Abstract
Skin burns are one of the most difficult medical problems. Recently, studies have been directed towards development of natural products in order to identify effective and safe remedies. In the present study, we evaluated the efficacy of a natural composite (formulated from honey and essential oils) compared with MEBO® (0.25% β-sitosterol) and DERMAZIN® creams (1% silver-sulfadiazine) in the treatment of thermally induced skin burns. For this purpose, four burn-wounds were created on the back of male New Zealand rabbits (n = 10) using a thermal stamp under the effect of general anesthesia. Each wound represents one of the following groups: non-treated, natural composite-cream, MEBO®-cream, and silver-sulfadiazine treated groups, respectively. Treatments were applied once a day topically until one of these wounds appeared to be healed grossly. The non-treated group received no treatment. Grossly, skin burns have been healed after 28 days of the treatment in all groups except of the non-treated group. The healing efficacy of the natural composite, MEBO® and silver-sulfadiazine creams was quite similar macroscopically. However, microscopically, the epidermal layer of the composite-cream treated group was more mature than those of both MEBO® and silver-sulfadiazine creams treated groups. In conclusion, the tested composite may be a promising effective and inexpensive treatment of skin burns.
1. Introduction
Burns are regarded as one of the most serious injuries. Physical impairments or even disabilities, as well as emotional and mental diseases, can result from burns. In developing countries, burns are one of the public health issues [1,2]. The World Health Organization (WHO) reported that each year approximately 11 million people suffer from burn wounds worldwide. About 180,000 of whom die because of such injuries and the non-fatal burn injuries are a leading cause of morbidity [3]. The majority of these cases occur in low- and middle-income countries in African and South-East Asia regions [3]. Additionally, skin burns are one of the complicated affections [4] which recorded in different animals [5,6,7,8]. However, to our knowledge, there is no documented data to measure the number of skin affections in animals, neither locally nor globally.
Burn injury can be classified according to its severity, depth, and size. Superficial burns (first-degree) are the type of burns that affect the uppermost layer of the skin (epidermis only), and skin becomes red with limited pain. Second-degree burns are divided into two types, superficial partial-thickness burns and deep partial-thickness burns. The superficial partial-thickness burns are characterized by painful, weep, and require dressing and wound care. However, the deep partial-thickness burns are less painful due to partial destruction of the pain receptors and require surgery. The third-degree burns affect the full thickness of the skin and are not typically painful due to the damage to the nerve endings and requires protection from becoming infected. Finally, the fourth-degree burns involve the underlying tissue such as muscle or bone and frequently accompanied by sloughing of burned tissues [9].
Skin burns can be induced by thermal, chemical, radioactive and electrical causes. Heat is one of the most common causes of skin burns which can induce a sudden coagulative necrosis of cells and tissues producing physical and physiological damage to the affected tissues resulting in enormous complications if not treated quickly and efficiently. Skin healing and regeneration of the complicated cases of skin burns need long hospitalization, expensive medications, and long rehabilitation programs [10,11,12].
Indeed, skin burns are quite common in animals, for example, dermal skin burns in pet animals has been reported after prolonged sun exposure especially in high ambient temperature as plaques and eschars. The microscopic features of theses lesions (like epidermal, vascular, and adnexal necrosis, and subepidermal vesiculation) were consistent with full-thickness thermal skin burns. The brown dachshund dogs are sensitive to direct sunlight exposure, which induce cutaneous skin burn [5,13,14]. Additionally, thermal skin burns have been recorded in small animals, most of these burns are caused by intentional or accidental exposure to a heat source, such as boiling liquids, electric heating pads, flames, hot air dryers, hot metals, and hot lamps [6]. Recently, other causes which induce skin injuries such as transdermal drug delivery has become a growing trend in veterinary medicine. Some substances that help in drug diffusion through the epidermis and subcutaneous tissues are known as penetration enhancers, sorption promoters, or accelerants. However, their uses in veterinary products with a high-absorbed dose may result in adverse side dermatological effects such as toxicological irritations [15].
Antibiotics are widely used in the treatment strategies of skin burns to prevent secondary infection, but unfortunately in some cases antibiotics may induce allergic reactions which delay the healing process [16]. Tissue engineered wound dressings and growth factors are also used in wound healing process but they are relatively too expensive. Therefore, studies for the discovery of new natural compounds that can be used in the healing process of skin burns are still required [10]. Several medicinal plants contain a vast diversity of phytogenic compound with antioxidant, anti-inflammatory and immunomodulatory properties [17] which can be the cornerstone of the future medication of burns.
Several natural products such as honey, rosemary and chamomile oils have many health benefits particularly in skin regeneration process. These natural bioactive substances were previously investigated individually as enhancers of wound healing [18,19,20]. In our previous study, combination between these materials enhanced the wound healing in equines [21]. Honey is one of the oldest known traditional treatments and it was declared in the Holy Quran since 1444 years ago. It has been employed for healing of several skin diseases including ulcers, wounds, eczema, and burns [22]. Honey has anti-inflammatory and antibacterial effects which decreasing edema and exudation. Additionally, honey stimulates tissue regeneration which promotes healing and diminishes the scar size [18].
Rosemary (Rosmarinus officinalis Linn.) is an aromatic plant which has therapeutic properties and has been used in pharmaceutical and cosmetics industries as well as in the folk medicine. Rosemary has antioxidant and anti-inflammatory properties due to the presence of carnosol/carnosic and ursolic acids. Rosemary has been used in the treatment of inflammatory diseases, wound healing, cancer, skin mycoses, cellulite, alopecia, ultraviolet damage, and aging [19,23,24,25,26,27,28,29]. Chamomile, family Asteraceae, is one of the most ancient herbal plants used in the herbal medicinal [30]. It contains several bioactive constituents [31]. Chamomile possesses antioxidant and anti-inflammatory properties in addition to its role in skin protection and regeneration of the necrosed skin [30,31,32,33,34,35,36]. Other oils of plant origin such as sesame oil [22,37,38] and olive oil [38,39] have been subjected to several experimental studies to evaluate their effects on the healing process of skin burns. Sesame oil is considered one of the most important sources of β-sitosterol which is one of the most powerful natural anti-inflammatory [7,40] which used to prevent and treat tissue necrosis [40]. Additionally, olive oil contains several bioactive ingredients which enhance the regeneration of necrosed tissues [8,41,42,43,44,45,46,47]. Recently, a mixture of rosemary and chamomile oils with honey, induced efficient and rapid skin regeneration of equine-skin wounds experimentally and clinically [21]. In this study, we compared the healing efficacy of a natural composite containing rosemary oil, chamomile oil, sesame oil, olive oil and clover flower bee honey to other commercial products on thermally induced skin burns in rabbits.
2. Materials and Methods
2.1. Commercial Creames and Composite Preparation
Commercial creams used in this study were the MEBO®-cream (0.25% β-sitosterol, Julphar, Ras Al-Khaimah, United Arab Emirates) and DERMAZIN®-cream (1% silver sulfadiazine, SANDOZ, Cairo, Egypt). The composite (formulated in this study) consists of 80% (w/w) active ingredients and 20% (w/w) vaseline base. The active ingredients were a mixture of the following: sesame oil 30%, olive oil 10%, rosemary oil 10% and chamomile oil 10% (Alcaptin Pharm Co., Cairo, Egypt), and clover flower bee honey 20% (Agriculture Ministry, Giza, Egypt).
2.2. Composite Preparation
Firstly, honey was stirred on magnetic Stirrer (MGS-1C, Shaoxing, China) at 1500 rpm at 37 °C for 10 min. Then the oil mixtures were added drop by drop until complete mixing. Finally, vaseline (37 °C) was added slowly until complete mixing with the composite.
2.3. Experimental Design
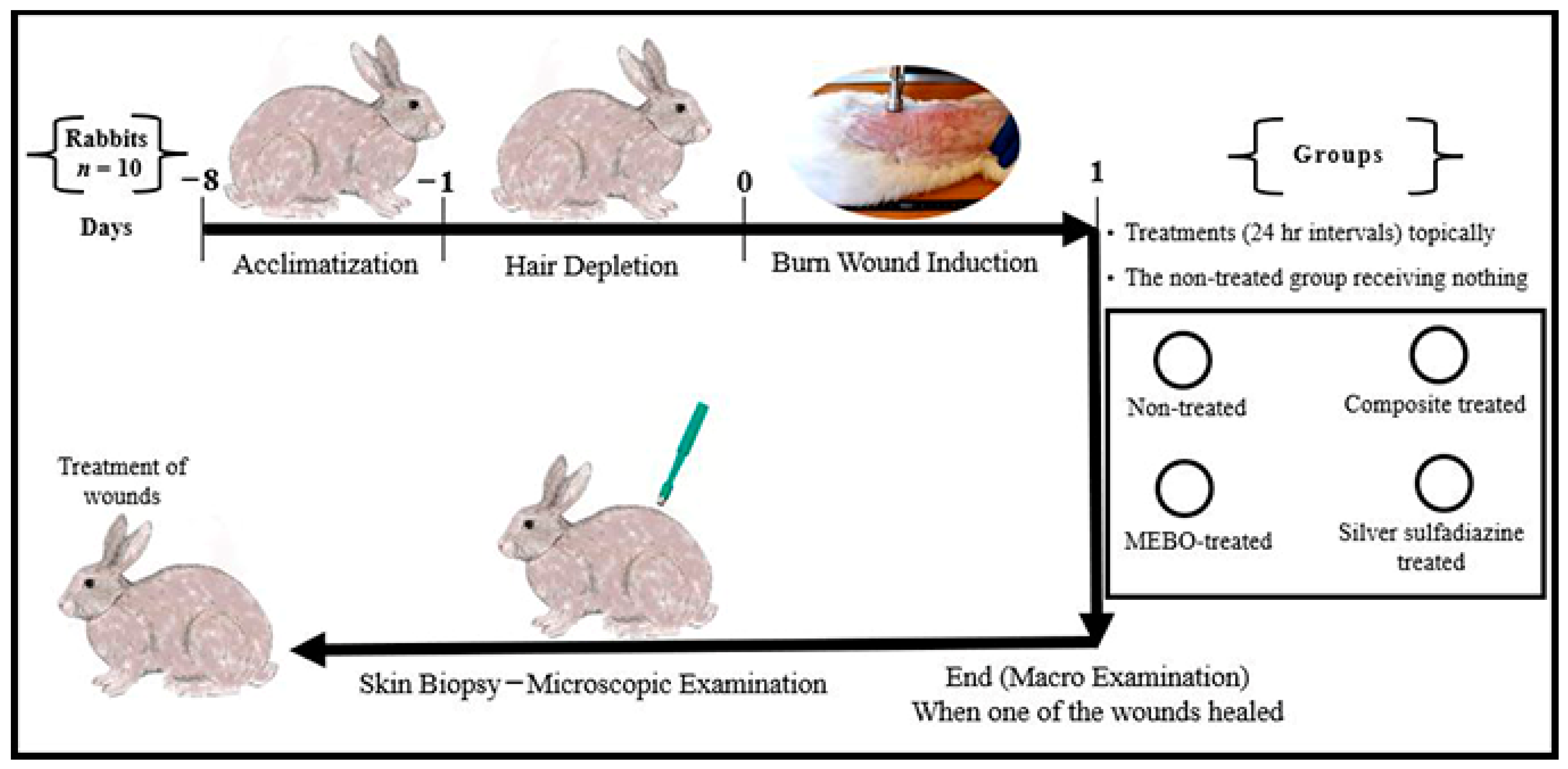
Animal-handling procedures as well as sample collection and disposal were done according to the regulations of Institutional Animal Care and Use Committee (IACUC), with approval number: VUSC-004-1-22. It was planned to kill animals humanely if they showed “a generalized dermatitis” or accompanied by complications that cannot be treated. Ten healthy adult male New Zealand Rabbit were used. The mean body weight was 3.6 kg (range from 3.2 to 4 kg). Animals were housed in rabbit cages (one animal per cage) and fed with commercial rabbit diet (Super Rabbit, Egyptian European Company, Cairo, Egypt) while water was dispensed ad libitum along the period of the study. Animal acclimatization was continued for one week, then hair depletion was done using Veet™ (Veet, Cairo, Egypt) cream topically for three minutes. The experimental design is illustrated in Figure 1.
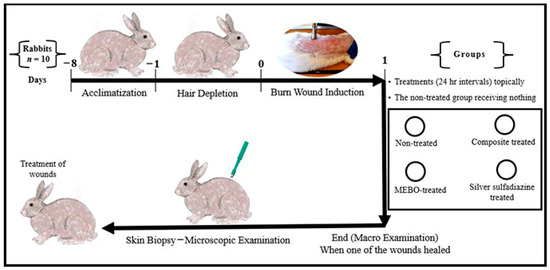
Figure 1.
Infograph representing the experimental design. Acclimatization was continued for one week. Hair depletion was done using Veet™ cream topically for three minutes. Burn wound induction was done under the effect of general anaesthesia, four burn-wounds were created by aluminum stamp (80 °C/14 s) on the back of the animal using electronic thermal aluminum stamp represents one of the following treatment groups; non-treated group, natural composite treated group, MEBO® cream treated group, and silver sulfadiazine® cream treated group, respectively. Skin biopsies were collected from the central part of the wounds using disposable skin biopsy punches 10 mm under the effect of general anaesthesia.
In the next day, anesthesia was induced in rabbits using xylazine and ketamine as method described by Oguntoye et al. [48]. Briefly, rabbits were premedicated first with xylazine (Xylaject®: 2% sol. Adwia Company, Cairo, Egypt) at a dose rate of 5 mg/kg. Anesthesia was induced with ketamine (Ketalar®: 5% sol. Amoun Company, Egypt) at a dose rate 35 mg/kg. All drugs were injected into Longissimus dorsi muscle at different locations. When the animal become anesthetized, four burn-wounds were created by aluminum stamp (80 °C/14 s) on the back of the animal by using electronic thermal aluminum stamp, manufactured locally as described by Knabl et al. [49] with some modifications by addition of electronic thermal controller (Figure 2).

Figure 2.
Adjustable electrical aluminum stamp for induction of thermal burn-wounds.
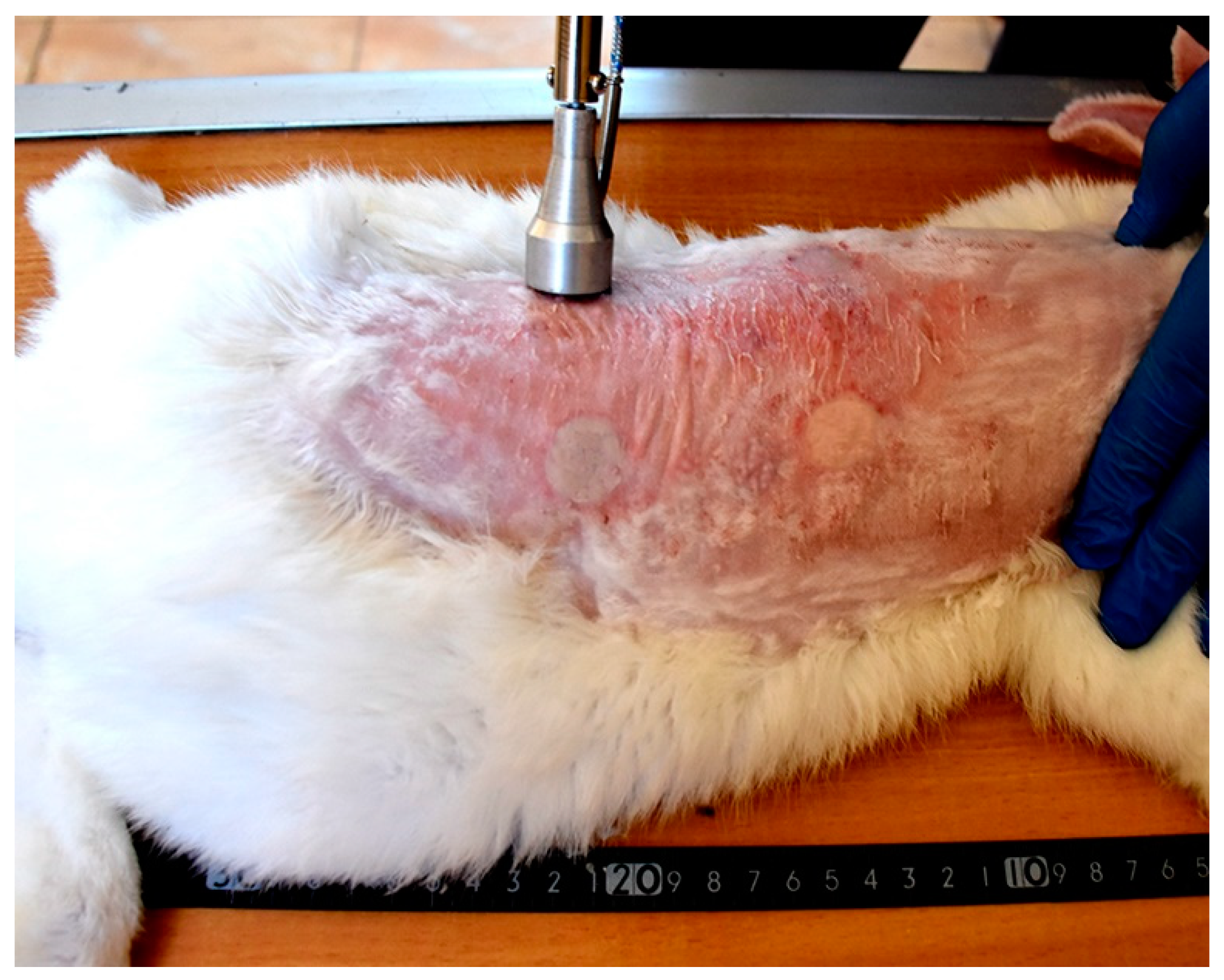
Each wound from the created four wounds represents one of the following treatment groups; non-treated group, natural composite treated, MEBO® cream treated, and silver sulfadiazine® cream treated groups, respectively. The site of wounds was anatomically fixed in all rabbits and the distance between the two wounds in the same side was 11 cm, while the distance between wounds from right to left sides was 7 cm (Figure 3).
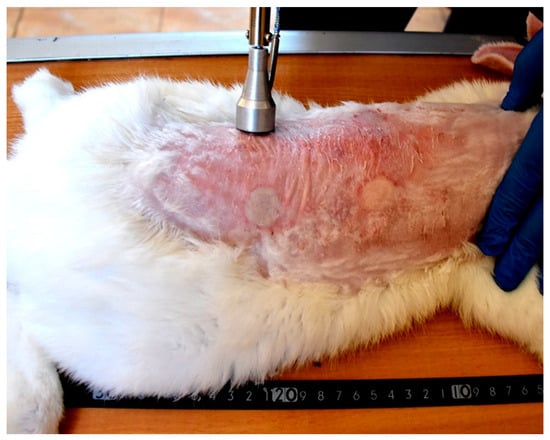
Figure 3.
Induction of skin burns in adult New Zealand rabbit. Skin burns were induced using aluminum stamp; the contact area was 4 cm2. The site of wounds was anatomically fixed in all rabbits and the distance between the two wounds in the same side was 11 cm, while the distance between wounds from right to left sides was 7 cm.
At the first day of burn induction, skin biopsies were taken from one rabbit at the center of the wound, by using disposable skin biopsy punches 10 mm (MentoK, Jaipur, India), under the effect of general anesthesia for histopathological evaluation of the wounds directly after burn induction. The other nine rabbits were used for the experiment. Treatments were applied once a day topically on the wound and the non-treated group received nothing. The experiment was terminated when one of the wounds appeared to be healed macroscopically. At the end of the experiment, skin biopsies were taken from the center of the wounds, by using disposable skin biopsy punches 10 mm (MentoK, India), under the effect of general anesthesia. After taking skin samples, all rabbits were subjected to intensive medical care to treat wounds created by the biopsy punches. Wounds were sutured and sprayed with betadine until complete healing and the animals were terminated by euthanization.
2.4. Histopathological Investigation
Samples were fixed with neutral buffered formalin 10% for 72 h then processed and sectioned at 3–5 um using Leica microtome. Tissues sections were stained with hematoxylin and eosin stain and the histological images were captured using Leica DMLB microscopes and Leica EC3 digital camera.
2.5. Morphometery
The morphometric evaluation was done according to methods described previously [50], with some modifications. Briefly, the images were taken with ×20 lens and saved in TIFF format. For each group, nine fields were taken (one field from each animal-skin sample). All fields were taken from the center of the wound. After the fields were saved, the Adobe photoshop program CS6 was used to improve them, using the lighting reference so that they all have the same quality. The full thickness of epidermis and the thickness of stratum corneum were calculated using ImageJ® (Bethesda, MD, USA) program and recorded in a Microsoft Excel spreadsheet.
2.6. Statistic Analysis
The data of epidermal measurements are presented as means ± standard error (S.E.) of the means. All morphometric data were subjected to One-way analysis of variance (ANOVA) followed by Duncan’s multiple range test for post hoc analysis using SPSS software, version 17 (IBM, Armonk, NY, USA). The significance level was set at p ≤ 0.05.
3. Results
3.1. Pathological Evaluation of Skin Burns at the First Day of Induction
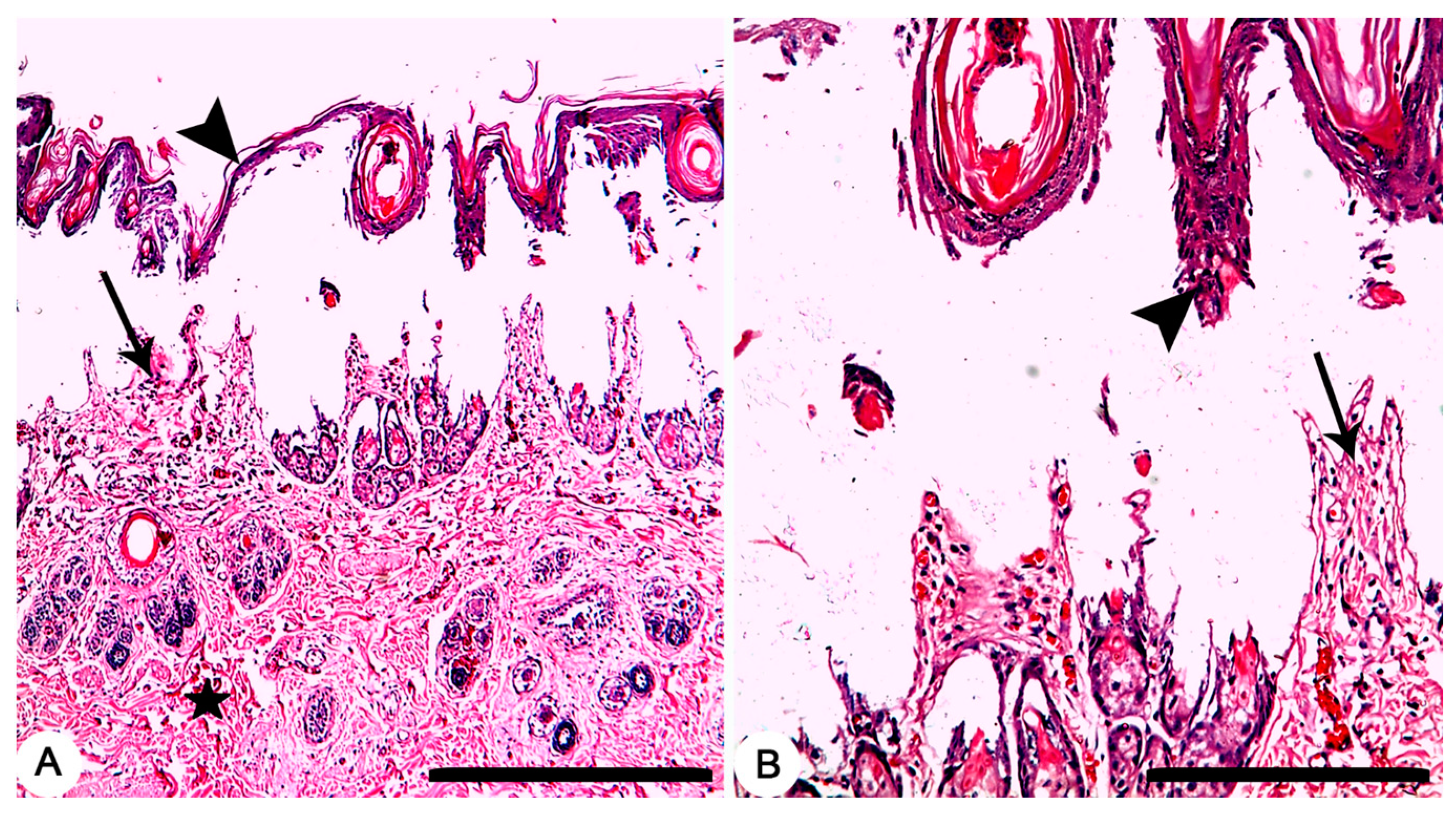
Grossly, circumscribed area of coagulative necrosis which was elevated above the skin surface and take a whitish color (Figure 3). Histopathologically, wounds were recorded as “second-degree burns” (superficial partial-thickness burns). Coagulative necrosis of both epidermal and dermal skin layers was found with a separation between epidermal and dermal layers, as well as denaturation of dermal collagens bundles (Figure 4A,B).
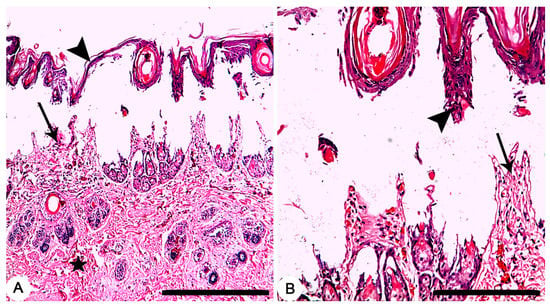
Figure 4.
Skin, Rabbit. (A,B) Thermally induced skin burns using aluminum stamp (80 °C/ 14 s), showing second-degree burns which characterized by necrosis of both epidermal (arrowheads) and dermal (arrows) skin layers with a separation between epidermal and dermal layers, as well as denaturation of dermal collagens bundles (star). HE stain; Bars (A) 500 µm; (B) 100 µm.
3.2. Pathological Evaluation of Skin Burns at the End of the Experiment
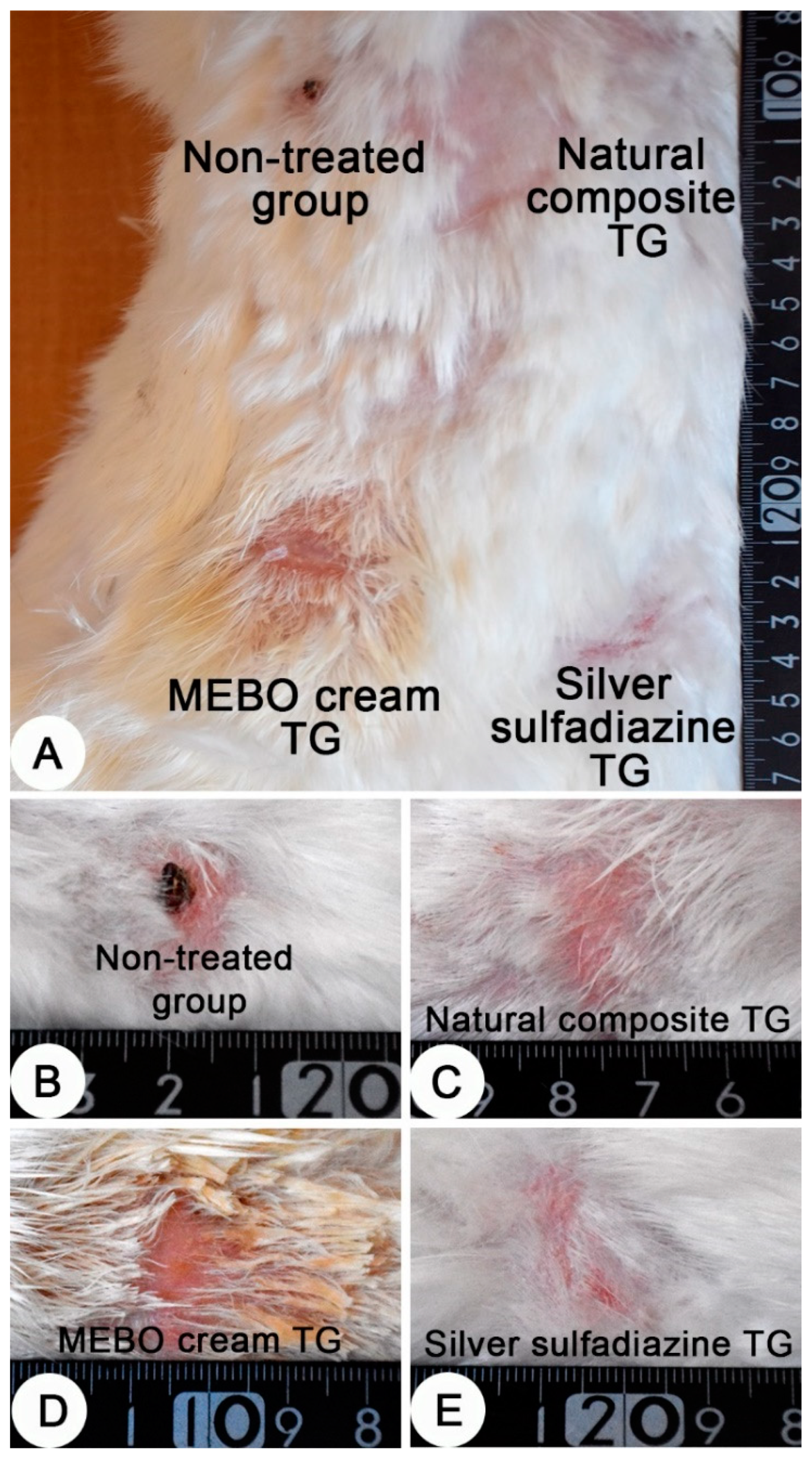
Grossly, after 28 days of the treatment, a dry crust on the burn sites of the non-treated group were observed and a complete healing of the burn sites of the other groups were recorded (Figure 5).
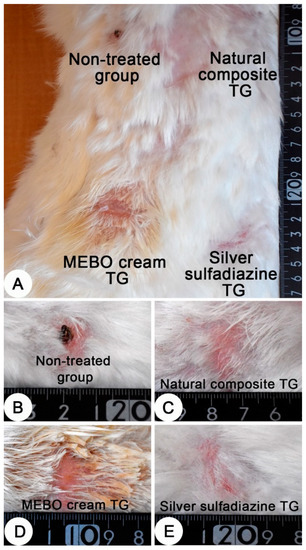
Figure 5.
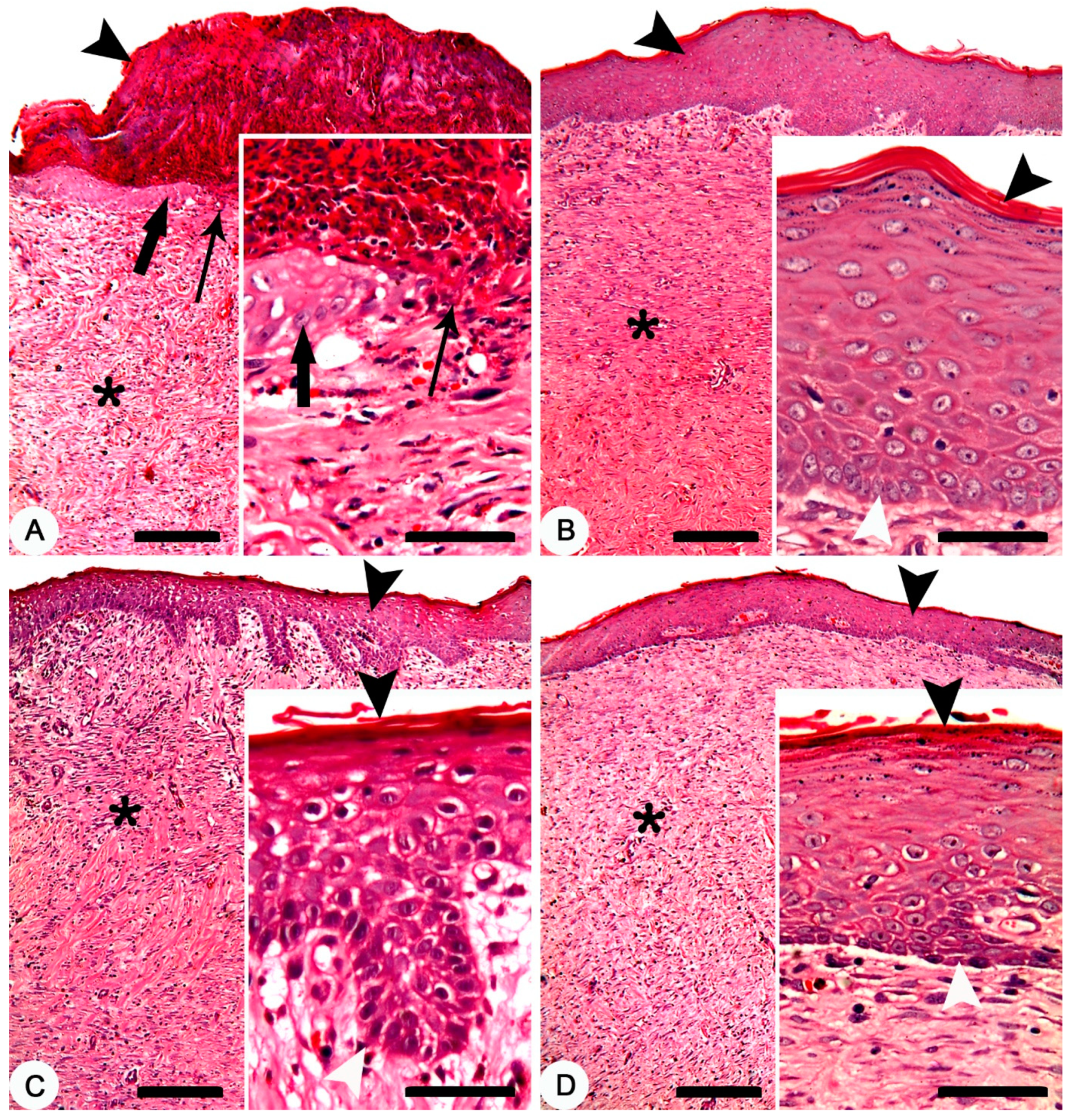
Skin, adult New Zealand rabbit. (A) Thermally induced skin burns after 28 days of treatment, showing the four burn sites in one animal. (B–E) a higher magnification of burn sites of figure A which representing the four treated groups. (B) dry crust on the burn site of the non-treated group. (C–E) complete healing of the burn sites of the treated groups. (C) composite cream treated group; (D) MEBO® cream treated group and (E) silver sulfadiazine cream treated group.
Histopathologically, after 28 days of the treatment, non-treated group exhibited crusts on the surface of the skin. The skin was denuded from the epidermal layer in some parts with advancing epidermal tongue growing over the granulation tissues in other parts (Figure 6A). In composite cream treated group, a mature and complete epidermal skin-layers which covering the granulation tissue was recorded (Figure 6B). In MEBO® cream treated group, a complete epidermal skin-layers, but with a thin stratum corneum, covering the granulation tissue was found (Figure 6C). Finally, a complete epidermal layer, but with a thin stratum corneum, covering a granulation tissue was observed in silver sulfadiazine cream treated group (Figure 6D).
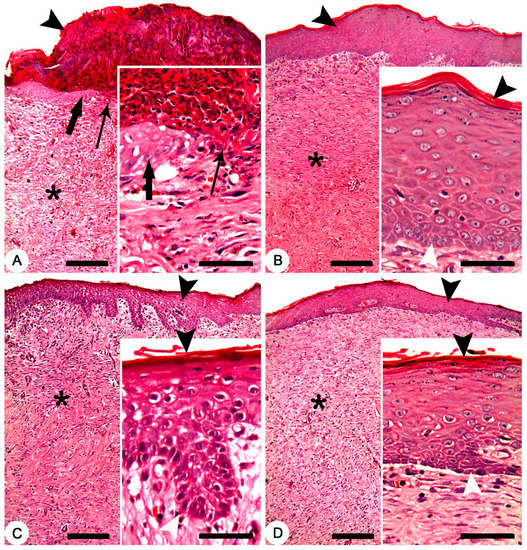
Figure 6.
Skin, New Zealand rabbit. Histopathology of thermally induced skin burns after 28 days of treatment. (A) non-treated group showing crusts on the surface of the skin (arrowhead), denuded skin surface from the epidermal layer (thin arrow) and a granulation tissue (asterisk). Inset showing crawling of the epidermal cells (thick arrow) over the granulation tissues of the denuded skin (thin arrow). (B) composite cream treated group showing complete epidermal layer (arrowhead) covering a granulation tissue (asterisk). Inset showing mature and complete epidermal layers between the stratum corneum (black arrowhead) and stratum germinativum (white arrowhead). (C) MEBO® cream treated group showing complete epidermal layer (arrowhead) covering a granulation tissue (asterisk). Inset showing complete epidermal layers between a thin stratum corneum (black arrowhead) and stratum germinativum (white arrowhead). (D) silver sulfadiazine cream treated group showing complete epidermal layer (arrowhead) covering a granulation tissue (asterisk). Inset showing complete epidermal layers between a thin stratum corneum (black arrowhead) and stratum germinativum (white arrowhead). HE stain; Bars: main figures 200 µm, Inset 50 µm.
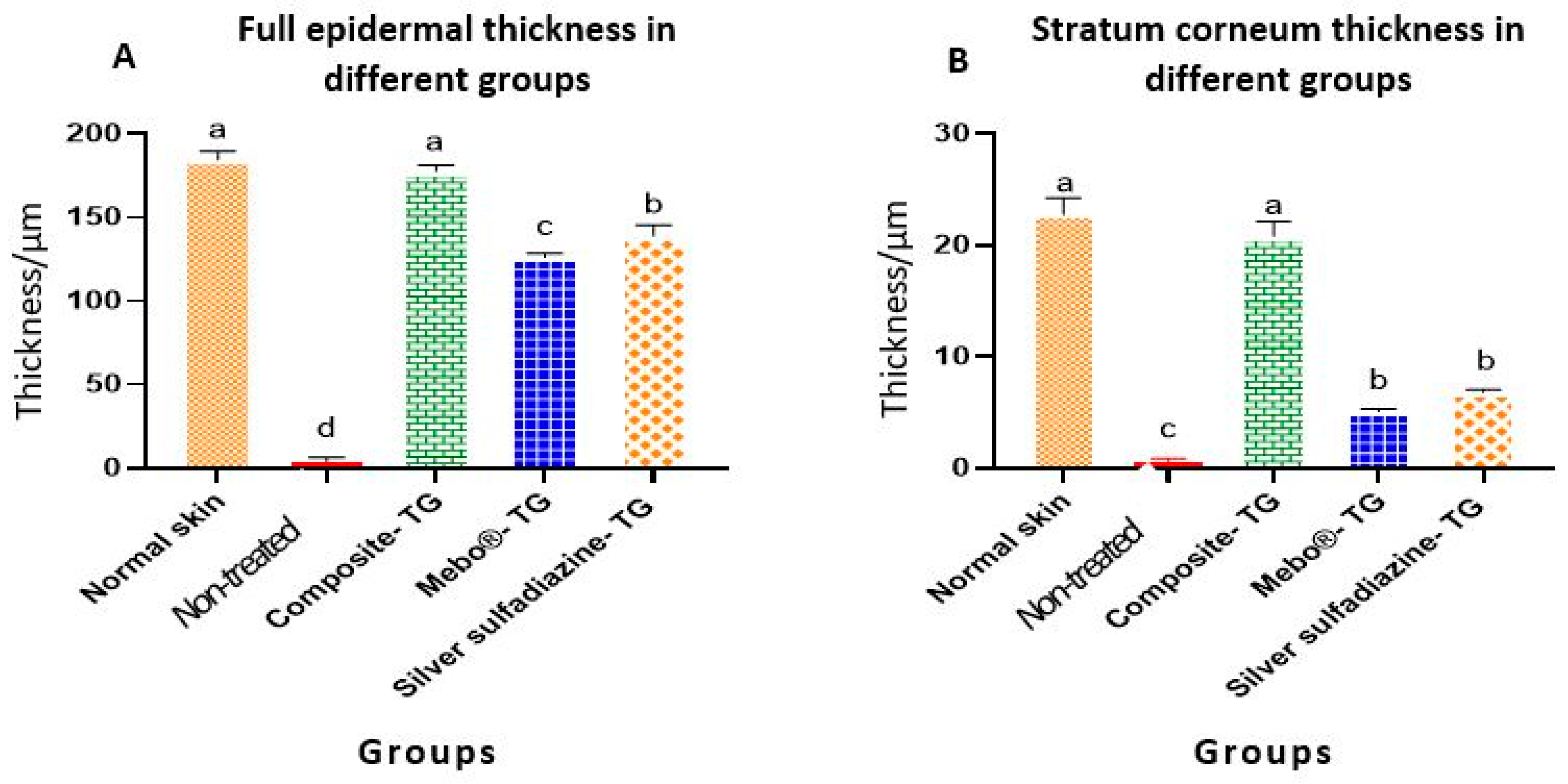
3.3. Morphometric Analysis
Table 1 and Figure 7 present the morphometric changes in the epidermal thickness of normal and different treated groups. According to the data shown, there was no significant differences in epidermal thickness between normal rabbit and composite- treated group. The epidermal thickness of composite-treated group was significantly higher than those of all other groups. Additionally, there was significant different between non-treated group, MEBO®-treated group and silver sulfadiazine-treated group (Figure 7A). Finally, the differences of the thickness of stratum corneum of both normal rabbit and composite- treated group was non-significant and both groups were highly significant than all other groups (Figure 7B).

Table 1.
Epidermal thickness of normal rabbit skin and different treated groups (TG) in induced skin burns. Values are presented as mean ± SE (n = 9). Different letters (a, b, c, and d) in the same column indicate significant differences at p ˂ 0.05.
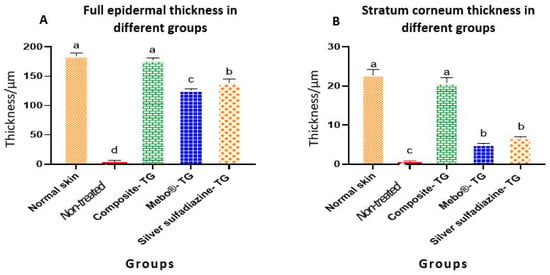
Figure 7.
Full epidermal and stratum corneum thickness of normal rabbit skin and different treated groups of experimentally induced skin burns. The measured histological cuts per treatment = 9. Bars with different letters (a, b, c, and d) are significantly different at p ˂ 0.05.
4. Discussion
Medication of skin burns are relatively too expensive, especially in poor countries; thus, a discovery of new compounds for skin protection and regeneration are still required [10]. Therefore, we tried to make our efforts to contribute in the discovery of new efficient and cheap phytogenic compounds to accelerate wound healing. This experiment was based on the fact that clover-flower bee honey, rosemary and chamomile oils accelerated wound healing in equines [21]. In this study, we have added sesame oil and olive oil to the above-mentioned composite to make a new composite that can be useful in the treatment of skin burns. Sesame oil is rich in many biological active ingredients especially β-sitosterol [7,8,37,51], which is the active ingredient of a commercially available cream (MEBO®-cream). Additionally, olive oil has been used in various previous studies investigating its role in the wound healing process [8,43,52,53].
Herein, the effect of our composite (sesame oil 30%, olive oil 10%, rosemary oil 10% and chamomile oil 10%, clover flower bee honey 20% and Vaseline base 20%) on the skin regeneration process was compared with the most famous commercially available creams used in the treatment of skin burns in Egypt, MEBO® and DERMAZIN®. MEBO® is composed mainly of 0.25% β-sitosterol in a base of sesame oil and beeswax [53], while DERMAZIN® is composed of 1% silver sulfadiazine, which was used previously in many studies on the matter of burn healing process [54,55,56,57].
Interestingly, thermally induced skin burns have been healed after 28 days of the treatment except the of negative control group (Figure 5). In all treated groups (except of the non-treated group), the healing process was grossly similar, but histologically, we can distinguish between the different treated groups according to the epidermal structures. In previous studies, MEBO® cream resulted in significant enhancement of wound healing in both healthy and immunocompromised dogs when compared to honey alone and the wound healing effect of MEBO® cream was superior to that of honey [7]. Additionally, silver sulfadiazine was subjected to many studies to evaluate its healing efficacy. It was revealed that silver sulfadiazine accelerated the healing process of burns [56,58]. On the other hand, some plant extracts like Alpinia officinarum (galangal) are significantly superior to silver sulfadiazine in the treatment of wound burns [55]. In this study, the healing efficacy of MEBO® cream, silver sulfadiazine cream and our composite were quite similar macroscopically (Figure 5). However, microscopically, the epidermal layer of composite cream treated groups was more mature than those of MEBO® cream and silver sulfadiazine treated groups (Figure 6). In addition, the morphometric analysis (Table 1 and Figure 7) showed that, epidermal thickness of the composite treated group was significantly higher than those of other treated groups. The thickness of stratum corneum of the composite treated wounds in addition to the arrangement and thickness of epidermal layers were quite similar to normal skin structures.
Although the bioactive substances of the natural composite were not analyzed (limitation of this study), the healing efficacy of the composite may be attributed to the nutrients and antibacterial potency of honey [59,60,61], growth promoters and nutrients of both rosemary and chamomile oils [62,63,64], anti-inflammatory and antioxidant properties of sesame oil [7] and vitamins and antioxidants in olive oil. The tested composite characterized by smooth and fast regeneration of the epidermal layers resulting in healthy and histologically uniformed epidermal layers which may be attributed to the several bioactive compounds which present in each component of the tested composite.
5. Conclusions
The non-treated group showed a slowed wound healing as compared to the other groups. However, the natural composite promotes the wound healing effectively, highlighting the potential use of natural compounds as effective and safe treatment of skin burns.
Author Contributions
Conceptualization, A.A., A.S. and S.E.H.; methodology, A.A., A.S. and S.E.H.; software, A.A.; validation, A.A., A.S., S.E.H. and A.A.S.; formal analysis, A.A.; resources, A.A.; data curation, A.A., A.S. and S.E.H.; writing—original draft preparation, A.A.; writing—review and editing, A.A., A.S., S.E.H. and A.A.S.; project administration, A.A.; funding acquisition, A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by University of Sadat City, Egypt, grant number 10.
Institutional Review Board Statement
The study was conducted in accordance with the regulations of Institutional Animal Care and Use Committee (IACUC), Faculty of Veterinary Medicine, University of Sadat city, with approval number: VUSC-004-1-22.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We acknowledge all the former and present leaders of University of Sadat City and the former and present leaders of Faculty of Veterinary Medicine, University of Sadat City for facilitating all the University and College capabilities to complete this research. We also want to thank Nermeen B. El-Borai, Department of Forensic Medicine and Toxicology, Faculty of Veterinary Medicine, University of Sadat City, for here help in statistical analysis. Additionally, we would like to thank Asmaa Lotfy and veterinarian Naeima Othman, Department of pathology, Faculty of Veterinary Medicine, University of Sadat City for their technical assistant.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Forjuoh, S.N. Burns in low- and middle-income countries: A review of available literature on descriptive epidemiology, risk factors, treatment, and prevention. Burns 2006, 32, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Peck, M.D.; Kruger, G.E.; van der Merwe, A.E.; Godakumbura, W.; Ahuja, R.B. Burns and fires from non-electric domestic appliances in low and middle income countries: Part I. The scope of the problem. Burns 2008, 34, 303–311. [Google Scholar] [CrossRef]
- World Health Organization. Burns. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/burns (accessed on 15 May 2022).
- Mssillou, I.; Agour, A.; Slighoua, M.; Chebaibi, M.; Amrati, F.E.; Alshawwa, S.Z.; Kamaly, O.A.; El Moussaoui, A.; Lyoussi, B.; Derwich, E. Ointment-based vombination of Dittrichia viscosa L. and Marrubium vulgare L. accelerate burn wound healing. Pharmaceuticals 2022, 15, 289. [Google Scholar] [CrossRef] [PubMed]
- Pavletic, M.M.; Trout, N.J. Bullet, bite, and burn wounds in dogs and cats. Vet. Clin. Small Anim. Pract. 2006, 36, 873–893. [Google Scholar] [CrossRef]
- Schwartz, S.L.; Schick, A.E.; Lewis, T.P.; Loeffler, D. Dorsal thermal necrosis in dogs: Aretrospective analysis of 16 cases in the southwestern USA (2009–2016). Vet. Dermatol. 2018, 29, 139-e155. [Google Scholar] [CrossRef]
- Alshehabat, M.; Hananeh, W.; Ismail, Z.B.; Rmilah, S.A.; Abeeleh, M.A. Wound healing in immunocompromised dogs: A comparison between the healing effects of moist exposed burn ointment and honey. Vet. World 2020, 13, 2793–2797. [Google Scholar] [CrossRef]
- Przadka, P.; Kuberka, M.; Skrzypczak, P.; Kielbowicz, Z. Healing of a large skin defect in a dog with concurrent ozonated olive oil application. J. Small Anim. Pract. 2022, 63, 42. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, M.G.; van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn injury. Nat. Rev. Dis. Primers 2020, 6, 11. [Google Scholar] [CrossRef]
- Heidari, M.; Bahramsoltani, R.; Abdolghaffari, A.H.; Rahimi, R.; Esfandyari, M.; Baeeri, M.; Hassanzadeh, G.; Abdollahi, M.; Farzaei, M.H. Efficacy of topical application of standardized extract of Tragopogon graminifolius in the healing process of experimental burn wounds. J. Tradit. Complement. Med. 2019, 9, 54–59. [Google Scholar] [CrossRef]
- Van Yperen, D.T.; van Lieshout, E.M.M.; Nugteren, L.H.T.; Plaisier, A.C.; Verhofstad, M.H.J.; van der Vlies, C.H. Adherence to the emergency management of severe burns referral criteria in burn patients admitted to a hospital with or without a specialized burn center. Burns 2021, 47, 1810–1817. [Google Scholar] [CrossRef]
- Arturson, G. Pathophysiology of the burn wound and pharmacological treatment. The Rudi Hermans Lecture, 1995. Burns 1996, 22, 255–274. [Google Scholar] [CrossRef]
- Hargis, A.; Lewis, T. Full-thickness cutaneous burn in black-haired skin on the dorsum of the body of a Dalmation puppy. Vet. Dermatol. 1999, 10, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Sumner, J.P.; Pucheu-Haston, C.M.; Fowlkes, N.; Merchant, S. Dorsal skin necrosis secondary to a solar-induced thermal burn in a brown-coated dachshund. Can. Vet. J. 2016, 57, 305–308. [Google Scholar] [PubMed]
- Magnusson, B.M.; Walters, K.A.; Roberts, M.S. Veterinary drug delivery: Potential for skin penetration enhancement. Adv. Drug Deliv. Rev. 2001, 50, 205–227. [Google Scholar] [CrossRef]
- Thomas, G.W.; Rael, L.T.; Bar-Or, R.; Shimonkevitz, R.; Mains, C.W.; Slone, D.S.; Craun, M.L.; Bar-Or, D. Mechanisms of delayed wound healing by commonly used antiseptics. J. Trauma Acute Care Surg. 2009, 66, 82–91. [Google Scholar] [CrossRef]
- Gupta, A.; Upadhyay, N.K.; Sawhney, R.; Kumar, R. A poly-herbal formulation accelerates normal and impaired diabetic wound healing. Wound Repair Regen. 2008, 16, 784–790. [Google Scholar] [CrossRef]
- Bulut, S.P.; Gurbuzel, M.; Karabela, S.N.; Pence, H.H.; Aksaray, S.; Topal, U. The investigation of biochemical and microbiological properties of four different honey types produced in turkey and the comparison of their effects with silver sulfadiazine on wound healing in a rat model of burn injury. Niger. J. Clin. Pract. 2021, 24, 1694–1705. [Google Scholar] [CrossRef]
- De Macedo, L.M.; Santos, E.M.D.; Militao, L.; Tundisi, L.L.; Ataide, J.A.; Souto, E.B.; Mazzola, P.G. Rosemary (Rosmarinus officinalis L., syn Salvia rosmarinus Spenn.) and its topical applications: A review. Plants 2020, 9, 651. [Google Scholar] [CrossRef]
- Kazemian, H.; Ghafourian, S.; Sadeghifard, N.; Houshmandfar, R.; Badakhsh, B.; Taji, A.; Shavalipour, A.; Mohebi, R.; Ebrahim-Saraie, H.S.; Houri, H.; et al. In vivo antibacterial and wound healing activities of Roman Chamomile (Chamaemelum nobile). Infect. Disord. Drug Targets 2018, 18, 41–45. [Google Scholar] [CrossRef]
- Anis, A.; Sharshar, A.; Hanbally, S.E.; Sadek, Y. A novel organic composite accelerates wound healing: Experimental and clinical study in equine. J. Equine Vet. Sci. 2021, 99, 103406. [Google Scholar] [CrossRef]
- Vaghardoost, R.; Mousavi Majd, S.G.; Tebyanian, H.; Babavalian, H.; Malaei, L.; Niazi, M.; Javdani, A. The healing fffect of sesame oil, camphor and honey on second degree burn wounds in Rat. World J. Plast. Surg. 2018, 7, 67–71. [Google Scholar] [PubMed]
- Auh, J.H.; Madhavan, J. Protective effect of a mixture of marigold and rosemary extracts on UV-induced photoaging in mice. Biomed. Pharm. 2021, 135, 111178. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Hillebrand, G.G.; Nunez, G. Rosmarinus officinalis L. (Rosemary) extracts containing carnosic acid and carnosol are potent quorum sensing inhibitors of Staphylococcus aureus virulence. Antibiotics 2020, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Marzo, N.; Perez-Sanchez, A.; Barrajon-Catalan, E.; Castillo, J.; Herranz-Lopez, M.; Micol, V. Rosemary diterpenes and flavanone aglycones provide improved genoprotection against UV-dnduced DNA damage in a human skin cell model. Antioxidants 2020, 9, 255. [Google Scholar] [CrossRef] [PubMed]
- Pazyar, N.; Yaghoobi, R.; Rafiee, E.; Mehrabian, A.; Feily, A. Skin wound healing and phytomedicine: A review. Ski. Pharmacol. Physiol. 2014, 27, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Ariffin, N.H.M.; Hasham, R. Potential dermatological application on Asian plants. Biotechnol. Bioprocess Eng. 2016, 21, 337–354. [Google Scholar] [CrossRef]
- Ojeda-Sana, A.M.; van Baren, C.M.; Elechosa, M.A.; Juárez, M.A.; Moreno, S. New insights into antibacterial and antioxidant activities of rosemary essential oils and their main components. Food Control 2013, 31, 189–195. [Google Scholar] [CrossRef]
- Fernández-Ochoa, Á.; Borras-Linares, I.; Pérez-Sánchez, A.; Barrajón-Catalán, E.; Gonzalez-Alvarez, I.; Arráez-Román, D.; Micol, V.; Segura-Carretero, A. Phenolic compounds in rosemary as potential source of bioactive compounds against colorectal cancer: In situ absorption and metabolism study. J. Funct. Foods 2017, 33, 202–210. [Google Scholar] [CrossRef]
- O’G’Li, F.J.S. Chamomile: A herbal medicine of the past with bright future. Eur. Int. J. Multidiscip. Res. Manag. Stud. 2022, 2, 251–254. [Google Scholar]
- Marderosian, A.D.; Liberti, L.E.; Krikorian, A.D. Natural product medicine: A scientific guide to foods, drugs, cosmetics. Q. Rev. Biol. 1989, 64, 106–107. Available online: https://www.journals.uchicago.edu/doi/abs/10.1086/416216 (accessed on 31 May 2022).
- Thornfeldt, C. Cosmeceuticals containing herbs: Fact, fiction, and future. Dermatol. Surg. 2005, 31, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Garbuio, D.C.; Ribeiro, V.D.S.; Hamamura, A.C.; Faustino, A.; Freitas, L.A.P.; Viani, G.; Carvalho, E.C. A Chitosan-coated chamomile microparticles formulation to prevent radiodermatitis in breast: A double-blinded, controlled, randomized, Phase II clinical trial. Am. J. Clin. Oncol. 2022, 45, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, E.B.; Ciol, M.A.; de Meneses, A.G.; Bontempo, P.S.M.; Hoffman, J.M.; Reis, P. Chamomile gel versus urea cream to prevent acute radiation dermatitis in head and neck cancer patients: Results from a preliminary clinical trial. Integr. Cancer 2020, 19, 1534735420962174. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Ishitsuka, Y.; Nakamura, Y.; Okiyama, N.; Watanabe, R.; Fujisawa, Y.; Fujimoto, M. Honey and chamomile activate keratinocyte antioxidative responses via the KEAP1/NRF2 system. Clin. Cosmet. Investig. Derm. 2020, 13, 657–660. [Google Scholar] [CrossRef]
- El-Salamouni, N.S.; Ali, M.M.; Abdelhady, S.A.; Kandil, L.S.; Elbatouti, G.A.; Farid, R.M. Evaluation of chamomile oil and nanoemulgels as a promising treatment option for atopic dermatitis induced in rats. Expert Opin. Drug Deliv. 2020, 17, 111–122. [Google Scholar] [CrossRef]
- Ebrahimpour, N.; Mehrabani, M.; Iranpour, M.; Kordestani, Z.; Mehrabani, M.; Nematollahi, M.H.; Asadipour, A.; Raeiszadeh, M.; Mehrbani, M. The efficacy of a traditional medicine preparation on second-degree burn wounds in rats. J. Ethnopharmacol. 2020, 252, 112570. [Google Scholar] [CrossRef]
- Saporito, F.; Sandri, G.; Bonferoni, M.C.; Rossi, S.; Boselli, C.; Icaro Cornaglia, A.; Mannucci, B.; Grisoli, P.; Vigani, B.; Ferrari, F. Essential oil-loaded lipid nanoparticles for wound healing. Int. J. Nanomed. 2018, 13, 175–186. [Google Scholar] [CrossRef]
- Bayir, Y.; Un, H.; Ugan, R.A.; Akpinar, E.; Cadirci, E.; Calik, I.; Halici, Z. The effects of beeswax, olive oil and butter impregnated bandage on burn wound healing. Burns 2019, 45, 1410–1417. [Google Scholar] [CrossRef]
- Gupta, E. β-Sitosterol: Predominant phytosterol of therapeutic potential. In Innovations in Food Technology; Springer: Singapore, 2020; pp. 465–477. [Google Scholar]
- Solt Kirca, A.; Kanza Gul, D. Effects of olive oil on Striae Gravidarum in primiparous women: A randomized controlled clinical study. Altern. Health Med. 2022, 28, 34–39. [Google Scholar]
- Shalaby, K. Effect of olive oil acidity on skin delivery of diclofenac: In vitro evaluation and ex vivo skin permeability studies. J. Biomed. Nanotechnol. 2022, 18, 234–242. [Google Scholar] [CrossRef]
- Massoud, D.; Fouda, M.M.A.; Sarhan, M.; Salama, S.G.; Khalifa, H.S. Topical application of aloe gel and/or olive oil combination promotes the wound healing properties of streptozotocin-induced diabetic rats. Environ. Sci. Pollut. Res. Int. 2022, 29, 59727–59735. [Google Scholar] [CrossRef] [PubMed]
- Gul, U.; Khan, M.I.; Madni, A.; Sohail, M.F.; Rehman, M.; Rasul, A.; Peltonen, L. Olive oil and clove oil-based nanoemulsion for topical delivery of terbinafine hydrochloride: In vitro and ex vivo evaluation. Drug Deliv. 2022, 29, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Donato-Trancoso, A.; Correa Atella, G.; Romana-Souza, B. Dietary olive oil intake aggravates psoriatic skin inflammation in mice via Nrf2 activation and polyunsaturated fatty acid imbalance. Int. Immunopharmacol. 2022, 108, 108851. [Google Scholar] [CrossRef] [PubMed]
- Baptista, S.; Pereira, J.R.; Gil, C.V.; Torres, C.A.V.; Reis, M.A.M.; Freitas, F. Development of olive oil and alpha-tocopherol containing emulsions stabilized by FucoPol: Rheological and textural analyses. Polymers 2022, 14, 2349. [Google Scholar] [CrossRef]
- Alnemer, F.; Aljohani, R.; Alajlan, A.; Aljohani, M.; Alozaib, I.; Masuadi, E.; Omair, A.; Al Jasser, M.I. The use of olive oil for skin health in a Saudi population: A cross-sectional study. Derm. Rep. 2022, 14, 9364. [Google Scholar] [CrossRef]
- Oguntoye, C.; Oke, B. A comparison of xylazine/ketamine, diazepam/ketamine and acepromazine/ketamine anaesthesia in rabbit. Sokoto J. Vet. Sci. 2014, 12, 21–25. [Google Scholar] [CrossRef]
- Knabl, J.S.; Bayer, G.S.; Bauer, W.A.; Schwendenwein, I.; Dado, P.F.; Kucher, C.; Horvat, R.; Turkof, E.; Schossmann, B.; Meissl, G. Controlled partial skin thickness burns: An animal model for studies of burnwound progression. Burns 1999, 25, 229–235. [Google Scholar] [CrossRef]
- Monaretti, G.L.; Costa, M.C.F.; Rocha, L.B.; Cintra, M.M.M.; da Cunha, M.T.R.; Pinheiro, N.M.; Noites, A.; Mendonca, A.C. Effect of capacitive radiofrequency on the dermis of the abdominal region. Lasers Med. Sci. 2022, 37, 619–625. [Google Scholar] [CrossRef]
- Hammam, W.E.; Gad, A.M.; Gad, M.K.; Kirollos, F.N.; Yassin, N.A.; Tantawi, M.E.E.; El Hawary, S.S.E. Pyrus communis L. (Pear) and Malus domestica Borkh. (apple) leaves lipoidal extracts as sources for beta-sitosterol rich formulae and their wound healing evaluation. Nat. Prod. Res. online ahead of print. 2022. [Google Scholar] [CrossRef]
- Moni, S.S.; Tripathi, P.; Sultan, M.H.; Alshahrani, S.; Alqahtani, S.S.; Madkhali, O.A.; Bakkari, M.A.; Pancholi, S.S.; Elmobark, M.E.; Jabeen, A.; et al. Wound-healing and cytokine-modulating potential of medicinal oil formulation comprising leaf extract of Murraya koenigii and olive oil. Braz. J. Biol. 2022, 82, e256158. [Google Scholar] [CrossRef]
- Taheri, M.; Amiri-Farahani, L.; Haghani, S.; Shokrpour, M.; Shojaii, A. The effect of olive cream on pain and healing of caesarean section wounds: A randomised controlled clinical trial. J. Wound Care 2022, 31, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Devi, M.V.; Poornima, V.; Sivagnanam, U.T. Wound healing in second-degree burns in rats treated with silver sulfadiazine: A systematic review and meta-analysis. J. Wound Care 2022, 31, S31–S45. [Google Scholar] [CrossRef] [PubMed]
- Kadam, K.; Kiyan, S.; Uyanikgil, Y.; Karabey, F.; Cetin, E.O. Investigation of acute effects of topical Alpinia officinarum (galangal) treatment in experimental contact type burns and comparison with topical silver sulfadiazine treatment. Ulus Travma Acil Cerrahi Derg. 2022, 28, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Roopmani, P.; Chauhan, M.; Basu, S.M.; Deeksha, W.; Kazem, M.D.; Hazra, S.; Rajakumara, E.; Giri, J. Silver sulfadiazine loaded core-shell airbrushed nanofibers for burn wound healing application. Int. J. Pharm. 2022, 613, 121358. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, B.; Zhang, X.H.; Liu, S.H.; Wu, W. The effectiveness of silver-containing hydrofiber dressing compared with topical silver sulfadiazine cream in pediatric patients with deep partial-thickness burns: A retrospective review. Wound Manag. Prev. 2022, 68, 29–36. [Google Scholar] [CrossRef]
- Al-Arjan, W.S.; Khan, M.U.A.; Almutairi, H.H.; Alharbi, S.M.; Razak, S.I.A. pH-Responsive PVA/BC-f-GO Dressing materials for burn and chronic wound healing with curcumin release kinetics. Polymers 2022, 14, 949. [Google Scholar] [CrossRef]
- Albaridi, N.A. Antibacterial potency of honey. Int. J. Microbiol. 2019, 2019, 2464507. [Google Scholar] [CrossRef]
- Algethami, J.S.; El-Wahed, A.A.A.; Elashal, M.H.; Ahmed, H.R.; Elshafiey, E.H.; Omar, E.M.; Naggar, Y.A.; Algethami, A.F.; Shou, Q.; Alsharif, S.M.; et al. Bee pollen: Clinical trials and patent applications. Nutrients 2022, 14, 2858. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; El-Wahed, A.A.A.; Zhao, C.; Saeed, A.; Zou, X.; Guo, Z.; Hegazi, A.G.; Shehata, A.A.; El-Seedi, H.H.R.; Algethami, A.F.; et al. A Spotlight on the Egyptian honeybee (Apis mellifera lamarckii). Animals 2022, 12, 2749. [Google Scholar] [CrossRef]
- Ghazalah, A.; Ali, A. Rosemary leaves as a dietary supplement for growth in broiler chickens. Int. J. Poult. Sci. 2008, 7, 234–239. [Google Scholar] [CrossRef]
- Das, S.; Horváth, B.; Šafranko, S.; Jokić, S.; Széchenyi, A.; Kőszegi, T. Antimicrobial activity of chamomile essential oil: Effect of different formulations. Molecules 2019, 24, 4321. [Google Scholar] [CrossRef] [PubMed]
- Shehata, A.A.; Yalçın, S.; Latorre, J.D.; Basiouni, S.; Attia, Y.A.; Abd El-Wahab, A.; Visscher, C.; El-Seedi, H.R.; Huber, C.; Hafez, H.M.; et al. Probiotics, prebiotics, and phytogenic substances for optimizing gut health in poultry. Microorganisms 2022, 10, 395. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).