A Four-Stepwise Electrocardiographic Algorithm for Differentiation of Ventricular Arrhythmias Originated from Left Ventricular Outflow Tract
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Study Populations
2.3. Electrophysiological Study
2.4. Mapping and Radiofrequency Catheter Ablation
2.5. ECG Analysis
2.6. Procedure Success and Follow-Up
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Mapping and Ablation
3.3. ECG Analysis
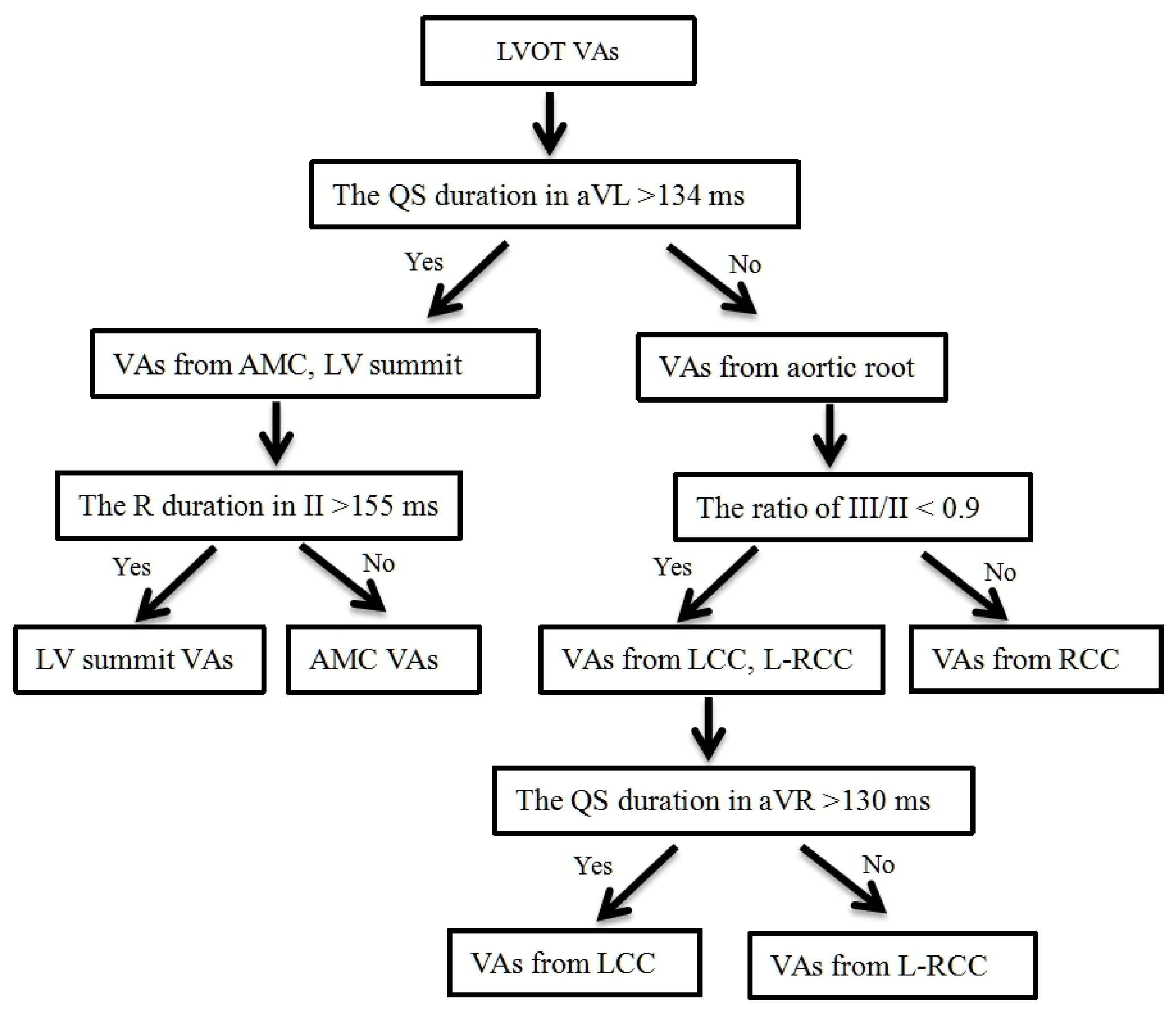
3.4. Develop a Stepwise ECG Algorithm to Differentiate LVOT VAs
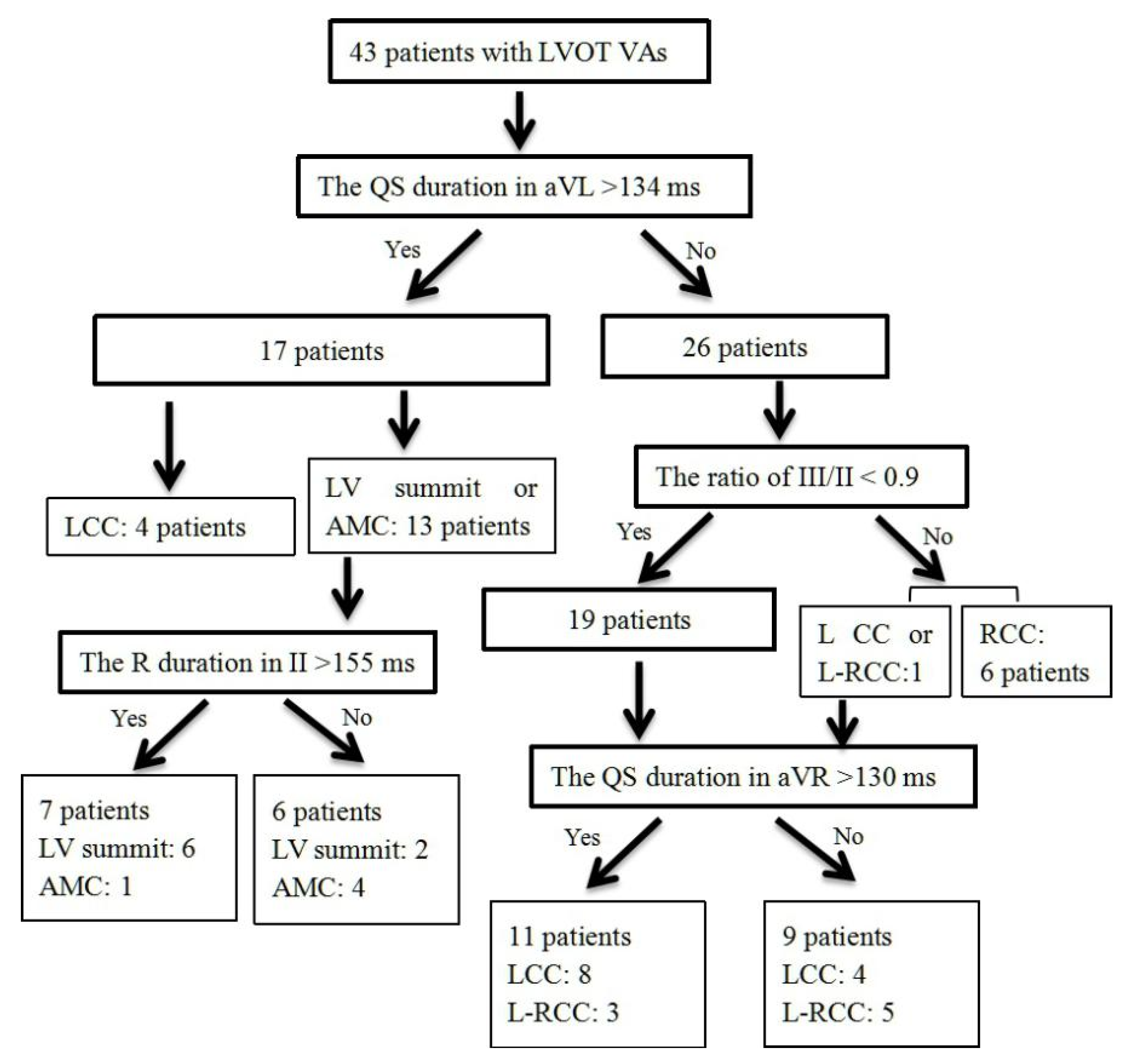
3.5. Validation of Four-Stepwise ECG Algorithm in the Prospective Study
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prystowsky, E.N.; Padanilam, B.J.; Joshi, S.; Fogel, R.I. Ventricular arrhythmias in the absence of structural heart disease. J. Am. Coll. Cardiol. 2012, 59, 1733–1744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chun, K.R.; Satomi, K.; Kuck, K.H.; Ouyang, F.; Antz, M. Left ventricular outflow tract tachycardia including ventricular tachycardia from the aortic cusps and epicardial ventricular tachycardia. Herz 2007, 32, 226–332. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.D.; Kumar, S.; Parameswaran, R.; Wong, G.; Voskoboinik, A.; Sugumar, H.; Watts, T.; Sparks, P.B.; Morton, J.B.; McLellan, A.; et al. Differentiating Right- and Left-Sided Outflow Tract Ventricular Arrhythmias: Classical ECG Signatures and Prediction Algorithms. Circ. Arrhythm. Electrophysiol. 2019, 12, e007392. [Google Scholar] [CrossRef] [PubMed]
- Reithmann, C.; Fiek, M. Left ventricular outflow tract arrhythmias with divergent QRS morphology: Mapping of different exits and ablation strategy. J. Interv. Card. Electrophysiol. 2018, 51, 61–69. [Google Scholar] [CrossRef]
- Cheung, J.W.; Anderson, R.H.; Markowitz, S.M.; Lerman, B.B. Catheter Ablation of Arrhythmias Originating From the Left Ventricular Outflow Tract. JACC Clin. Electrophysiol. 2019, 5, 1–12. [Google Scholar] [CrossRef]
- Xie, S.; Kubala, M.; Liang, J.J.; Hayashi, T.; Park, J.; Padros, I.L.; Garcia, F.C.; Santangeli, P.; Supple, G.E.; Frankel, D.S.; et al. Lead I R-wave amplitude to differentiate idiopathic ventricular arrhythmias with left bundle branch block right inferior axis originating from the left versus right ventricular outflow tract. J. Cardiovasc. Electrophysiol. 2018, 29, 1515–1522. [Google Scholar] [CrossRef]
- Cheng, D.; Ju, W.; Zhu, L.; Chen, K.; Zhang, F.; Chen, H.; Yang, G.; Li, X.; Li, M.; Gu, K.; et al. V3R/V7 Index: A Novel Electrocardiographic Criterion for Differentiating Left From Right Ventricular Outflow Tract Arrhythmias Origins. Circ. Arrhythm. Electrophysiol. 2018, 11, e006243. [Google Scholar] [CrossRef]
- Yoshida, N.; Yamada, T.; McElderry, H.T.; Inden, Y.; Shimano, M.; Murohara, T.; Kumar, V.; Doppalapudi, H.; Plumb, V.J.; Kay, G.N. A novel electrocardiographic criterion for differentiating a left from right ventricular outflow tract tachycardia origin: The V2S/V3R index. J. Cardiovasc. Electrophysiol. 2014, 25, 747–753. [Google Scholar] [CrossRef]
- Di, C.; Wan, Z.; Tse, G.; Letsas, K.P.; Liu, T.; Efremidis, M.; Li, J.; Lin, W. The V1-V3 transition index as a novel electrocardiographic criterion for differentiating left from right ventricular outflow tract ventricular arrhythmias. J. Interv. Card. Electrophysiol. 2019, 56, 37–43. [Google Scholar] [CrossRef]
- Igarashi, M.; Nogami, A.; Sekiguchi, Y.; Kuroki, K.; Yamasaki, H.; Machino, T.; Yui, Y.; Ogawa, K.; Talib, A.K.; Murakoshi, N.; et al. The QRS morphology pattern in V5R is a novel and simple parameter for differentiating the origin of idiopathic outflow tract ventricular arrhythmias. Europace 2015, 17, 1107–1116. [Google Scholar] [CrossRef]
- Enriquez, A.; Baranchuk, A.; Briceno, D.; Saenz, L.; Garcia, F. How to use the 12-lead ECG to predict the site of origin of idiopathic ventricular arrhythmias. Heart Rhythm. 2019, 16, 1538–1544. [Google Scholar] [CrossRef]
- Ouyang, F.; Mathew, S.; Wu, S.; Kamioka, M.; Metzner, A.; Xue, Y.; Ju, W.; Yang, B.; Zhan, X.; Rillig, A.; et al. Ventricular arrhythmias arising from the left ventricular outflow tract below the aortic sinus cusps: Mapping and catheter ablation via transseptal approach and electrocardiographic characteristics. Circ Arrhythm. Electrophysiol. 2014, 7, 445–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, T.; McElderry, H.T.; Doppalapudi, H.; Murakami, Y.; Yoshida, Y.; Yoshida, N.; Okada, T.; Tsuboi, N.; Inden, Y.; Murohara, T.; et al. Idiopathic ventricular arrhythmias originating from the aortic root: Prevalence, electrocardiographic and electrophysiologic characteristics, and results of radiofrequency catheter ablation. J. Am. Coll. Cardiol. 2008, 52, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Lau, Y.R.; Litovsky, S.H.; Thomas McElderry, H.; Doppalapud, I.H.; Osorio, J.; Plumb, V.J.; Neal Kay, G. Prevalence and clinical, electrocardiographic, and electrophysiologic characteristics of ventricular arrhythmias originating from the noncoronary sinus of Valsalva. Heart Rhythm. 2013, 10, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Anter, E.; Frankel, D.S.; Marchlinski, F.E.; Dixit, S. Effect of electrocardiographic lead placement on localization of outflow tract tachycardias. Heart Rhythm. 2012, 9, 697–703. [Google Scholar] [CrossRef]
- Betensky, B.P.; Park, R.E.; Marchlinski, F.E.; Hutchinson, M.D.; Garcia, F.C.; Dixit, S.; Callans, D.J.; Cooper, J.M.; Bala, R.; Lin, D.; et al. The V(2) transition ratio: A new electrocardiographic criterion for distinguishing left from right ventricular outflow tract tachycardia origin. J. Am. Coll. Cardiol. 2011, 57, 2255–2262. [Google Scholar] [CrossRef] [Green Version]
- Hasdemir, C.; Aktas, S.; Govsa, F.; Aktas, E.O.; Kocak, A.; Bozkaya, Y.T.; Demirbas, M.I.; Ulucan, C.; Ozdogan, O.; Kayikcioglu, M.; et al. Demonstration of ventricular myocardial extensions into the pulmonary artery and aorta beyond the ventriculo-arterial junction. Pacing Clin. Electrophysiol. 2007, 30, 534–549. [Google Scholar] [CrossRef]
- Chen, J.; Hoff, P.I.; Rossvoll, O.; De Bortoli, A.; Solheim, E.; Sun, L.; Schuster, P.; Larsen, T.; Ohm, O.J. Ventricular arrhythmias originating from the aortomitral continuity: An uncommon variant of left ventricular outflow tract tachycardia. Europace 2012, 14, 388–395. [Google Scholar] [CrossRef]
- Steven, D.; Roberts-Thomson, K.C.; Seiler, J.; Inada, K.; Tedrow, U.B.; Mitchell, R.N.; Sobieszczyk, P.S.; Eisenhauer, A.C.; Couper, G.S.; Stevenson, W.G. Ventricular tachycardia arising from the aortomitral continuity in structural heart disease: Characteristics and therapeutic considerations for an anatomically challenging area of origin. Circ. Arrhythm. Electrophysiol. 2009, 2, 660–666. [Google Scholar] [CrossRef] [Green Version]
- Chung, F.P.; Lin, C.Y.; Shirai, Y.; Futyma, P.; Santangeli, P.; Lin, Y.J.; Chang, S.L.; Lo, L.W.; Hu, Y.F.; Chang, H.Y.; et al. Outcomes of catheter ablation of ventricular arrhythmia originating from the left ventricular summit: A multicenter study. Heart Rhythm. 2020, 17, 1077–1083. [Google Scholar] [CrossRef]
- Szili-Torok, T.; van Malderen, S.; de Groot, N. ‘Born’ with a ‘dead’-end-tract resulting in arrhythmias in the aorto-mitral continuity: Coincidence, causation, and ‘commensuration’. Europace 2012, 14, 308–389. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, F.; Fotuhi, P.; Ho, S.Y.; Hebe, J.; Volkmer, M.; Goya, M.; Burns, M.; Antz, M.; Ernst, S.; Cappato, R.; et al. Repetitive monomorphic ventricular tachycardia originating from the aortic sinus cusp: Electrocardiographic characterization for guiding catheter ablation. J. Am. Coll. Cardiol. 2002, 39, 500–508. [Google Scholar] [CrossRef] [Green Version]
- Lin, D.; Ilkhanoff, L.; Gerstenfeld, E.; Dixit, S.; Beldner, S.; Bala, R.; Garcia, F.; Callans, D.; Marchlinski, F.E. Twelve-lead electrocardiographic characteristics of the aortic cusp region guided by intracardiac echocardiography and electroanatomic mapping. Heart Rhythm. 2008, 5, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, Y.; Nogami, A.; Shinoda, Y.; Masuda, K.; Machino, T.; Kuroki, K.; Yamasaki, H.; Sekiguchi, Y.; Aonuma, K. Idiopathic Ventricular Arrhythmias Originating From the Vicinity of the Communicating Vein of Cardiac Venous Systems at the Left Ventricular Summit. Circ. Arrhythm. Electrophysiol. 2018, 11, e005386. [Google Scholar] [CrossRef] [PubMed]
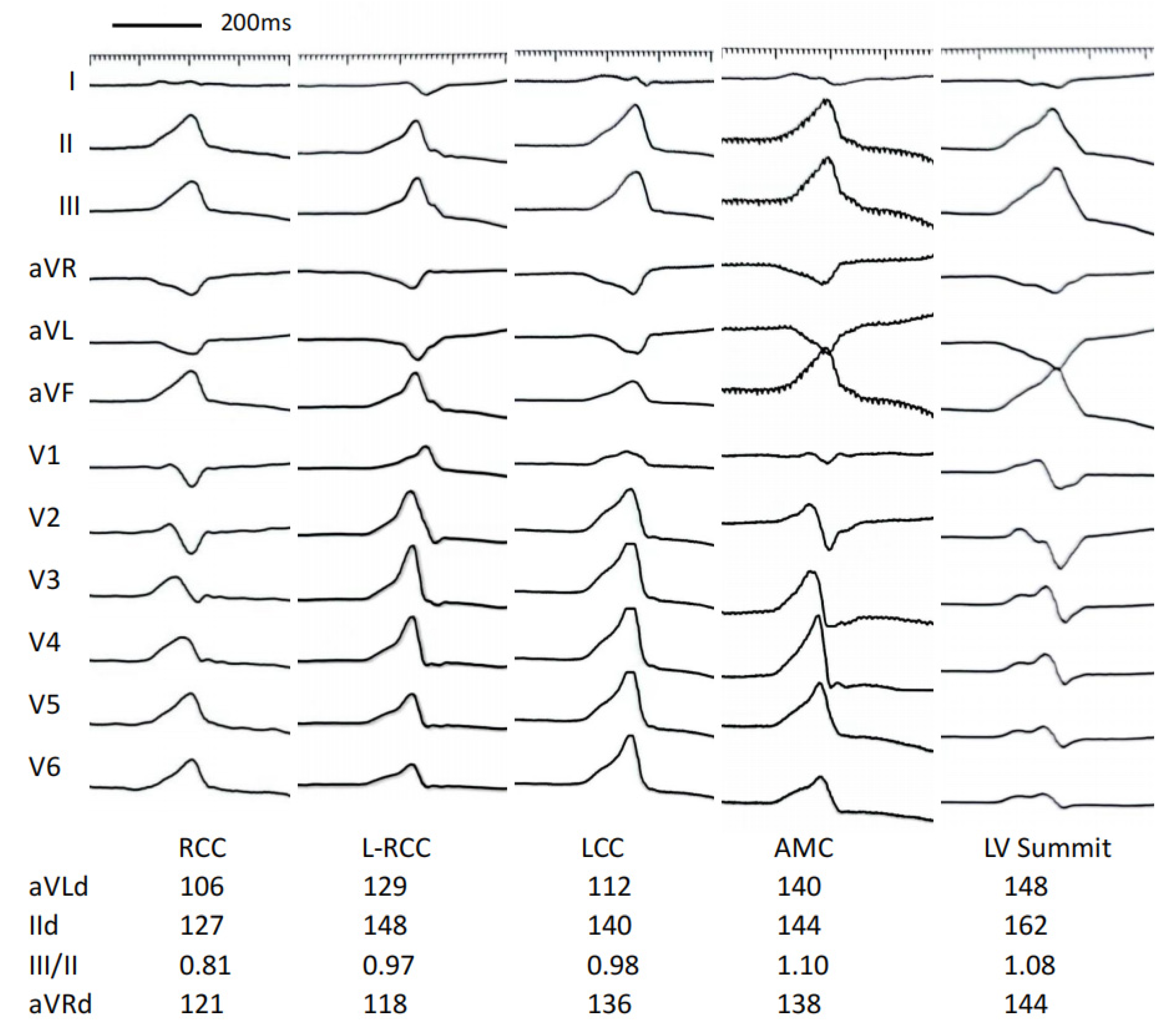

| Characteristic | RCC Group (n = 8) | L-RCC Group (n = 21) | LCC Group (n = 24) | AMC Group (n = 9) | LV Summit Group (n = 6) |
|---|---|---|---|---|---|
| Age (year) | 45 ± 19 | 50 ± 16 | 56 ± 16 | 66 ± 15 | 54 ± 20 |
| Gender (male) | 1 (13%) | 7 (33%) | 11 (46%) | 5 (56%) | 4 (67%) |
| BMI (kg/m2) | 24.9 ± 4.8 | 26.3 ± 6.2 | 25.2 ± 5.3 | 27.0 ± 7.1 | 25.3 ± 4.8 |
| Hypertension (n,%) | 3 (37%) | 7 (33%) | 9 (37%) | 4 (44%) | 2 (33%) |
| Diabetes | 1 (14%) | 2 (9%) | 3 (14%) | 1 (11%) | 0 |
| LVd (mm) | 45 ± 5 | 48 ± 4 | 50 ± 5 | 51 ± 3 | 49 ± 3 |
| LVEF (%) | 59 ± 3 | 65 ± 6 | 61 ± 5 | 61 ± 6 | 61 ± 7 |
| History (month) | 17 ± 13 | 21 ± 28 | 30 ± 31 | 30 ± 26 | 65 ± 58 *# |
| PVC burden (n/24 h) | 20,653 ± 13,707 | 23,279 ± 12,299 | 22,302 ± 8208 | 23,751 ± 11,356 | 34,843 ± 13,899 |
| VT (%) | 1 (13%) | 2 (9%) | 2 (8%) | 0 | 2 (33%) |
| Antiarrhythmics (n) | 2.1 ± 1.1 | 1.9 ± 1.3 | 2.0 ± 1.2 | 2.2 ± 1.2 | 1.8 ± 1.3 |
| Characteristic | RCC Group (n = 8) | L-RCC Group (n = 21) | LCC Group (n = 24) | AMC Group (n = 9) | LV Summit Group (n = 6) |
|---|---|---|---|---|---|
| Total QRS duration (ms) | 150 ± 28 | 142 ± 15 | 147 ± 13 | 158 ± 13 # | 178 ± 26 *#&$ |
| R wave duration (ms) | |||||
| Lead I | 100 ± 35 | 88 ± 33 | 83 ± 35 | 73 ± 26 | 103 ± 41 |
| Lead II | 132 ± 13 | 134 ± 16 | 142 ± 14 | 152 ± 11 *# | 171 ± 30 *#&$ |
| Lead III | 131 ± 11 | 129 ± 19 | 139 ± 16 | 152 ± 11 *# | 169 ± 34 *#& |
| Lead aVF | 130 ± 10 | 133 ± 17 | 141 ± 16 | 151 ± 11 *# | 168 ± 30 *#& |
| QS wave duration (ms) | |||||
| Lead I (S wave) | 35 ± 7 | 59 ± 23 | 64 ± 27 | 81 ± 29 | 71 ± 46 |
| Lead aVL | 113 ± 19 | 124 ± 18 | 130 ± 17 * | 147 ± 13 *#& | 153 ± 24 *#& |
| Lead aVR | 125 ± 14 | 128 ± 14 | 139 ± 15 *# | 146 ± 14 *# | 155 ± 15 *#& |
| R wave amplitude (mV) | |||||
| Lead I | 0.46 ± 0.27 | 0.43 ± 0.30 | 0.32 ± 0.22 | 0,20 ± 0.11 | 0.29 ± 0.21 |
| Lead II | 1.75 ± 0.33 | 2.07 ± 0.81 | 2.11 ± 0.57 | 1.71 ± 0.28 | 1.84 ± 0.53 |
| Lead III | 1.40 ± 0.45 | 1.95 ± 0.86 | 2.18 ± 0.69 * | 1.89 ± 0.39 | 1.93 ± 0.55 |
| Lead aVF | 1.60 ± 0.42 | 2.05 ± 0.89 | 2.14 ± 0.59 * | 1.79 ± 0.34 | 1.86 ± 0.53 |
| The ratio of III/II | 0.78 ± 0.15 | 0.94 ± 0.16* | 1.03 ± 0.13 * | 1.10 ± 0.16 *# | 1.06 ± 0.10 * |
| QS wave amplitude (mV) | |||||
| Lead I (S wave) | 0.07 ± 0.03 | 0.22 ± 0.13 | 0.31 ± 0.17 | 0.39 ± 0.11 | 0.24 ± 0.16 |
| Lead aVL | 0.58 ± 0.29 | 1.14 ± 0.53 * | 1.24 ± 0.35 * | 1.12 ± 0.21 * | 1.12 ± 0.30 * |
| Lead aVR | 1.02 ± 0.18 | 1.09 ± 0.42 | 1.09 ± 0.21 | 0.79 ± 0.15 #& | 0.96 ± 0.25 |
| The ratio of aVL/aVR | 0.58 ± 0.28 | 1.12 ± 0.36 * | 1.20 ± 0.36 * | 1.45 ± 0.38 *# | 1.18 ± 0.18 * |
| Characteristic | RCC Group (n = 8) | L-RCC Group (n = 21) | LCC Group (n = 24) | AMC Group (n = 9) | LV Summit Group (n = 6) |
|---|---|---|---|---|---|
| R wave duration (ms) | |||||
| Lead V1 | 46 ± 10 | 52 ± 24 | 72 ± 32 * | 101 ± 52 *#& | 134 ± 17 *#& |
| Lead V2 | 54 ± 14 | 72 ± 33 | 88 ± 27 | 113 ± 35 *#& | 127 ± 12 *#& |
| Lead V3 | 92 ± 25 | 108 ± 24 | 112 ± 23 * | 124 ± 31 * | 132 ± 18 * |
| Lead V4 | 129 ± 15 | 127 ± 16 | 131 ± 14 | 137 ± 23 | 148 ± 22 *#& |
| Lead V5 | 134 ± 14 | 130 ± 14 | 135 ± 14 | 139 ± 22 | 150 ± 23 |
| Lead V6 | 113 ± 41 | 130 ± 13 | 134 ± 12 * | 142 ± 16 * | 147 ± 28 * |
| S wave duration (ms) | |||||
| Lead V1 | 79 ± 23 | 69 ± 23 | 60 ± 23 | 30 ± 1 *#& | 44 ± 7 * |
| Lead V2 | 73 ± 18 | 62 ± 29 | 56 ± 23 | 44 ± 13 * | 43 ± 6 * |
| Lead V3 | 45 ± 15 | 47 ± 21 | 40 ± 21 | 37 ± 12 | 42 ± 15 |
| R wave amplitude (mV) | |||||
| Lead V1 | 0.22 ± 0.07 | 0.34 ± 0.24 | 0.53 ± 0.42 * | 0.68 ± 0.57 *# | 0.83 ± 0.26 *# |
| Lead V2 | 0.45 ± 0.17 | 0.98 ± 0.65 | 1.07 ± 0.58 | 1.22 ± 0.76 | 1.47 ± 0.58 |
| Lead V3 | 0.93 ± 0.32 | 1.57 ± 0.97 | 1.85 ± 0.83 * | 1.77 ± 0.71 * | 2.42 ± 0.94 *# |
| Lead V4 | 1.79 ± 0.53 | 2.06 ± 1.11 | 2.25 ± 0.68 | 1.84 ± 0.52 | 2.68 ± 0.88 |
| Lead V5 | 1.92 ± 0.30 | 2.10 ± 0.88 | 2.18 ± 0.68 | 1.33 ± 0.43 #& | 2.28 ± 0.89 $ |
| Lead V6 | 1.75 ± 0.33 | 1.84 ± 0.67 | 1.77 ± 0.56 | 0.91 ± 0.34 *#& | 1.22 ± 0.37 #& |
| S wave amplitude (mV) | |||||
| Lead V1 | 1.28 ± 0.67 | 1.00 ± 0.58 | 0.61 ± 0.48 *# | 0.16 ± 0.11 *# | 0.37 ± 0.28 * |
| Lead V2 | 1.36 ± 0.61 | 1.25 ± 0.86 | 1.12 ± 0.66 | 0.48 ± 0.35 *#& | 0.50 ± 0.16 * |
| Lead V3 | 0.55 ± 0.43 | 0.69 ± 0.49 | 0.81 ± 0.52 | 0.58 ± 0.51 | 0.45 ± 0.17 |
| Sensitivity, % | Specificity, % | PPV, % | NPV, % | |
|---|---|---|---|---|
| The QS duration in aVL > 134 ms for differentiating VAs from AMC, LV summit and VAs from aortic root | 100.0 | 86.7 | 76.5 | 100.0 |
| The R duration in II > 155 ms for differentiating VAs from AMC and VAs from LV summit | 75.0 | 80.0 | 85.7 | 66.7 |
| The ratio of III/II < 0.9 for differentiating VAs from RCC and VAs from L-RCC, LCC | 100.0 | 95.0 | 85.7 | 100.0 |
| The QS duration in aVR > 130 ms for differentiating VAs from LCC and VAs from L-RCC | 66.7 | 62.5 | 72.7 | 55.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, H.-W.; Gao, W.-D.; Wang, X.-H.; Chen, Z.-S.; Liu, X.-B. A Four-Stepwise Electrocardiographic Algorithm for Differentiation of Ventricular Arrhythmias Originated from Left Ventricular Outflow Tract. J. Clin. Med. 2022, 11, 6398. https://doi.org/10.3390/jcm11216398
Tan H-W, Gao W-D, Wang X-H, Chen Z-S, Liu X-B. A Four-Stepwise Electrocardiographic Algorithm for Differentiation of Ventricular Arrhythmias Originated from Left Ventricular Outflow Tract. Journal of Clinical Medicine. 2022; 11(21):6398. https://doi.org/10.3390/jcm11216398
Chicago/Turabian StyleTan, Hong-Wei, Wei-Dong Gao, Xin-Hua Wang, Zhi-Song Chen, and Xue-Bo Liu. 2022. "A Four-Stepwise Electrocardiographic Algorithm for Differentiation of Ventricular Arrhythmias Originated from Left Ventricular Outflow Tract" Journal of Clinical Medicine 11, no. 21: 6398. https://doi.org/10.3390/jcm11216398





