High Triglyceride-Glucose Index with Renal Hyperfiltration and Albuminuria in Young Adults: The Korea National Health and Nutrition Examination Survey (KNHANES V, VI, and VIII)
Abstract
:1. Introduction
2. Methods
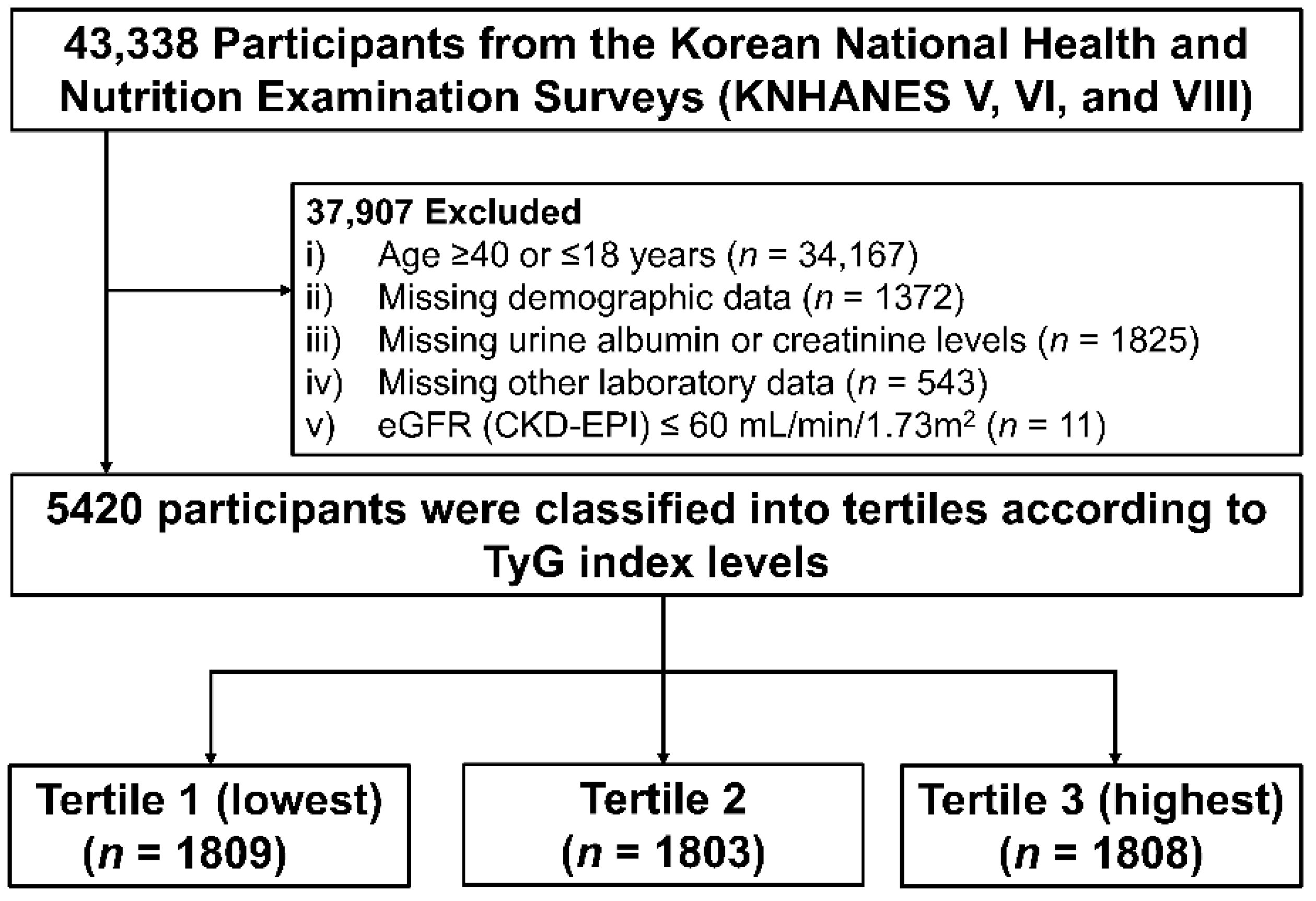
2.1. Study Population
2.2. Data Collection
2.3. Definitions of Exposure and Outcome
2.4. Statistical Analyses
3. Results
3.1. Baseline Characteristics
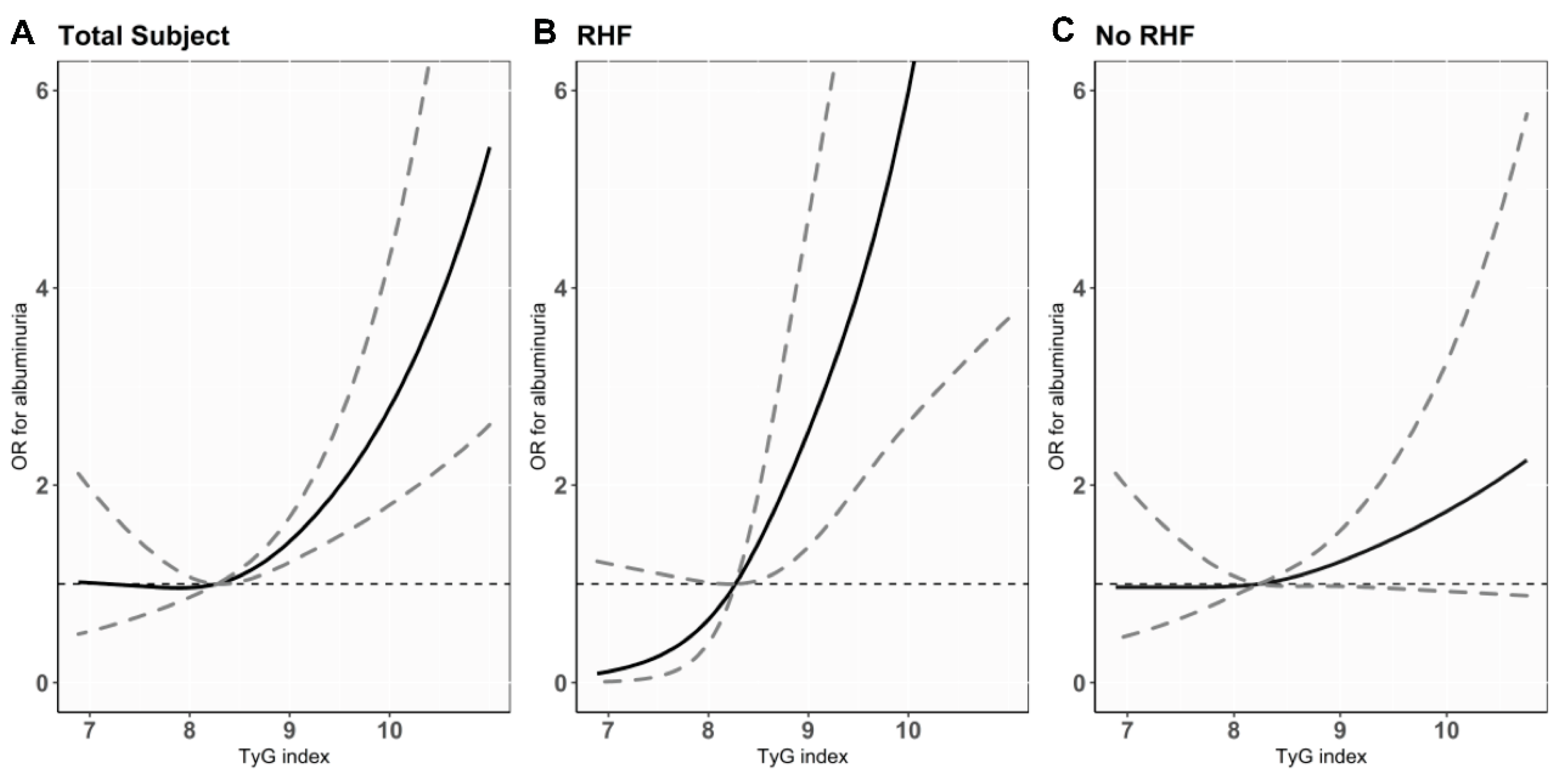
3.2. Association of TyG Index with Albuminuria
3.3. Association of TyG Index with RHF
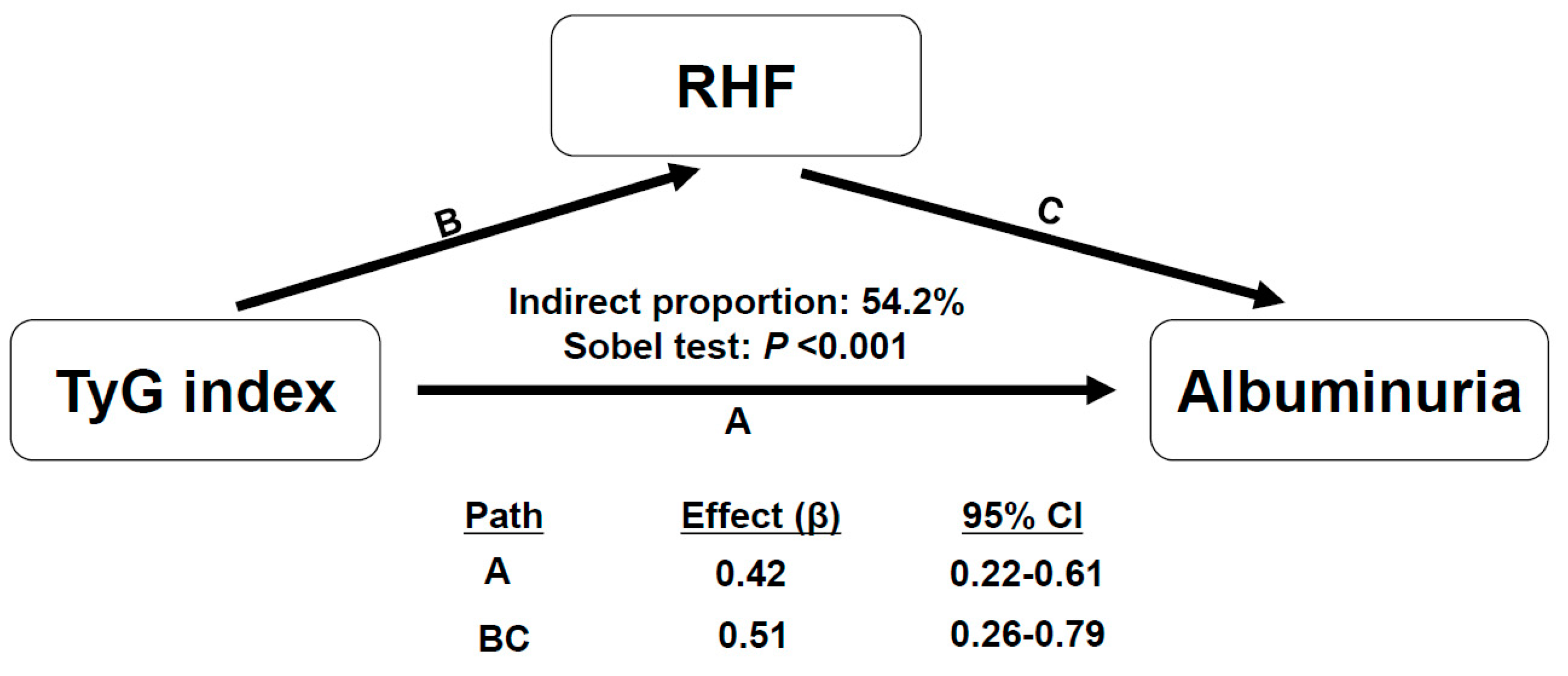
3.4. Association between TyG Index with the Presence of RHF and the Risk of Albuminuria
3.5. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the global burden of disease study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [Green Version]
- Kula, A.J.; Prince, D.K.; Flynn, J.T.; Bansal, N. Bp in young adults with ckd and associations with cardiovascular events and decline in kidney function. J. Am. Soc. Nephrol. 2021, 32, 1200–1209. [Google Scholar] [CrossRef] [PubMed]
- Palit, S.; Chonchol, M.; Cheung, A.K.; Kaufman, J.; Smits, G.; Kendrick, J. Association of bp with death, cardiovascular events, and progression to chronic dialysis in patients with advanced kidney disease. Clin. J. Am. Soc. Nephrol. 2015, 10, 934–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Modi, Z.J.; Lu, Y.; Ji, N.; Kapke, A.; Selewski, D.T.; Dietrich, X.; Abbott, K.; Nallamothu, B.K.; Schaubel, D.E.; Saran, R.; et al. Risk of cardiovascular disease and mortality in young adults with end-stage renal disease: An analysis of the us renal data system. JAMA Cardiol. 2019, 4, 353–362. [Google Scholar] [CrossRef] [Green Version]
- Ogden, C.L.; Carroll, M.D.; Lawman, H.G.; Fryar, C.D.; Kruszon-Moran, D.; Kit, B.K.; Flegal, K.M. Trends in obesity prevalence among children and adolescents in the united states, 1988–1994 through 2013–2014. JAMA 2016, 315, 2292–2299. [Google Scholar] [CrossRef] [Green Version]
- Mayer-Davis, E.J.; Lawrence, J.M.; Dabelea, D.; Divers, J.; Isom, S.; Dolan, L.; Imperatore, G.; Linder, B.; Marcovina, S.; Pettitt, D.J.; et al. Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. N. Engl. J. Med. 2017, 376, 1419–1429. [Google Scholar] [CrossRef] [Green Version]
- Robinson, C.H.; Chanchlani, R. High blood pressure in children and adolescents: Current perspectives and strategies to improve future kidney and cardiovascular health. Kidney Int. Rep. 2022, 7, 954–970. [Google Scholar] [CrossRef]
- Neuhauser, H.; Diederichs, C.; Starker, A.; Scheidt-Nave, C. Health of young adults: Blind spot in public health? Eur. J. Public Health 2018, 28, cky213–cky575. [Google Scholar] [CrossRef]
- Lambers Heerspink, H.J.; Gansevoort, R.T. Albuminuria is an appropriate therapeutic target in patients with ckd: The pro view. Clin. J. Am. Soc. Nephrol. 2015, 10, 1079–1088. [Google Scholar] [CrossRef] [Green Version]
- van Bommel, E.J.M.; Ruiter, D.; Muskiet, M.H.A.; van Baar, M.J.B.; Kramer, M.H.H.; Nieuwdorp, M.; Joles, J.A.; Bjornstad, P.; van Raalte, D.H. Insulin sensitivity and renal hemodynamic function in metformin-treated adults with type 2 diabetes and preserved renal function. Diabetes Care 2019, 43, 228–234. [Google Scholar]
- Melsom, T.; Mathisen, U.D.; Ingebretsen, O.C.; Jenssen, T.G.; Njølstad, I.; Solbu, M.D.; Toft, I.; Eriksen, B.O. Impaired fasting glucose is associated with renal hyperfiltration in the general population. Diabetes Care 2011, 34, 1546–1551. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, A.; Ishimura, E.; Uedono, H.; Ochi, A.; Nakatani, S.; Morioka, T.; Mori, K.; Uchida, J.; Emoto, M.; Nakatani, T.; et al. Association of albuminuria with intraglomerular hydrostatic pressure and insulin resistance in subjects with impaired fasting glucose and/or impaired glucose tolerance. Diabetes Care 2018, 41, 2414–2420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dengel, D.R.; Goldberg, A.P.; Mayuga, R.S.; Kairis, G.M.; Weir, M.R. Insulin resistance, elevated glomerular filtration fraction, and renal injury. Hypertension 1996, 28, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Tonneijck, L.; Muskiet, M.H.A.; Smits, M.M.; van Bommel, E.J.; Heerspink, H.J.L.; van Raalte, D.H.; Joles, J.A. Glomerular hyperfiltration in diabetes: Mechanisms, clinical significance, and treatment. J. Am. Soc. Nephrol. 2017, 28, 1023–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helal, I.; Fick-Brosnahan, G.M.; Reed-Gitomer, B.; Schrier, R.W. Glomerular hyperfiltration: Definitions, mechanisms and clinical implications. Nat. Rev. Nephrol. 2012, 8, 293–300. [Google Scholar] [CrossRef]
- Melsom, T.; Nair, V.; Schei, J.; Mariani, L.; Stefansson, V.T.N.; Harder, J.L.; Jenssen, T.G.; Solbu, M.D.; Norvik, J.V.; Looker, H.; et al. Correlation between baseline gfr and subsequent change in gfr in norwegian adults without diabetes and in pima indians. Am. J. Kidney Dis. 2019, 73, 777–785. [Google Scholar] [CrossRef] [Green Version]
- Bjornstad, P.; Nehus, E.; El Ghormli, L.; Bacha, F.; Libman, I.M.; McKay, S.; Willi, S.M.; Laffel, L.; Arslanian, S.; Nadeau, K.J. Insulin sensitivity and diabetic kidney disease in children and adolescents with type 2 diabetes: An observational analysis of data from the today clinical trial. Am. J. Kidney Dis. 2018, 71, 65–74. [Google Scholar] [CrossRef]
- Bjornstad, P.; Maahs, D.M.; Cherney, D.Z.; Cree-Green, M.; West, A.; Pyle, L.; Nadeau, K.J. Insulin sensitivity is an important determinant of renal health in adolescents with type 2 diabetes. Diabetes Care 2014, 37, 3033–3039. [Google Scholar] [CrossRef] [Green Version]
- Lv, L.; Zhou, Y.; Chen, X.; Gong, L.; Wu, J.; Luo, W.; Shen, Y.; Han, S.; Hu, J.; Wang, Y.; et al. Relationship between the tyg index and diabetic kidney disease in patients with type-2 diabetes mellitus. Diabetes Metab Syndr Obes 2021, 14, 3299–3306. [Google Scholar] [CrossRef]
- Fritz, J.; Brozek, W.; Concin, H.; Nagel, G.; Kerschbaum, J.; Lhotta, K.; Ulmer, H.; Zitt, E. The association of excess body weight with risk of eskd is mediated through insulin resistance, hypertension, and hyperuricemia. J. Am. Soc. Nephrol. 2022, 33, 1377–1389. [Google Scholar] [CrossRef]
- Fritz, J.; Brozek, W.; Concin, H.; Nagel, G.; Kerschbaum, J.; Lhotta, K.; Ulmer, H.; Zitt, E. The triglyceride-glucose index and obesity-related risk of end-stage kidney disease in austrian adults. JAMA Netw. Open 2021, 4, e212612. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Schmid, C.H.; Tighiouart, H.; Eckfeldt, J.H.; Feldman, H.I.; Greene, T.; Kusek, J.W.; Manzi, J.; Van Lente, F.; Zhang, Y.L.; et al. Estimating glomerular filtration rate from serum creatinine and cystatin c. N. Engl. J. Med. 2012, 367, 20–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnston, R.; Jones, K.; Manley, D. Confounding and collinearity in regression analysis: A cautionary tale and an alternative procedure, illustrated by studies of british voting behaviour. Qual. Quant. 2018, 52, 1957–1976. [Google Scholar] [CrossRef] [Green Version]
- Vittinghoff, E.G.D.; Shiboski, S.C.; McCulloch, C.E. Regression Methods in Biostastics: Linear, Logistic, Survival, and Repeated Measures Models, 2nd ed.; Springer: New York, NY, USA, 2011. [Google Scholar] [CrossRef]
- Dikaiakou, E.; Vlachopapadopoulou, E.A.; Paschou, S.A.; Athanasouli, F.; Panagiotopoulos, Ι.; Kafetzi, M.; Fotinou, A.; Michalacos, S. Τriglycerides-glucose (tyg) index is a sensitive marker of insulin resistance in greek children and adolescents. Endocrine 2020, 70, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Yang, Y.; Lee, E.Y.; Yang, H.K.; Kim, H.S.; Lim, S.Y.; Lee, J.H.; Lee, S.S.; Suh, B.K.; Yoon, K.H. Triglycerides/glucose index is a useful surrogate marker of insulin resistance among adolescents. Int. J. Obes. 2017, 41, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Vasques, A.C.; Novaes, F.S.; de Oliveira Mda, S.; Souza, J.R.; Yamanaka, A.; Pareja, J.C.; Tambascia, M.A.; Saad, M.J.; Geloneze, B. Tyg index performs better than homa in a brazilian population: A hyperglycemic clamp validated study. Diabetes Res. Clin. Pract. 2011, 93, e98–e100. [Google Scholar] [CrossRef] [Green Version]
- Guerrero-Romero, F.; Villalobos-Molina, R.; Jiménez-Flores, J.R.; Simental-Mendia, L.E.; Méndez-Cruz, R.; Murguía-Romero, M.; Rodríguez-Morán, M. Fasting triglycerides and glucose index as a diagnostic test for insulin resistance in young adults. Arch. Med. Res. 2016, 47, 382–387. [Google Scholar] [CrossRef]
- Ding, X.; Wang, X.; Wu, J.; Zhang, M.; Cui, M. Triglyceride-glucose index and the incidence of atherosclerotic cardiovascular diseases: A meta-analysis of cohort studies. Cardiovasc. Diabetol. 2021, 20, 76. [Google Scholar] [CrossRef]
- Bae, E.H.; Lim, S.Y.; Jung, J.H.; Oh, T.R.; Choi, H.S.; Kim, C.S.; Ma, S.K.; Han, K.D.; Kim, S.W. Obesity, abdominal obesity and chronic kidney disease in young adults: A nationwide population-based cohort study. J. Clin. Med. 2021, 10, 1065. [Google Scholar] [CrossRef]
- De Cosmo, S.; Menzaghi, C.; Prudente, S.; Trischitta, V. Role of insulin resistance in kidney dysfunction: Insights into the mechanism and epidemiological evidence. Nephrol. Dial. Transpl. 2013, 28, 29–36. [Google Scholar] [CrossRef]
- Park, S.; Lee, S.; Kim, Y.; Lee, Y.; Kang, M.W.; Han, K.; Lee, H.; Lee, J.P.; Joo, K.W.; Lim, C.S.; et al. Reduced risk for chronic kidney disease after recovery from metabolic syndrome: A nationwide population-based study. Kidney Res. Clin. Pract. 2020, 39, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Dronavalli, S.; Duka, I.; Bakris, G.L. The pathogenesis of diabetic nephropathy. Nat. Clin. Pract. Endocrinol. Metab. 2008, 4, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Ko, G.J.; Rhee, C.M.; Kalantar-Zadeh, K.; Joshi, S. The effects of high-protein diets on kidney health and longevity. J. Am. Soc. Nephrol. 2020, 31, 1667–1679. [Google Scholar] [CrossRef] [PubMed]
- Jhee, J.H.; Kee, Y.K.; Park, S.; Kim, H.; Park, J.T.; Han, S.H.; Kang, S.W.; Yoo, T.H. High-protein diet with renal hyperfiltration is associated with rapid decline rate of renal function: A community-based prospective cohort study. Nephrol. Dial. Transpl. 2020, 35, 98–106. [Google Scholar] [CrossRef]
- Hong, S.; Choi, Y.M.; Ihm, S.H.; Kim, D.; Choi, M.G.; Yu, J.M.; Hong, E.G. Association between metabolic parameters and glomerular hyperfiltration in a representative korean population without chronic kidney disease. PLoS ONE 2018, 13, e0207843. [Google Scholar] [CrossRef] [Green Version]
- Magen, D.; Halloun, R.; Galderisi, A.; Caprio, S.; Weiss, R. Relation of glomerular filtration to insulin resistance and related risk factors in obese children. Int. J. Obes. 2022, 46, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Melsom, T.; Schei, J.; Stefansson, V.T.; Solbu, M.D.; Jenssen, T.G.; Mathisen, U.D.; Wilsgaard, T.; Eriksen, B.O. Prediabetes and risk of glomerular hyperfiltration and albuminuria in the general nondiabetic population: A prospective cohort study. Am. J. Kidney Dis. 2016, 67, 841–850. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.M.; Charlton, J.R.; Carmody, J.B.; Gurka, M.J.; DeBoer, M.D. Metabolic risk factors in nondiabetic adolescents with glomerular hyperfiltration. Nephrol. Dial. Transpl. 2017, 32, 1517–1524. [Google Scholar] [CrossRef] [Green Version]
- Skinner, A.C.; Perrin, E.M.; Moss, L.A.; Skelton, J.A. Cardiometabolic risks and severity of obesity in children and young adults. N. Engl. J. Med. 2015, 373, 1307–1317. [Google Scholar] [CrossRef]
- Simental-Mendía, L.E.; Rodríguez-Morán, M.; Guerrero-Romero, F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab. Syndr. Relat. Disord. 2008, 6, 299–304. [Google Scholar] [CrossRef]
- Gao, W.; Wang, J.; Chen, Y.; Qiao, H.; Qian, X.; Xin, Z.; Zhao, Z.; Wang, T.; Xu, Y.; Xu, M.; et al. Discordance between the triglyceride glucose index and homa-ir in incident albuminuria: A cohort study from china. Lipids Health Dis. 2021, 20, 176. [Google Scholar] [CrossRef] [PubMed]

| TyG Index Tertile | ||||
|---|---|---|---|---|
| Characteristics | Tertile 1 (Lowest) (n = 1809) | Tertile 2 (n = 1803) | Tertile 3 (Highest) (n = 1808) | p |
| Demographic data | ||||
| Age, years, mean (SD) | 29.4 ± 6.2 | 30.3 ± 6.1 | 32.2 ± 5.4 | <0.001 |
| Male, n (%) | 462 (25.5%) | 820 (45.5%) | 1234 (68.3%) | <0.001 |
| Smoking status, n (%) | 446 (24.7%) | 739 (41.0%) | 1060 (58.6%) | <0.001 |
| Alcohol status, n (%) | 804 (44.4%) | 929 (51.5%) | 1133 (62.7%) | <0.001 |
| Education, n (%) | <0.001 | |||
| Low | 344 (19.0%) | 451 (25.0%) | 533 (29.5%) | |
| High | 1465 (81.0%) | 1352 (75.0%) | 1275 (70.5%) | |
| Income, n (%) | 0.02 | |||
| Low | 837 (46.3%) | 889 (49.3%) | 921 (50.9%) | |
| High | 972 (53.7%) | 914 (50.7%) | 887 (49.1%) | |
| BMI, kg/m2, mean (SD) | 21.4 ± 2.9 | 22.6 ± 3.4 | 25.5 ± 4.0 | <0.001 |
| SBP, mmHg, mean (SD) | 105.4 ± 10.0 | 109.1 ± 11.3 | 115.1 ± 13.0 | <0.001 |
| DBP, mmHg, mean (SD) | 69.5 ± 8.3 | 72.2 ± 9.2 | 77.7 ± 11.0 | <0.001 |
| Comorbidities | ||||
| Hypertension, n (%) | 32 (1.8%) | 85 (4.7%) | 265 (14.7%) | <0.001 |
| Diabetes, n (%) | 3 (0.2%) | 7 (0.4%) | 109 (6.0%) | <0.001 |
| Dyslipidemia, n (%) | 6 (0.3%) | 16 (0.9%) | 68 (3.8%) | <0.001 |
| Laboratory data | ||||
| TyG index, mean (SD) | 7.7 ± 0.3 | 8.3 ± 0.2 | 9.1 ± 0.5 | <0.001 |
| eGFR, mL/min/1.73 m2, mean (SD) | 110.6 ± 11.9 | 108.4 ± 12.7 | 106.1 ± 13.2 | <0.001 |
| Microalbuminuria, n (%) | 44 (2.4%) | 64 (3.5%) | 125 (6.9%) | <0.001 |
| Hemoglobin, g/dL, mean (SD) | 13.5 ± 1.5 | 14.2 ±1.6 | 14.9 ± 1.6 | <0.001 |
| HbA1c, %, mean (SD) | 5.3 ± 0.3 | 5.4 ± 0.4 | 5.7 ± 0.8 | <0.001 |
| Fasting plasma glucose, g/dL, mean (SD) | 86.3 ± 6.9 | 89.8 ± 7.7 | 97.6 ± 24.5 | <0.001 |
| Total cholesterol, mg/dL, mean (SD) | 166.9 ± 27.1 | 177.9 ± 29.1 | 197.3 ± 36.5 | <0.001 |
| LDL-C, mg/dL, mean (SD) | 97.3 ± 23.7 | 106.7 ± 27.3 | 116.5 ± 33.2 | <0.001 |
| HDL-C, mg/dL, mean (SD) | 59.4 ± 11.1 | 53.8 ± 11.4 | 46.5 ± 11.2 | <0.001 |
| Triglyceride, mg/dL, mean (SD) | 52.0 ± 11.8 | 89.1 ± 14.8 | 206.4 ± 140.6 | <0.001 |
| Prevalence * n (%) | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|
| Variable | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| TyG index (per 1.0 increase) | 233 (4.3) | 2.41 (2.03–2.86) | <0.001 | 2.63 (2.18–3.18) | <0.001 | 1.56 (1.24–1.95) | <0.001 |
| Tertile of TyG index | |||||||
| Tertile 1 (lowest) | 44 (2.4) | (Reference) | |||||
| Tertile 2 | 64 (3.5) | 1.64 (1.12–2.40) | 0.01 | 1.66 (1.13–2.44) | 0.01 | 1.49 (1.01–2.21) | 0.04 |
| Tertile 3 (highest) | 125 (6.9) | 3.05 (2.15–4.33) | <0.001 | 3.08 (2.12–4.49) | <0.001 | 1.65 (1.08–2.52) | 0.02 |
| Prevalence * n (%) | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|
| Variable | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| TyG index (per 1.0 increase) | 543 (10) | 2.03 (1.79–2.30) | <0.001 | 1.49 (1.30–1.72) | <0.001 | 1.56 (1.32–1.84) | <0.001 |
| Tertile of TyG index | |||||||
| Tertile 1 (lowest) | 107 (5.9) | (Reference) | |||||
| Tertile 2 | 161 (8.9) | 1.56 (1.21–2.01) | <0.001 | 1.14 (0.88–1.48) | 0.33 | 1.19 (0.91–1.55) | 0.21 |
| Tertile 3 (highest) | 275 (15.2) | 2.84 (2.24–3.58) | <0.001 | 1.56 (1.21–2.02) | <0.001 | 1.73 (1.31–2.30) | <0.001 |
| Prevalence * n (%) | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|
| Variable | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| With RHF | |||||||
| TyG index (per 1.0 increase) | 64 (11.8) | 4.22 (2.90–6.15) | <0.001 | 4.20 (2.86–6.16) | <0.001 | 2.75 (1.74–4.35) | <0.001 |
| Tertile of TyG index | |||||||
| Tertile 1 (lowest, n = 181) | 5 (2.8) | (reference) | |||||
| Tertile 2 (n = 180) | 14 (7.8) | 2.97 (1.05–8.42) | 0.04 | 2.89 (1.02–8.23) | 0.04 | 2.44 (0.82–7.30) | 0.11 |
| Tertile 3 (highest, n = 182) | 45 (24.7) | 11.56 (4.47–29.9) | <0.001 | 10.90 (4.16–28.58) | <0.001 | 5.25 (1.82–15.14) | 0.002 |
| Without RHF | |||||||
| TyG index (per 1.0 increase) | 169 (3.5) | 1.65 (1.33–2.05) | <0.001 | 1.93 (1.52–2.45) | <0.001 | 1.23 (0.92–1.65) | 0.16 |
| Tertile of TyG index | |||||||
| Tertile 1 (lowest, n = 1627) | 43 (2.6) | (reference) | |||||
| Tertile 2 (n = 1625) | 53 (3.3) | 1.24 (0.83–1.87) | 0.09 | 1.36 (0.90–2.05) | 0.15 | 1.25 (0.82–1.90) | 0.31 |
| Tertile 3 (highest, n = 1625) | 73 (4.5) | 1.73 (1.18–2.54) | 0.005 | 2.10 (1.39–3.18) | <0.001 | 1.18 (0.74–1.91) | 0.49 |
| Prevalence of Albuminuria *, n (%) | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|
| Variable | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| RHF + Tertile of TyG | |||||||
| No RHF + T1 (n = 1627) | 43 (2.6) | (Reference) | |||||
| No RHF + T2 (n = 1625) | 53 (3.3) | 1.24 (0.83–1.87) | 0.30 | 1.33 (0.88–2.01) | 0.18 | 1.18 (0.78–1.80) | 0.43 |
| No RHF + T3 (n = 1625) | 73 (4.5) | 1.73 (1.18–2.54) | 0.005 | 1.99 (1.32–2.99) | 0.001 | 1.07 (0.68–1.68) | 0.78 |
| RHF + T1 (n = 181) | 5 (2.8) | 1.05 (0.41–2.68) | 0.92 | 1.33 (0.51–3.45) | 0.56 | 0.95 (0.35–2.60) | 0.92 |
| RHF + T2 (n = 180) | 14 (7.8) | 3.11 (1.67–5.80) | <0.001 | 3.87 (2.03–7.40) | <0.001 | 2.40 (1.15–5.00) | <0.001 |
| RHF + T3 (n = 182) | 45 (24.7) | 12.10 (7.69–19.03) | <0.001 | 15.47 (9.36–25.58) | <0.001 | 5.77 (3.04–10.95) | <0.001 |
| Variable | AUC | 95% CI | p-value |
|---|---|---|---|
| With RHF | |||
| Basic model * | 0.823 | 0.765–0.880 | |
| Basic model + TyG index | 0.849 | 0.794–0.904 | 0.03 # |
| Without RHF | |||
| Basic model * | 0.673 | 0.622–0.717 | |
| Basic model + TyG index | 0.673 | 0.629–0.717 | 0.94 # |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, D.; Park, S.H.; Lee, S.; Yang, E.; Choi, H.Y.; Park, H.C.; Jhee, J.H. High Triglyceride-Glucose Index with Renal Hyperfiltration and Albuminuria in Young Adults: The Korea National Health and Nutrition Examination Survey (KNHANES V, VI, and VIII). J. Clin. Med. 2022, 11, 6419. https://doi.org/10.3390/jcm11216419
Oh D, Park SH, Lee S, Yang E, Choi HY, Park HC, Jhee JH. High Triglyceride-Glucose Index with Renal Hyperfiltration and Albuminuria in Young Adults: The Korea National Health and Nutrition Examination Survey (KNHANES V, VI, and VIII). Journal of Clinical Medicine. 2022; 11(21):6419. https://doi.org/10.3390/jcm11216419
Chicago/Turabian StyleOh, Donghwan, Sang Ho Park, Seoyoung Lee, Eunji Yang, Hoon Young Choi, Hyeong Cheon Park, and Jong Hyun Jhee. 2022. "High Triglyceride-Glucose Index with Renal Hyperfiltration and Albuminuria in Young Adults: The Korea National Health and Nutrition Examination Survey (KNHANES V, VI, and VIII)" Journal of Clinical Medicine 11, no. 21: 6419. https://doi.org/10.3390/jcm11216419
APA StyleOh, D., Park, S. H., Lee, S., Yang, E., Choi, H. Y., Park, H. C., & Jhee, J. H. (2022). High Triglyceride-Glucose Index with Renal Hyperfiltration and Albuminuria in Young Adults: The Korea National Health and Nutrition Examination Survey (KNHANES V, VI, and VIII). Journal of Clinical Medicine, 11(21), 6419. https://doi.org/10.3390/jcm11216419





