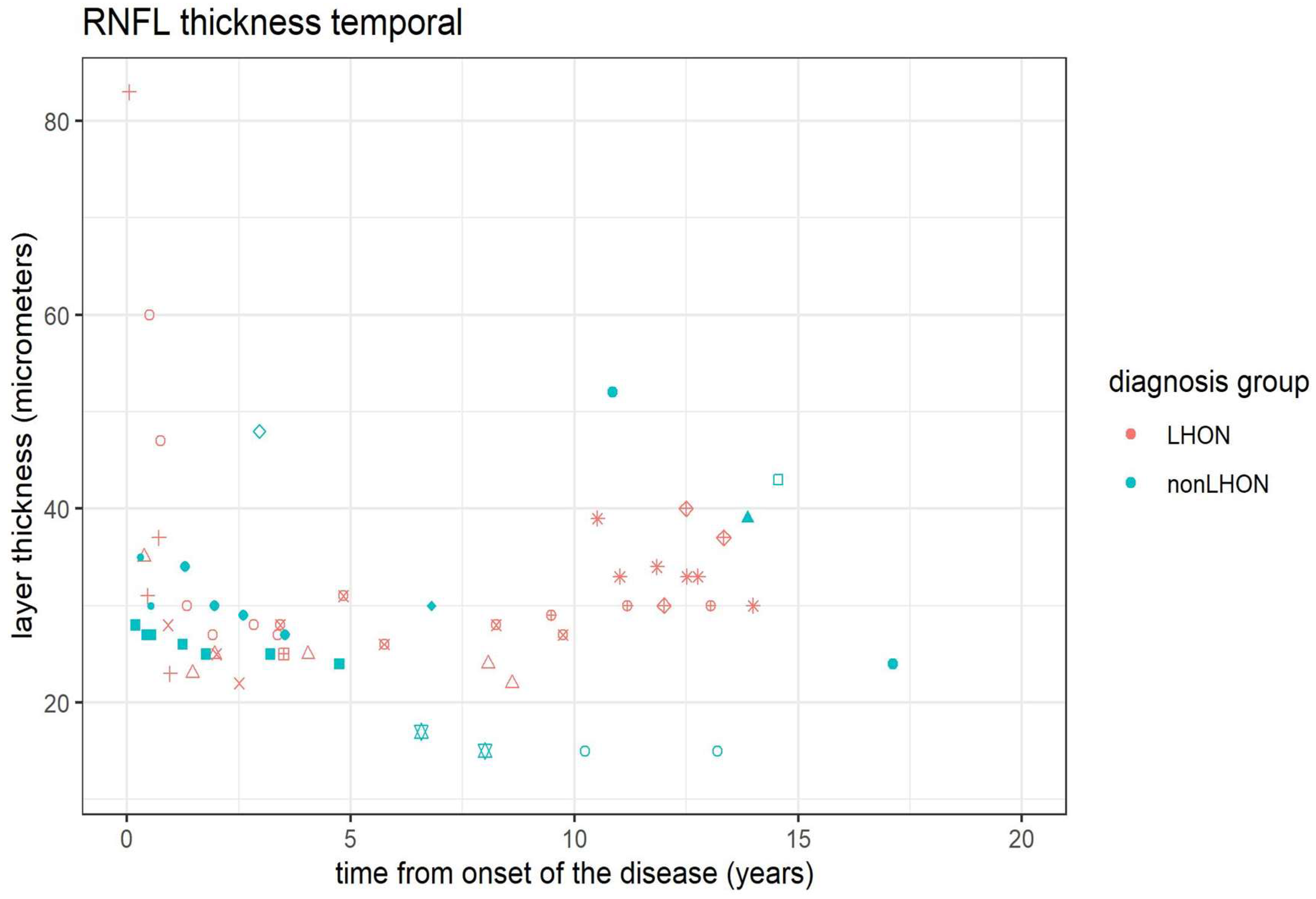
3.4. Peripapillary RNFL (pRNFL) Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu-Wai-Man, P.; Griffiths, P.G.; Chinnery, P.F. Mitochondrial Optic Neuropathies—Disease Mechanisms and Therapeutic Strategies. Prog. Retin. Eye Res. 2011, 30, 81–114. [Google Scholar] [CrossRef] [Green Version]
- Stenton, S.L.; Sheremet, N.L.; Catarino, C.B.; Andreeva, N.A.; Assouline, Z.; Barboni, P.; Barel, O.; Berutti, R.; Bychkov, I.; Caporali, L.; et al. Impaired Complex I Repair Causes Recessive Leber’s Hereditary Optic Neuropathy. J. Clin. Investig. 2021, 131, e138267. [Google Scholar] [CrossRef]
- Sadun, A.A.; Win, P.H.; Ross-Cisneros, F.N.; Walker, S.O.; Carelli, V. Leber’s Hereditary Optic Neuropathy Differentially Affects Smaller Axons in the Optic Nerve. Trans. Am. Ophthalmol. Soc. 2000, 98, 223–232; discussion 232–235. [Google Scholar]
- Carelli, V.; Carbonelli, M.; de Coo, I.F.; Kawasaki, A.; Klopstock, T.; Lagrèze, W.A.; La Morgia, C.; Newman, N.J.; Orssaud, C.; Pott, J.W.R.; et al. International Consensus Statement on the Clinical and Therapeutic Management of Leber Hereditary Optic Neuropathy. J. Neuroophthalmol. 2017, 37, 371–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carelli, V.; Rugolo, M.; Sgarbi, G.; Ghelli, A.; Zanna, C.; Baracca, A.; Lenaz, G.; Napoli, E.; Martinuzzi, A.; Solaini, G. Bioenergetics Shapes Cellular Death Pathways in Leber’s Hereditary Optic Neuropathy: A Model of Mitochondrial Neurodegeneration. Biochim. Biophys. Acta BBA Bioenerg. 2004, 1658, 172–179. [Google Scholar] [CrossRef] [Green Version]
- Levin, L.A. Superoxide Generation Explains Common Features of Optic Neuropathies Associated with Cecocentral Scotomas. J. Neuro-Ophthalmol. Off. J. North Am. Neuro-Ophthalmol. Soc. 2015, 35, 152–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, J.; Yang, Q.; Yu, D.; Wu, J.; Zhu, Y.; Guo, W. Vulnerability Study of Myelinated and Unmyelinated Nerve Fibers in Acute Ocular Hypertension in Rabbit. Mol. Med. Rep. 2017, 16, 6794–6802. [Google Scholar] [CrossRef] [Green Version]
- Carelli, V.; La Morgia, C.; Ross-Cisneros, F.N.; Sadun, A.A. Optic Neuropathies: The Tip of the Neurodegeneration Iceberg. Hum. Mol. Genet. 2017, 26, R139–R150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Huang, H.; Wei, S.; Gong, Y.; Li, H.; Dai, Y.; Zhao, S.; Wang, Y.; Yan, H. Characterization of Macular Thickness Changes in Leber’s Hereditary Optic Neuropathy by Optical Coherence Tomography. BMC Ophthalmol. 2014, 14, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balducci, N.; Cascavilla, M.L.; Ciardella, A.; La Morgia, C.; Triolo, G.; Parisi, V.; Bandello, F.; Sadun, A.A.; Carelli, V.; Barboni, P. Peripapillary Vessel Density Changes in Leber’s Hereditary Optic Neuropathy: A New Biomarker. Clin. Experiment. Ophthalmol. 2018, 46, 1055–1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizoguchi, A.; Hashimoto, Y.; Shinmei, Y.; Nozaki, M.; Ishijima, K.; Tagawa, Y.; Ishida, S. Macular Thickness Changes in a Patient with Leber’s Hereditary Optic Neuropathy. BMC Ophthalmol. 2015, 15, 27. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.Y.; Wang, X.W.; Hong, N.; Gu, Y.S. A Meta-Analysis of the Association between Different Genotypes (G11778A, T14484C and G3460A) of Leber Hereditary Optic Neuropathy and Visual Prognosis. Int. J. Ophthalmol. 2016, 9, 1493–1498. [Google Scholar] [CrossRef]
- Majander, A.; Robson, A.G.; João, C.; Holder, G.E.; Chinnery, P.F.; Moore, A.T.; Votruba, M.; Stockman, A.; Yu-Wai-Man, P. The Pattern of Retinal Ganglion Cell Dysfunction in Leber Hereditary Optic Neuropathy. Mitochondrion 2017, 36, 138–149. [Google Scholar] [CrossRef]
- Pemp, B.; Kircher, K.; Reitner, A. Visual Function in Chronic Leber’s Hereditary Optic Neuropathy during Idebenone Treatment Initiated 5 to 50 Years after Onset. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 2751–2757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pemp, B.; Mitsch, C.; Kircher, K.; Reitner, A. Changes in Visual Function and Correlations with Inner Retinal Structure in Acute and Chronic Leber’s Hereditary Optic Neuropathy Patients after Treatment with Idebenone. J. Clin. Med 2021, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Jarc-Vidmar, M.; Tajnik, M.; Brecelj, J.; Fakin, A.; Sustar, M.; Naji, M.; Stirn-Kranjc, B.; Glavač, D.; Hawlina, M. Clinical and Electrophysiology Findings in Slovene Patients with Leber Hereditary Optic Neuropathy. Doc. Ophthalmol. 2015, 130, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi, A.; Pellegrini, M.; Acquistapace, A.; Benatti, E.; Erba, S.; Cozzi, M.; Cigada, M.; Viola, F.; Gillies, M.; Staurenghi, G. Normative Data for Retinal-Layer Thickness Maps Generated by Spectral-Domain OCT in a White Population. Ophthalmol. Retina 2018, 2, 808–815.e1. [Google Scholar] [CrossRef] [PubMed]
- Staurenghi, G.; Sadda, S.; Chakravarthy, U.; Spaide, R.F. Proposed Lexicon for Anatomic Landmarks in Normal Posterior Segment Spectral-Domain Optical Coherence Tomography. Ophthalmology 2014, 121, 1572–1578. [Google Scholar] [CrossRef] [PubMed]
- Demidenko, E. Mixed Models: Theory and Applications with R, 2nd ed.; Wiley Series in Probability and Statistics; Wiley: Hoboken, NJ, USA, 2013; ISBN 978-1-118-59299-1. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-PROJECT.org/ (accessed on 27 August 2022).
- Chen, A.T.; Brady, L.; Bulman, D.E.; Sundaram, A.N.E.; Rodriguez, A.R.; Margolin, E.; Waye, J.S.; Tarnopolsky, M.A. An Evaluation of Genetic Causes and Environmental Risks for Bilateral Optic Atrophy. PLoS ONE 2019, 14, e0225656. [Google Scholar] [CrossRef] [PubMed]
- Carbonelli, M.; La Morgia, C.; Savini, G.; Cascavilla, M.L.; Borrelli, E.; Chicani, F.; do Val Ferreira Ramos, C.; Salomao, S.R.; Parisi, V. Macular Microcysts in Mitochondrial Optic Neuropathies: Prevalence and Retinal Layer Thickness Measurements. PLoS ONE 2015, 10, e0127906. [Google Scholar] [CrossRef]
- Asanad, S.; Tian, J.J.; Frousiakis, S.; Jiang, J.P.; Kogachi, K.; Felix, C.M.; Fatemeh, D.; Irvine, A.G.; Ter-Zakarian, A.; Falavarjani, K.G.; et al. Optical Coherence Tomography of the Retinal Ganglion Cell Complex in Leber’s Hereditary Optic Neuropathy and Dominant Optic Atrophy. Curr. Eye Res. 2019, 44, 638–644. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, W.B.; Wang, Y.X.; Yan, Y.N.; Yang, J.Y.; Zhou, W.J.; Chan, S.Y.; Xu, L.; Jonas, J.B. Thickness of Individual Layers at the Macula and Associated Factors: The Beijing Eye Study 2011. BMC Ophthalmol. 2020, 20, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.T. Foveal Pit Morphological Changes in Asymptomatic Carriers of the G11778A Mutation with Leber’s Hereditary Optic Neuropathy. Int. J. Ophthalmol. 2020, 13, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Barboni, P.; Savini, G.; Parisi, V.; Carbonelli, M.; La Morgia, C.; Maresca, A.; Sadun, F.; De Negri, A.M.; Carta, A.; Sadun, A.A.; et al. Retinal Nerve Fiber Layer Thickness in Dominant Optic Atrophy. Ophthalmology 2011, 118, 2076–2080. [Google Scholar] [CrossRef] [PubMed]
- Barboni, P.; Amore, G.; Cascavilla, M.L.; Battista, M.; Frontino, G.; Romagnoli, M.; Caporali, L.; Baldoli, C.; Gramegna, L.L.; Sessagesimi, E.; et al. The Pattern of Retinal Ganglion Cell Loss in Wolfram Syndrome Is Distinct from Mitochondrial Optic Neuropathies. Am. J. Ophthalmol. 2022, 241, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Masri, R.A.; Weltzien, F.; Purushothuman, S.; Lee, S.C.S.; Martin, P.R.; Grünert, U. Composition of the Inner Nuclear Layer in Human Retina. Investig. Opthalmol. Vis. Sci. 2021, 62, 22. [Google Scholar] [CrossRef] [PubMed]
- Cesareo, M.; Ciuffoletti, E.; Martucci, A.; Sebastiani, J.; Sorge, R.P.; Lamantea, E.; Garavaglia, B.; Ricci, F.; Cusumano, A.; Nucci, C.; et al. Assessment of the Retinal Posterior Pole in Dominant Optic Atrophy by Spectral-Domain Optical Coherence Tomography and Microperimetry. PLoS ONE 2017, 12, e0174560. [Google Scholar] [CrossRef] [Green Version]
- Lam, B.L.; Burke, S.P.; Wang, M.X.; Nadayil, G.A.; Rosa, P.R.; Gregori, G.; Feuer, W.J.; Cuprill-Nilson, S.; Vandenbroucke, R.; Zhang, X.; et al. Macular Retinal Sublayer Thicknesses in G11778A Leber Hereditary Optic Neuropathy. Ophthalmic. Surg. Lasers Imaging Retina 2016, 47, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Barboni, P.; Carbonelli, M.; Savini, G.; do Val Ferreira Ramos, C.; Carta, A.; Berezovsky, A.; Salomao, S.R.; Carelli, V.; Sadun, A.A. Natural History of Leber’s Hereditary Optic Neuropathy: Longitudinal Analysis of the Retinal Nerve Fiber Layer by Optical Coherence Tomography. Ophthalmology 2010, 117, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, H.L.; Du, Y.Y.; Yuan, J.; Li, X.; Tian, Z.; Zhou, H.; Wang, S.; Song, L.; Sun, J.; et al. Characterisation of Thickness Changes in the Peripapillary Retinal Nerve Fibre Layer in Patients with Leber’s Hereditary Optic Neuropathy. Br. J. Ophthalmol. 2021, 105, 1166–1171. [Google Scholar] [CrossRef]
- Mashima, Y. Macular Nerve Fibers Temporal to Fovea May Have a Greater Potential to Recover Function in Patients With Leber’s Hereditary Optic Neuropathy. Jpn. J. Ophthalmol. 2002, 46, 660–667. [Google Scholar] [CrossRef]
- Moster, S.J.; Moster, M.L.; Scannell Bryan, M.; Sergott, R.C. Retinal Ganglion Cell and Inner Plexiform Layer Loss Correlate with Visual Acuity Loss in LHON: A Longitudinal, Segmentation OCT Analysis. Investig. Opthalmol. Vis. Sci. 2016, 57, 3872. [Google Scholar] [CrossRef] [PubMed]


| Control vs. LHON (Corrected p-Value) | Control vs. nonLHON (Corrected p-Value) | |||||
|---|---|---|---|---|---|---|
| ETDRS Ring | ETDRS Ring | |||||
| Retinal Layer | Center | Middle | Outer | Center | Middle | Outer |
| Retina | 0.101 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| GCC | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| INL | 0.234 | 1 | 0.275 | 1 | 1 | 1 |
| OPL | 1 | 1 | 0.245 | 0.454 | 0.846 | 0.257 |
| ONL | 1 | 0.5 | 1 | 1 | 1 | 1 |
| Center | Middle | Outer | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Retinal Layer | c2 | p | Corrected p-Value | c2 | p | Corrected p-Value | c2 | p | Corrected p-Value |
| GCC | 11.92 | 0.001 | 0.016 | 5.95 | 0.015 | 0.360 | 4.34 | 0.037 | 0.820 |
| INL | 7.3 | 0.007 | 0.200 | 0.45 | 0.503 | 1 | 1.82 | 0.177 | 1 |
| OPL | 1.4 | 0.237 | 1 | 0.86 | 0.354 | 1 | 1.12 | 0.289 | 1 |
| ONL | 0 | 0.988 | 1 | 1.64 | 0.201 | 1 | 0.1 | 0.754 | 1 |
| Outcome | χ2 (1) | df | p | Corrected p Value |
|---|---|---|---|---|
| RNFL thickness general (G) trend | 1.09 | 1 | 0.297 | 1 |
| RNFL thickness inferior nasal trend | 0.53 | 1 | 0.468 | 1 |
| RNFL thickness inferior temporal trend | 1.29 | 1 | 0.257 | 1 |
| RNFL thickness nasal trend | 1.43 | 1 | 0.232 | 1 |
| RNFL thickness superior nasal trend | 0.16 | 1 | 0.687 | 1 |
| RNFL thickness superior temporal trend | 10.57 | 1 | 0.001 | 0.008 |
| RNFL thickness temporal trend | 4.03 | 1 | 0.045 | 0.268 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrovic Pajic, S.; Lapajne, L.; Vratanar, B.; Fakin, A.; Jarc-Vidmar, M.; Sustar Habjan, M.; Volk, M.; Maver, A.; Peterlin, B.; Hawlina, M. The Relative Preservation of the Central Retinal Layers in Leber Hereditary Optic Neuropathy. J. Clin. Med. 2022, 11, 6045. https://doi.org/10.3390/jcm11206045
Petrovic Pajic S, Lapajne L, Vratanar B, Fakin A, Jarc-Vidmar M, Sustar Habjan M, Volk M, Maver A, Peterlin B, Hawlina M. The Relative Preservation of the Central Retinal Layers in Leber Hereditary Optic Neuropathy. Journal of Clinical Medicine. 2022; 11(20):6045. https://doi.org/10.3390/jcm11206045
Chicago/Turabian StylePetrovic Pajic, Sanja, Luka Lapajne, Bor Vratanar, Ana Fakin, Martina Jarc-Vidmar, Maja Sustar Habjan, Marija Volk, Ales Maver, Borut Peterlin, and Marko Hawlina. 2022. "The Relative Preservation of the Central Retinal Layers in Leber Hereditary Optic Neuropathy" Journal of Clinical Medicine 11, no. 20: 6045. https://doi.org/10.3390/jcm11206045






