Clinical Utility of Three-Dimensional Speckle-Tracking Echocardiography in Heart Failure
Abstract
:1. Introduction
2. Evolution of Strain Imaging
3. Tissue Doppler Imaging
4. Two-Dimensional Speckle-Tracking Echocardiography
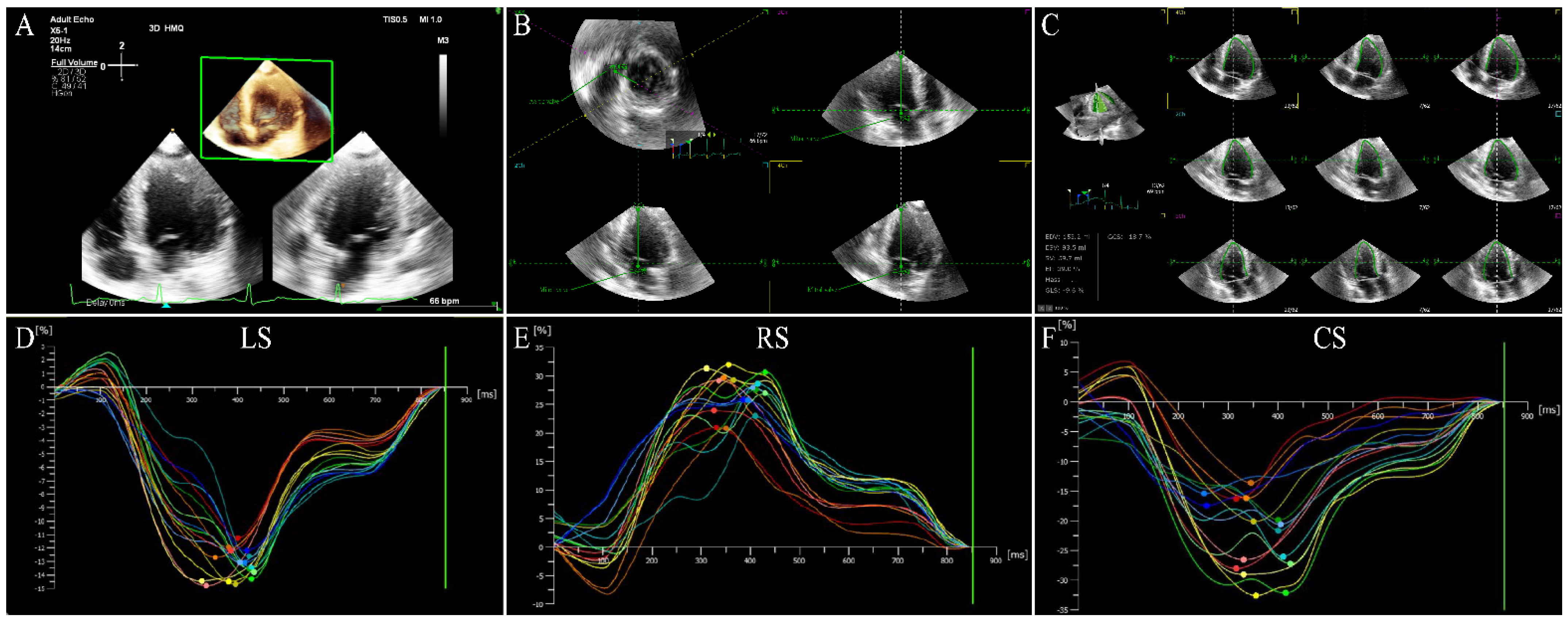
5. Three-Dimensional Speckle-Tracking Echocardiography
6. Three-Dimensional Speckle-Tracking Echocardiography in HF Patients
6.1. Left Ventricular Global Systolic Function
6.2. Left Ventricular Regional Systolic Function
6.3. Left Ventricular Diastolic Function
6.4. Cardiac Resynchronization Therapy
6.5. Left Atrial Function
6.6. Right Ventricular Function
6.7. Myocardial Fibrosis
7. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 37, 2129–2200. [Google Scholar] [CrossRef]
- Baman, J.R.; Ahmad, F.S. Heart Failure. JAMA 2020, 324, 1015. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.S.; Gao, R.L.; Liu, L.S.; Zhu, M.L.; Wang, W.; Wang, Y.J.; Wu, Z.S.; Li, H.J.; Gu, D.F.; Yang, Y.J. Summary of the 2018 report on cardiovascular diseases in China. Chin. Circ. J. 2019, 34, 209–220. [Google Scholar] [CrossRef]
- Mishra, S.; Mohan, J.C.; Nair, T.; Chopra, V.K.; Harikrishnan, S.; Guha, S.; Ramakrishnan, S.; Ray, S.; Sethi, R.; Samal, U.; et al. Management protocols for chronic heart failure in India. Indian Hearth J. 2018, 70, 105–127. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2020 Update: A Report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- Dalmau, R. Heart failure: A likely horizon in the elderly that could be prevented by avoiding obesity in middle age. Eur. J. Prev. Cardiol. 2020, 27, 715–716. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidis, I.; Aboyans, V.; Blacher, J.; Brodmann, M.; Brutsaert, D.L.; Chirinos, J.A.; De Carlo, M.; Delgado, V.; Lancellotti, P.; Lekakis, J.; et al. The role of ventricular–arterial coupling in cardiac disease and heart failure: Assessment, clinical implications and therapeutic interventions. A consensus document of the European Society of Cardiology Working Group on Aorta & Peripheral Vascular Diseases, European Association of Cardiovascular Imaging, and Heart Failure Association. Eur. J. Heart Fail. 2019, 21, 402–424. [Google Scholar] [CrossRef] [Green Version]
- Urbano-Moral, J.A.; Patel, A.R.; Maron, M.S.; Arias-Godínez, J.A.; Pandian, N.G. Three-Dimensional Speckle-Tracking Echocardiography: Methodological Aspects and Clinical Potential. Echocardiography 2012, 29, 997–1010. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Negishi, K.; Wang, Y.; Nolan, M.; Saito, M.; Marwick, T.H. Echocardiographic screening for non-ischaemic stage B heart failure in the community. Eur. J. Hearth Fail. 2016, 18, 1331–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tops, L.F.; Delgado, V.; Marsan, N.A.; Bax, J.J. Myocardial strain to detect subtle left ventricular systolic dysfunction. Eur. J. Heart Fail. 2017, 19, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Ishizu, T.; Atsumi, A.; Kawamura, R.; Aonuma, K. Three-Dimensional Speckle Tracking Echocardiography. Circ. J. 2014, 78, 1290–1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reant, P.; Reynaud, A.; Pillois, X.; Dijos, M.; Arsac, F.; Touche, C.; Landelle, M.; Rooryck, C.; Roudaut, R.; Lafitte, S. Comparison of Resting and Exercise Echocardiographic Parameters as Indicators of Outcomes in Hypertrophic Cardiomyopathy. J. Am. Soc. Echocardiogr. 2015, 28, 194–203. [Google Scholar] [CrossRef]
- Voigt, J.U.; Cvijic, M. 2- and 3-Dimensional Myocardial Strain in Cardiac Health and Disease. JACC Cardiovasc. Imaging 2019, 12, 1849–1863. [Google Scholar] [CrossRef]
- Seo, Y.; Ishizu, T.; Aonuma, K. Current Status of 3-Dimensional Speckle Tracking Echocardiography: A Review from Our Experiences. J. Cardiovasc. Ultrasound 2014, 22, 49–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smiseth, O.A.; Torp, H.; Opdahl, A.; Haugaa, K.H.; Urheim, S. Myocardial strain imaging: How useful is it in clinical decision making? Eur. Heart J. 2016, 37, 1196–1207. [Google Scholar] [CrossRef] [Green Version]
- Čelutkienė, J.; Plymen, C.M.; Flachskampf, F.A.; De Boer, R.A.; Grapsa, J.; Manka, R.; Anderson, L.; Garbi, M.; Barberis, V.; Filardi, P.P.; et al. Innovative imaging methods in heart failure: A shifting paradigm in cardiac assessment. Position statement on behalf of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 1615–1633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nabeshima, Y.; Seo, Y.; Takeuchi, M. A review of current trends in three-dimensional analysis of left ventricular myocardial strain. Cardiovasc. Ultrasound 2020, 18, 351–369. [Google Scholar] [CrossRef]
- Cheung, Y.F. The role of 3D wall motion tracking in heart failure. Nat. Rev. Cardiol. 2012, 9, 644–657. [Google Scholar] [CrossRef]
- Pellerin, D.; Sharma, R.; Elliott, P.; Veyrat, C. Tissue Doppler, strain, and strain rate echocardiography for the assessment of left and right systolic ventricular function. Heart 2003, 89 (Suppl. S3), iii9–iii17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cameli, M.; Mandoli, G.E.; Sciaccaluga, C.; Mondillo, S. More than 10 years of speckle tracking echocardiography: Still a novel technique or a definite tool for clinical practice? Echocardiography 2019, 36, 958–970. [Google Scholar] [CrossRef] [PubMed]
- Varghese, T.; Zagzebski, J.A.; Rahko, P.S.; Breburda, C. Ultrasonic imaging of myocardial strain using cardiac elastography. Ultrason. Imaging 2003, 25, 1–16. [Google Scholar] [CrossRef]
- Vijiiac, A.; Onciul, S.; Guzu, C.; Scarlatescu, A.; Petre, I.; Zamfir, D.; Onut, R.; Deaconu, S.; Dorobantu, M. Forgotten No More—The Role of Right Ventricular Dysfunction in Heart Failure with Reduced Ejection Fraction: An Echocardiographic Perspective. Diagnostics 2021, 11, 548. [Google Scholar] [CrossRef]
- Cameli, M.; Mondillo, S.; Galderisi, M.; Mandoli, G.E.; Ballo, P.; Nistri, S.; Capo, V.; D’Ascenzi, F.; D’Andrea, A.; Esposito, R.; et al. Speckle Tracking Imaging: A Practical Guide. G. Ital. Cardiol. 2017, 18, 253–269. [Google Scholar] [CrossRef]
- Houard, L.; Militaru, S.; Tanaka, K.; Pasquet, A.; Vancraeynest, D.; Vanoverschelde, J.L.; Pouleur, A.-C.; Gerber, B.L. Test–retest reliability of left and right ventricular systolic function by new and conventional echocardiographic and cardiac magnetic resonance parameters. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 1157–1167. [Google Scholar] [CrossRef]
- Aurich, M.; Keller, M.; Greiner, S.; Steen, H.; Aus dem Siepen, F.; Riffel, J.; Katus, H.A.; Buss, S.J.; Mereles, D. Left ventricular mechanics assessed by two-dimensional echocardiography and cardiac magnetic resonance imaging: Comparison of high-resolution speckle tracking and feature tracking. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1370–1378. [Google Scholar] [CrossRef] [Green Version]
- Kalam, K.; Otahal, P.; Marwick, T.H. Prognostic implications of global LV dysfunction: A systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart 2014, 100, 1673–1680. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Guo, R.Q.; Ping, J. Research progress of using three-dimensional speckle tracking imaging to evaluate the left ventricular structure and function. Med. Recapitul. 2018, 24, 787–793. [Google Scholar] [CrossRef]
- Vachalcova, M.; Valočik, G.; Kurečko, M.; Grapsa, J.; Taha, V.A.; Michalek, P.; Jankajová, M.; Sabol, F.; Kubikova, L.; Orban, M.; et al. The three-dimensional speckle tracking echocardiography in distinguishing between ischaemic and non-ischaemic aetiology of heart failure. ESC Heart Fail. 2020, 7, 2297–2304. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, Y.; Guan, X.; Zhang, Y.; Li, J. Value of three dimensional-speckle tracking imaging for predicting left ventricular function after non-ST-segment elevation myocardial infarction with percutaneous coronary intervention. J. X-Ray Sci. Technol. 2018, 26, 331–339. [Google Scholar] [CrossRef]
- Muraru, D.; Niero, A.; Rodriguez-Zanella, H.; Cherata, D.; Badano, L. Three-dimensional speckle-tracking echocardiography: Benefits and limitations of integrating myocardial mechanics with three-dimensional imaging. Cardiovasc. Diagn. Ther. 2018, 8, 101–117. [Google Scholar] [CrossRef]
- Howard-Quijano, K.; Methangkool, E.; Scovotti, J.C.; Mazor, E.; Grogan, T.R.; Kratzert, W.B.; Mahajan, A. Regional Left Ventricular Myocardial Dysfunction After Cardiac Surgery Characterized by 3-Dimensional Strain. Anesth. Analg. 2019, 128, 854–864. [Google Scholar] [CrossRef]
- Ma, C.; Chen, J.; Yang, J.; Tang, L.; Chen, X.; Li, N.; Liu, S.; Zhang, Y. Quantitative Assessment of Left Ventricular Function by 3-Dimensional Speckle-Tracking Echocardiography in Patients with Chronic Heart Failure: A Meta-Analysis. J. Ultrasound Med. 2014, 33, 287–295. [Google Scholar] [CrossRef]
- Nunes, M.C.P.; Badano, L.P.; Marin-Neto, J.A.; Edvardsen, T.; Fernández-Golfín, C.; Bucciarelli-Ducci, C.; Popescu, B.A.; Underwood, R.; Habib, G.; Zamorano, J.L.; et al. Multimodality imaging evaluation of Chagas disease: An expert consensus of Brazilian Cardiovascular Imaging Department (DIC) and the European Association of Cardiovascular Imaging (EACVI). Eur. Heart J. Cardiovasc. Imaging 2018, 19, 459n–460n. [Google Scholar] [CrossRef]
- Ünlü, S.; Duchenne, J.; Mirea, O.; Pagourelias, E.D.; Bézy, S.; Cvijic, M.; Beela, A.S.; Thomas, J.D.; Badano, L.P.; Voigt, J.-U.; et al. Impact of apical foreshortening on deformation measurements: A report from the EACVI-ASE Strain Standardization Task Force. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 337–343. [Google Scholar] [CrossRef]
- Smolarek, D.; Gruchała, M.; Sobiczewski, W. Echocardiographic evaluation of right ventricular systolic function: The traditional and innovative approach. Cardiol. J. 2017, 24, 563–572. [Google Scholar] [CrossRef] [Green Version]
- Jasaityte, R.; Heyde, B.; D’Hooge, J. Current State of Three-Dimensional Myocardial Strain Estimation Using Echocardiography. J. Am. Soc. Echocardiogr. 2013, 26, 15–28. [Google Scholar] [CrossRef] [Green Version]
- Inciardi, R.M.; Galderisi, M.; Nistri, S.; Santoro, C.; Cicoira, M.; Rossi, A. Echocardiographic advances in hypertrophic cardiomyopathy: Three-dimensional and strain imaging echocardiography. Echocardiography 2018, 35, 716–726. [Google Scholar] [CrossRef]
- Nemes, A. Three-dimensional speckle-tracking echocardiography offers complete volumetric and functional assessment of the left atrium. Int. J. Cardiovasc. Imaging 2021, 37, 2235. [Google Scholar] [CrossRef]
- Ajmone Marsan, N.; Michalski, B.; Cameli, M.; Podlesnikar, T.; Manka, R.; Sitges, M.; Dweck, M.R.; Haugaa, K. EACVI survey on standardization of cardiac chambers quantification by transthoracic echocardiography. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 119–123. [Google Scholar] [CrossRef] [Green Version]
- Collier, P.; Phelan, D.; Klein, A. A Test in Context: Myocardial Strain Measured by Speckle-Tracking Echocardiography. J. Am. Coll. Cardiol. 2017, 69, 1043–1056. [Google Scholar] [CrossRef]
- Rösner, A.; Barbosa, D.; Aarsæther, E.; Kjønås, D.; Schirmer, H.; D’Hooge, J. The influence of frame rate on two-dimensional speckle-tracking strain measurements: A study on silico-simulated models and images recorded in patients. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Voigt, J.U.; Pedrizzetti, G.; Lysyansky, P.; Marwick, T.H.; Houle, H.; Baumann, R.; Pedri, S.; Ito, Y.; Abe, Y.; Matz, S.; et al. Definitions for a common standard for 2D speckle tracking echocardiography: Consensus document of the EACVI/ASE/Industry Task Force to standardize defor-mation imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ünlü, S.; Mirea, O.; Duchenne, J.; Pagourelias, E.D.; Bézy, S.; Thomas, J.D.; Badano, L.; Voigt, J.-U. Comparison of Feasibility, Accuracy, and Reproducibility of Layer-Specific Global Longitudinal Strain Measurements Among Five Different Vendors: A Report from the EACVI-ASE Strain Standardization Task Force. J. Am. Soc. Echocardiogr. 2018, 31, 374–380.e1. [Google Scholar] [CrossRef]
- Pastore, M.C.; Mandoli, G.E.; Aboumarie, H.S.; Santoro, C.; Bandera, F.; D’Andrea, A.; Benfari, G.; Esposito, R.; Evola, V.; Sorrentino, R.; et al. Basic and advanced echocardiography in advanced heart failure: An overview. Heart Fail. Rev. 2019, 25, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Kamperidis, V.; Marsan, N.A.; Delgado, V.; Bax, J.J. Left ventricular systolic function assessment in secondary mitral regurgitation: Left ventricular ejection fraction vs. speckle tracking global longitudinal strain. Eur. Heart J. 2016, 37, 811–816. [Google Scholar] [CrossRef]
- Leosco, D.; Parisi, V.; Pellegrino, T.; Pagano, G.; Femminella, G.D.; Bevilacqua, A.; Paolillo, S.; Formisano, R.; Ferro, G.; De Lucia, C.; et al. Alterations of left ventricular deformation and cardiac sympathetic derangement in patients with systolic heart failure: A 3D speckle tracking echocardiography and cardiac 123I-MIBG study. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1601–1611. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.J.; Wang, Z.B.; Wang, W.G.; Li, J.F.; Li, Y. Evaluation of left ventricular systolic function in patients with heart failure with preserved ejection fraction by speckle tracking imaging. Chin. J. Ultrasound Med. 2018, 34, 609–612. [Google Scholar] [CrossRef]
- Li, C.M.; Li, C.; Bai, W.J.; Zhang, X.L.; Tang, H.; Qing, Z.; Li, R. Value of Three-Dimensional Speckle-Tracking in Detecting Left Ventricular Dysfunction in Patients with Aortic Valvular Diseases. J. Am. Soc. Echocardiogr. 2013, 26, 1245–1252. [Google Scholar] [CrossRef]
- Nagata, Y.; Takeuchi, M.; Wu, V.C.; Izumo, M.; Suzuki, K.; Sato, K.; Seo, Y.; Akashi, Y.J.; Aonuma, K.; Otsuji, Y. Prognostic Value of LV Deformation Parameters Using 2D and 3D Speckle-Tracking Echocardiography in Asymptomatic Patients with Severe Aortic Stenosis and Preserved LV Ejection Fraction. JACC Cardiovasc. Imaging 2015, 8, 235–245. [Google Scholar] [CrossRef] [Green Version]
- Rady, M.; Ulbrich, S.; Heidrich, F.; Jellinghaus, S.; Ibrahim, K.; Linke, A.; Sveric, K.M. Left Ventricular Torsion—A New Echocardiographic Prognosticator in Patients with Non-Ischemic Dilated Cardiomyopathy. Circ. J. 2019, 83, 595–603. [Google Scholar] [CrossRef]
- Negishi, T.; Negishi, K.; Thavendiranathan, P.; Cho, G.-Y.; Popescu, B.A.; Vinereanu, D.; Kurosawa, K.; Penicka, M.; Marwick, T.H.; Aakhus, S.; et al. Effect of Experience and Training on the Concordance and Precision of Strain Measurements. JACC Cardiovasc. Imaging 2016, 10, 518–522. [Google Scholar] [CrossRef]
- Iwahashi, N.; Kirigaya, J.; Gohbara, M.; Abe, T.; Horii, M.; Hanajima, Y.; Toya, N.; Takahashi, H.; Minamimoto, Y.; Kimura, Y.; et al. Global Strain Measured by Three-Dimensional Speckle Tracking Echocardiography Is a Useful Predictor for 10-Year Prognosis After a First ST-Elevation Acute Myocardial Infarction. Circ. J. 2021, 85, 1735–1743. [Google Scholar] [CrossRef]
- Iwahashi, N.; Horii, M.; Kirigaya, J.; Abe, T.; Gohbara, M.; Toya, N.; Hanajima, Y.; Takahashi, H.; Minamimoto, Y.; Kimura, Y.; et al. Clinical Usefulness of the Serial Examination of Three-Dimensional Global Longitudinal Strain After the Onset of ST-Elevation Acute Myocardial Infarction. Circ. J. 2022, 86, 611–619. [Google Scholar] [CrossRef]
- Altman, M.; Bergerot, C.; Aussoleil, A.; Davidsen, E.S.; Sibellas, F.; Ovize, M.; Bonnefoy-Cudraz, E.; Thibault, H.; Derumeaux, G. Assessment of left ventricular systolic function by deformation imaging derived from speckle tracking: A comparison between 2D and 3D echo modalities. Eur. Heart J. Cardiovasc. Imaging 2013, 15, 316–323. [Google Scholar] [CrossRef]
- Malagoli, A.; Albini, A.; Mandoli, G.E.; Baggiano, A.; Vinco, G.; Bandera, F.; D’Andrea, A.; Esposito, R.; D’Ascenzi, F.; Sorrentino, R.; et al. Multimodality imaging of the ischemic right ventricle: An overview and proposal of a diagnostic algorithm. Int. J. Cardiovasc. Imaging 2021, 37, 3343–3354. [Google Scholar] [CrossRef]
- Wang, N.; Hung, C.-L.; Shin, S.-H.; Claggett, B.; Skali, H.; Thune, J.J.; Køber, L.; Shah, A.; Mcmurray, J.; Pfeffer, M.A.; et al. Regional cardiac dysfunction and outcome in patients with left ventricular dysfunction, heart failure, or both after myocardial infarction. Eur. Heart J. 2016, 37, 466–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimitriu-Leen, A.C.; Scholte, A.J.; Katsanos, S.; Hoogslag, G.E.; van Rosendael, A.R.; van Zwet, E.W.; Bax, J.J.; Delgado, V. Influence of Myocardial Ischemia Extent on Left Ventricular Global Longitudinal Strain in Patients After ST-Segment Elevation Myocardial Infarction. Am. J. Cardiol. 2017, 119, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Huang, X.; Ma, J.; Huang, J.; Fan, Y.; Li, H.; Qiu, J.; Zhang, H.; Huang, W. Value of three-dimensional strain parameters for predicting left ventricular remodeling after ST-elevation myocardial infarction. Int. J. Cardiovasc. Imaging 2017, 33, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Maffessanti, F.; Nesser, H.J.; Weinert, L.; Steringer-Mascherbauer, R.; Niel, J.; Gorissen, W.; Sugeng, L.; Lang, R.M.; Mor-Avi, V. Quantitative Evaluation of Regional Left Ventricular Function Using Three-Dimensional Speckle Tracking Echocardiography in Patients with and Without Heart Disease. Am. J. Cardiol. 2009, 104, 1755–1762. [Google Scholar] [CrossRef]
- Zhu, W.; Liu, W.; Tong, Y.; Xiao, J. Three-Dimensional Speckle Tracking Echocardiography for the Evaluation of the Infarct Size and Segmental Transmural Involvement in Patients with Acute Myocardial Infarction. Echocardiography 2014, 31, 58–66. [Google Scholar] [CrossRef]
- Kleijn, S.A.; Aly, M.F.; Terwee, C.B.; van Rossum, A.C.; Kamp, O. Three-Dimensional Speckle Tracking Echocardiography for Automatic Assessment of Global and Regional Left Ventricular Function Based on Area Strain. J. Am. Soc. Echocardiogr. 2011, 24, 314–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.C.; Jin, F.L.; Jing, C.; Xiao, Q.; Liu, Y.; Ran, Z.S.; Zhang, J.-J. Predictive Value of Left Ventricular Remodeling by Area Strain Based on Three-Dimensional Wall-Motion Tracking After PCI in Patients with Recent NSTEMI. Ultrasound Med. Biol. 2012, 38, 1491–1501. [Google Scholar] [CrossRef]
- Aly, M.F.; Kleijn, S.A.; Menken-Negroiu, R.F.; Robbers, L.F.; Beek, A.M.; Kamp, O. Three-dimensional speckle tracking echocardiography and cardiac magnetic resonance for left ventricular chamber quantification and identification of myocardial transmural scar. Neth. Heart J. 2016, 24, 600–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tatsumi, K.; Tanaka, H.; Matsumoto, K.; Sawa, T.; Miyoshi, T.; Imanishi, J.; Motoji, Y.; Mochizuki, Y.; Fukuda, Y.; Shinke, T.; et al. Global endocardial area change rate for the assessment of left ventricular relaxation and filling pressure: Using 3-dimensional speckle-tracking study. Int. J. Cardiovasc. Imaging 2014, 30, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Li, R. Application of three-dimensional speckle tracking echocardiography in evaluation of left ventricular diastolic function in patients with coronary heart disease. Chin. Foreign Med. Res. 2021, 19, 74–77. [Google Scholar] [CrossRef]
- Li, M.; Lu, Y.; Fang, C.; Zhang, X. Correlation between myocardial deformation on three-dimensional speckle tracking echocardiography and cardiopulmonary exercise testing. Echocardiography 2017, 34, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.A.; Nanda, N.C.; Sorrell, V.L. Role of Echocardiography in the Diagnostic Assessment and Etiology of Heart Failure in Older Adults: Opacify, Quantify, and Rectify. Heart Fail. Clin. 2017, 13, 445–466. [Google Scholar] [CrossRef]
- Jiang, F.X.; Guo, R.Q.; Chen, J.L. Evaluation of left ventricular mechanical dyssynchrony in chronic heart failure patients by two-dimensional speckle tracking imaging. Kaohsiung J. Med. Sci. 2013, 29, 374–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gkhan, S.; Klettas, D.; Kapetanakis, S.; Monaghan, M.J. Clinical utility of speckle-tracking echocardiography in cardiac resynchronisation therapy. Echo Res. Pract. 2016, 3, R1–R11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, Y.; Sun, M.M.; Cui, J.; Chen, H.Y.; Su, Y.G.; Pan, C.Z.; Shu, X.H. Three-dimensional speckle tracking echocardiography for the assessment of left ventricular function and mechanical dyssynchrony. Acta Cardiol. 2012, 67, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Szulik, M.; Śliwiñska, A.; Lenarczyk, R.; Szyma£a, M.; Kalinowski, M.E.; Markowicz-Pawlus, E.; Kalarus, Z.; Kukulski, T. 3D and 2D left ventricular systolic function imaging—From ejection fraction to deformation. Cardiac resynchronization therapy—Substudy. Acta Cardiol. 2015, 70, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Chen, H.; Fulati, Z.; Liu, Y.; Su, Y.; Shu, X. The value of left ventricular strain-volume loops in predicting response to cardiac resynchronization therapy. Cardiovasc. Ultrasound 2019, 17, 3. [Google Scholar] [CrossRef] [PubMed]
- Cameli, M.; Ciccone, M.M.; Maiello, M.; Modesti, P.A.; Muiesan, M.L.; Scicchitano, P.; Novo, S.; Palmiero, P.; Saba, P.S.; Pedrinelli, R. Speckle tracking analysis: A new tool for left atrial function analysis in systemic hypertension: An overview. J. Cardiovasc. Med. 2016, 17, 339–343. [Google Scholar] [CrossRef]
- Mandoli, G.E.; D’Ascenzi, F.; Vinco, G.; Benfari, G.; Ricci, F.; Focardi, M.; Cavigli, L.; Pastore, M.C.; Sisti, N.; De Vivo, O.; et al. Novel Approaches in Cardiac Imaging for Non-invasive Assessment of Left Heart Myocardial Fibrosis. Front. Cardiovasc. Med. 2021, 8, 614235. [Google Scholar] [CrossRef]
- Fan, J.L.; Su, B.; Zhao, X.; Zhou, B.Y.; Ma, C.S.; Wang, H.P.; Hu, S.D.; Zhou, Y.F.; Ju, Y.J.; Wang, M.H. Correlation of left atrial strain with left ventricular end-diastolic pressure in patients with normal left ventricular ejection fraction. Int. J. Cardiovasc. Imaging 2020, 36, 1659–1666. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Ma, H.; Gao, L.; Wang, Y.; Wang, J.; Zhu, Z.; Pang, K.; Wang, H.; Wu, W. Left atrial reservoir strain combined with E/E’ as a better single measure to predict elevated LV filling pressures in patients with coronary artery disease. Cardiovasc. Ultrasound 2020, 18, 11. [Google Scholar] [CrossRef] [PubMed]
- Hiebert, J.B.; Vacek, J.; Shah, Z.; Rahman, F.; Pierce, J.D. Use of speckle tracking to assess heart failure with preserved ejection fraction. J. Cardiol. 2019, 74, 397–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, L.; Marwick, T.H.; Popescu, B.A.; Donal, E.; Badano, L.P. LA Structure and Function, and Left Ventricular Diastolic Dysfunction: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 1961–1977. [Google Scholar] [CrossRef]
- Mandoli, G.E.; Sisti, N.; Mondillo, S.; Cameli, M. LA strain in left ventricular diastolic dysfunction: Have we finally found the missing piece of the puzzle? Heart Fail. Rev. 2020, 25, 409–417. [Google Scholar] [CrossRef]
- Reddy, Y.N.V.; Obokata, M.; Egbe, A.; Yang, J.H.; Pislaru, S.; Lin, G.; Carter, R.; Borlaug, B.A. Left atrial strain and compliance in the diagnostic evaluation of heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2019, 21, 891–900. [Google Scholar] [CrossRef]
- Teng, H.; Ran, H.T.; Ren, J.L. Research progress of three-dimensional speckle tracking imaging in evaluation of LA structure and function. Chin. J. Interv. Imaging Ther. 2019, 16, 368–371. [Google Scholar] [CrossRef]
- Yuda, S. Current clinical applications of speckle tracking echocardiography for assessment of left atrial function. J. Echocardiogr. 2021, 19, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Tsujiuchi, M.; Yamauchi, T.; Ebato, M.; Maezawa, H.; Nogi, A.; Ikeda, N.; Mizukami, T.; Nagumo, S.; Iso, Y.; Nakadate, T.; et al. Prognostic Value of LA Size and Functional Indices Measured by 3-Dimensional Speckle-Tracking Analysis. Circ. J. 2019, 83, 801–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsujiuchi, M.; Ebato, M.; Maezawa, H.; Ikeda, N.; Mizukami, T.; Nagumo, S.; Iso, Y.; Yamauchi, T.; Suzuki, H. The Prognostic Value of Left Atrial Reservoir Functional Indices Measured by Three-Dimensional Speckle-Tracking Echocardiography for Major Cardiovascular Events. Circ. J. 2021, 85, 631–639. [Google Scholar] [CrossRef]
- Liu, J.S.; Wang, J.; Zhang, B.; Liu, B.; Lü, W.; Xu, Y.; Zhi, G. Evaluation of LA function by three-dimensional speckle-tracking echocardiography in heart failure with preserved ejection fraction patients. Natl. Med. J. Chin. 2015, 95, 2919–2923. [Google Scholar] [CrossRef]
- Ben-Arzi, A.; Hazanov, E.; Ghanim, D.; Rozen, G.; Marai, I.; Grosman-Rimon, L.; Kachel, E.; Amir, O.; Carasso, S. LA minimal volume: Association with diastolic dysfunction and heart failure in patients in sinus rhythm or atrial fibrillation with preserved ejection fraction. BMC Med. Imaging 2021, 21, 76. [Google Scholar] [CrossRef]
- Kotecha, D.; Lam, C.S.; Van Veldhuisen, D.J.; Van Gelder, I.C.; Voors, A.A.; Rienstra, M. Heart Failure with Preserved Ejection Fraction and Atrial Fibrillation: Vicious Twins. J. Am. Coll. Cardiol. 2016, 68, 2217–2228. [Google Scholar] [CrossRef]
- Furukawa, A.; Ishii, K.; Hyodo, E.; Shibamoto, M.; Komasa, A.; Nagai, T.; Tada, E.; Seino, Y.; Yoshikawa, J. Three-Dimensional Speckle Tracking Imaging for Assessing Left Atrial Function in Hypertensive Patients with Paroxysmal Atrial Fibrillation. Int. Heart J. 2016, 57, 705–711. [Google Scholar] [CrossRef] [Green Version]
- Mochizuki, A.; Yuda, S.; Fujito, T.; Kawamukai, M.; Muranaka, A.; Nagahara, D.; Shimoshige, S.; Hashimoto, A.; Miura, T. Left atrial strain assessed by three-dimensional speckle tracking echocardiography predicts atrial fibrillation recurrence after catheter ablation in patients with paroxysmal atrial fibrillation. J. Echocardiogr. 2017, 15, 79–87. [Google Scholar] [CrossRef]
- Esposito, G.; Piras, P.; Evangelista, A.; Nuzzi, V.; Nardinocchi, P.; Pannarale, G.; Torromeo, C.; Puddu, P.E. Improving performance of 3D speckle tracking in arterial hypertension and paroxysmal atrial fibrillation by using novel strain parameters. Sci. Rep. 2019, 9, 7382. [Google Scholar] [CrossRef]
- Tadic, M.; Pieske-Kraigher, E.; Cuspidi, C.; Morris, D.A.; Burkhardt, F.; Baudisch, A.; Haßfeld, S.; Tschöpe, C.; Pieske, B. Right ventricular strain in heart failure: Clinical perspective. Arch. Cardiovasc. Dis. 2017, 110, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Ishizu, T.; Ieda, M.; Ohte, N. Right ventricular three-dimensional echocardiography: The current status and future perspectives. J. Echocardiogr. 2020, 18, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Kovács, A.; Lakatos, B.; Tokodi, M.; Merkely, B. Right ventricular mechanical pattern in health and disease: Beyond longitudinal shortening. Heart Fail. Rev. 2019, 24, 511–520. [Google Scholar] [CrossRef] [Green Version]
- Ishizu, T.; Seo, Y.; Atsumi, A.; Tanaka, Y.O.; Yamamoto, M.; Machino-Ohtsuka, T.; Horigome, H.; Aonuma, K.; Kawakami, Y. Global and Regional Right Ventricular Function Assessed by Novel Three-Dimensional Speckle-Tracking Echocardiography. J. Am. Soc. Echocardiogr. 2017, 30, 1203–1213. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Zhu, S.; Xie, Y.; Zhang, Y.; Qian, M.; Gao, L.; Li, M.; Lin, Y.; Wu, W.; Wang, J.; et al. Prognostic Value of Right Ventricular 3D Speckle-Tracking Strain and Ejection Fraction in Patients with HFpEF. Front. Cardiovasc. Med. 2021, 8, 694365. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Cao, X.; Guo, Y.; Tan, X.; Dong, L.; Pan, C.; Shu, X. Long-term impacts of hemodialysis on the right ventricle: Assessment via 3-dimensional speckle-tracking echocardiography. Clin. Cardiol. 2018, 41, 87–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitarelli, A.; Mangieri, E.; Terzano, C.; Gaudio, C.; Salsano, F.; Rosato, E.; Capotosto, L.; D’Orazio, S.; Azzano, A.; Truscelli, G.; et al. Three-Dimensional Echocardiography and 2D-3D Speckle-Tracking Imaging in Chronic Pulmonary Hypertension: Diagnostic Accuracy in Detecting Hemodynamic Signs of Right Ventricular (RV) Failure. J. Am. Heart Assoc. 2015, 4, e001584. [Google Scholar] [CrossRef] [Green Version]
- Lisi, M.; Cameli, M.; Righini, F.M.; Malandrino, A.; Tacchini, D.; Focardi, M.; Tsioulpas, C.; Bernazzali, S.; Tanganelli, P.; Maccherini, M.; et al. RV Longitudinal Deformation Correlates with Myocardial Fibrosis in Patients with End-Stage Heart Failure. JACC Cardiovasc. Imaging 2015, 8, 514–522. [Google Scholar] [CrossRef] [Green Version]
- Plaksej, R.; Kosmala, W.; Frantz, S.; Herrmann, S.; Niemann, M.; Störk, S.; Wachter, R.; Angermann, C.E.; Ertl, G.; Bijnens, B.; et al. Relation of circulating markers of fibrosis and progression of left and right ventricular dysfunction in hypertensive patients with heart failure. J. Hypertens. 2009, 27, 2483–2491. [Google Scholar] [CrossRef]
- Krämer, J.; Niemann, M.; Liu, D.; Hu, K.; Machann, W.; Beer, M.; Wanner, C.; Ertl, G.; Weidemann, F. Two-dimensional speckle tracking as a non-invasive tool for identification of myocardial fibrosis in Fabry disease. Eur. Heart J. 2013, 34, 1587–1596. [Google Scholar] [CrossRef]
- Popović, Z.B.; Kwon, D.H.; Mishra, M.; Buakhamsri, A.; Greenberg, N.L.; Thamilarasan, M.; Flamm, S.D.; Thomas, J.D.; Lever, H.M.; Desai, M.Y. Association Between Regional Ventricular Function and Myocardial Fibrosis in Hypertrophic Cardiomyopathy Assessed by Speckle Tracking Echocardiography and Delayed Hyperenhancement Magnetic Resonance Imaging. J. Am. Soc. Echocardiogr. 2008, 21, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Karamitsos, T.D.; Arvanitaki, A.; Karvounis, H.; Neubauer, S.; Ferreira, V.M. Myocardial Tissue Characterization and Fibrosis by Imaging. JACC Cardiovasc. Imaging 2020, 13, 1221–1234. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.; Zhang, L.; Tian, F.; Wang, B.; Xie, Y.; Sun, W.; Sun, Z.; Yang, Y.; Lv, Q.; et al. Assessment of Myocardial Fibrosis Using Two-Dimensional and Three-Dimensional Speckle Tracking Echocardiography in Dilated Cardiomyopathy with Advanced Heart Failure. J. Card. Fail. 2021, 27, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.Y.; Zhang, L.; Xie, Y.J.; Zhang, Y.; Zhu, S.; Wu, C.; Li, M.; Gao, Y.; Wang, J.; Yang, Y.; et al. 3-dimensional versus 2-dimensional speckle-tracking echo-cardiography for right ventricular myocardial fibrosis in patients with end-stage heart failure. JACC Cardiovasc. Imaging 2021, 14, 1309–1320. [Google Scholar] [CrossRef] [PubMed]

| References | Sample Size | Age (Years) | Men, n (%) | LVEF (%) | Strain Parameters | Main Findings |
|---|---|---|---|---|---|---|
| Sun et al. [47] | 65 | 62 ± 8 a 61 ± 9 b | Not reported | 59.5 ± 4.2 a 36.2 ± 5.3 b | GS, GLS, GCS, GRS | GS, GLS, GCS, and GRS were progressively decreased from HFpEF to HFrEF compared with normal controls. |
| Li et al. [48] | 34 | 55.6 ± 16.7 | 19 (55.9) | 59.1 ± 7.5 | GLS, GAS, GRS | GLS, GAS, and GRS were impaired in the aortic valve stenosis group compared with healthy controls. |
| Nagata et al. [49] | 104 | 78 ± 10 | 43 (41.3) | 60 ± 5 | GLS, GRS | 3D GLS, 3D GRS, and 2D GLS had significant predictive power for MACE. Three-dimensional GLS was the most powerful independent predictor of MACE. |
| Rady et al. [50] | 91 | 53 ± 13 | 74 (81.3) | 33 ± 10 | GLS, GCS, LV torsion | The patients with a reduced LV torsion (<0.59 degrees/cm) had higher risk of HF hospitalization. |
| Luo et al. [29] | 44 | 62.3 ± 8.4 a 64.9 ± 4.8 b 61.1 ± 9.3 c | 11 (78.6) a 7 (63.6) b 12 (63.2) c | 56.42 ± 4.61 a 43.89 ± 6.19 b 59.34 ± 2.20 c | GLS, GCS, LV torsion | GLS, GCS, and LV systolic torsion were significantly decreased in HF patients with MI. |
| Iwahashi et al. [52] | 270 | 65 | 222 (82.2) | 52 | GLS, GCS, GRS, global principal strain | The model using 3D GLS was found to predict adverse outcomes stronger than those using 2D GLS. Three-dimensional GLS of >−11.1 was associated with primary endpoint events. |
| Iwahashi et al. [53] | 248 | 64 | 206 (83.1) | 56 ± 12.1 | GLS | The reduced 3D GLS at 1 year was an independent predictor for the primary endpoint events. |
| Altman et al. [54] | 147 | 54 ± 15 | Not reported | 21–72 | GLS, GRS, GCS, AS | GLS, GRS, GCS, and AS showed good accuracy in the detection of 2D LVEF < 55%, with AS indicating superiority over GRS and GCS but not GLS. |
| References | Sample Size | Age (Years) | Men, n (%) | LVEF (%) | Strain Parameters | Main Findings |
|---|---|---|---|---|---|---|
| Maffessanti et al. [59] | 32 | 59 ± 17 | 20 (62.5) | Not reported | Longitudinal strain, circumferential strain, radial strain, rotation | All 3D-STE indexes were reduced in the abnormal myocardial segments. |
| Zhu et al. [60] | 26 | 56.3 ± 11.1 | 15 (57.7) | 51.3 ± 5.73 a 44.69 ± 6.73 b 41.22 ± 9.29 c | GLS, GCS, GRS, longitudinal strain, circumferential strain, radial strain | LV GLS, GRS, and GCS correlated with the infarct area of myocardium measured by CMR. Transmural infarct segments displayed markedly lower longitudinal, radial, and circumferential strains than normal segments. |
| Kleijn et al. [61] | 114 | 59 ± 16 | 67 (58.8) | 51 ± 13 | LV AS | AS was a promising index to quantitative evaluation of LV regional function and identify regional wall motion abnormalities. |
| Li et al. [62] | 61 | 54.2 ± 9.2 d 53.2 ± 8.7 e | 15 (60) d 21 (58.3) e | 44.7 ± 4.3 | LV GAS, LV regional peak AS | Regional AS were significantly lower in the infarcted segments than those in the non-infrared segments. |
| Vachalcova et al. [28] | 40 | 63 ± 9 f 64 ± 11g | 19 (95) f 15 (75) g | 29.0 ± 11.3 f 27.3 ± 7.5 g | GLS, twist, LV apical rotation | LV apical rotation in HF patients with ischemic etiology is significantly higher than in those non-ischemic etiology. |
| Aly et al. [63] | 120 | 63 ± 12 f 59 ± 14 g | 65 (79) f 27 (61) g | 40 ± 8 f 37 ± 9 g | Longitudinal strain, circumferential strain, radial strain, 3D strain, AS, LV twist | No significant differences were observed in global or regional strain parameters between the two groups, except for lower twist in non-ischemic group. |
| References | Sample Size | Age (Years) | Men, n (%) | LVEF (%) | Strain Parameters | Main Findings |
|---|---|---|---|---|---|---|
| Tsujiuchi et al. [83] | 514 | 66 ± 15 | 320 (62) | 55 ± 16 | Maximal LA volume index, minimal LA volume index, LAEmpF | 3D-STE-derived LAEmpF was an independent predictor of hospitalization in HF patients, providing higher prognostic power than that measured by 2D echocardiography. |
| Tsujiuchi et al. [84] | 264 | 65 ± 16 | 159 (60) | 55 ± 16 | Maximal LA volume index, minimal LA volume index, LAEmpF, LA longitudinal strain, LA circumferential strain | 2D LV longitudinal strain, 3D LA longitudinal strain, and 3D LAEmpF showed higher hazard ratio than other parameters. Three-dimensional LAEmpF displayed incremental prognostic values in major cardiovascular events. |
| Liu et al. [85] | 43 | 55.7 ± 6.9 a 54.8 ± 7.4 b | 11 (57.9) a 12 (50.0) b | 58 ± 10 a 60 ± 6 b | Maximal LA volume index, minimal LA volume index, longitudinal strain, LA stiffness index | LA reservoir, conduit, pump function in HFpEF patients were significantly reduced, and strains of LA middle level were powerful parameters for assessing LA function. |
| Mochizuki et al. [89] | 42 | 58 ± 10 | 29 (69) | 66 ± 7 | LA GLS, LA GCS, LA GAS | Systolic 3D GCS and age may predict the recurrence of AF, and 3D LA strain was a better predictor of AF recurrence after catheter ablation than other predictors. |
| Esposito et al. [90] | 48 | 59.78 ± 13.8 c 56.39 ± 7.58 d 57.5 ± 9.62 e 71.64 ± 3.96 f | 8 (89) c 10 (56) d 9 (90) e 6 (55) f | 60.11 ± 4.46 c 60.39 ± 5.08 d 55.7 ± 3.86 e 60 ± 5.1 f | LA GLS, LA GCS, LA GAS, strain rate | Impairment of LA strain and strain rates were observed in both hypertensive and paroxysmal AF groups compared with control subjects, and LA strain was an independent predictor of poor outcomes in patients with paroxysmal AF. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, L.; Lin, Y.; Ji, M.; Wu, W.; Li, H.; Qian, M.; Zhang, L.; Xie, M.; Li, Y. Clinical Utility of Three-Dimensional Speckle-Tracking Echocardiography in Heart Failure. J. Clin. Med. 2022, 11, 6307. https://doi.org/10.3390/jcm11216307
Gao L, Lin Y, Ji M, Wu W, Li H, Qian M, Zhang L, Xie M, Li Y. Clinical Utility of Three-Dimensional Speckle-Tracking Echocardiography in Heart Failure. Journal of Clinical Medicine. 2022; 11(21):6307. https://doi.org/10.3390/jcm11216307
Chicago/Turabian StyleGao, Lang, Yixia Lin, Mengmeng Ji, Wenqian Wu, He Li, Mingzhu Qian, Li Zhang, Mingxing Xie, and Yuman Li. 2022. "Clinical Utility of Three-Dimensional Speckle-Tracking Echocardiography in Heart Failure" Journal of Clinical Medicine 11, no. 21: 6307. https://doi.org/10.3390/jcm11216307
APA StyleGao, L., Lin, Y., Ji, M., Wu, W., Li, H., Qian, M., Zhang, L., Xie, M., & Li, Y. (2022). Clinical Utility of Three-Dimensional Speckle-Tracking Echocardiography in Heart Failure. Journal of Clinical Medicine, 11(21), 6307. https://doi.org/10.3390/jcm11216307








