Deep Learning Improves Prediction of Cardiovascular Disease-Related Mortality and Admission in Patients with Hypertension: Analysis of the Korean National Health Information Database
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Study Variables
2.3. Clinical Outcomes
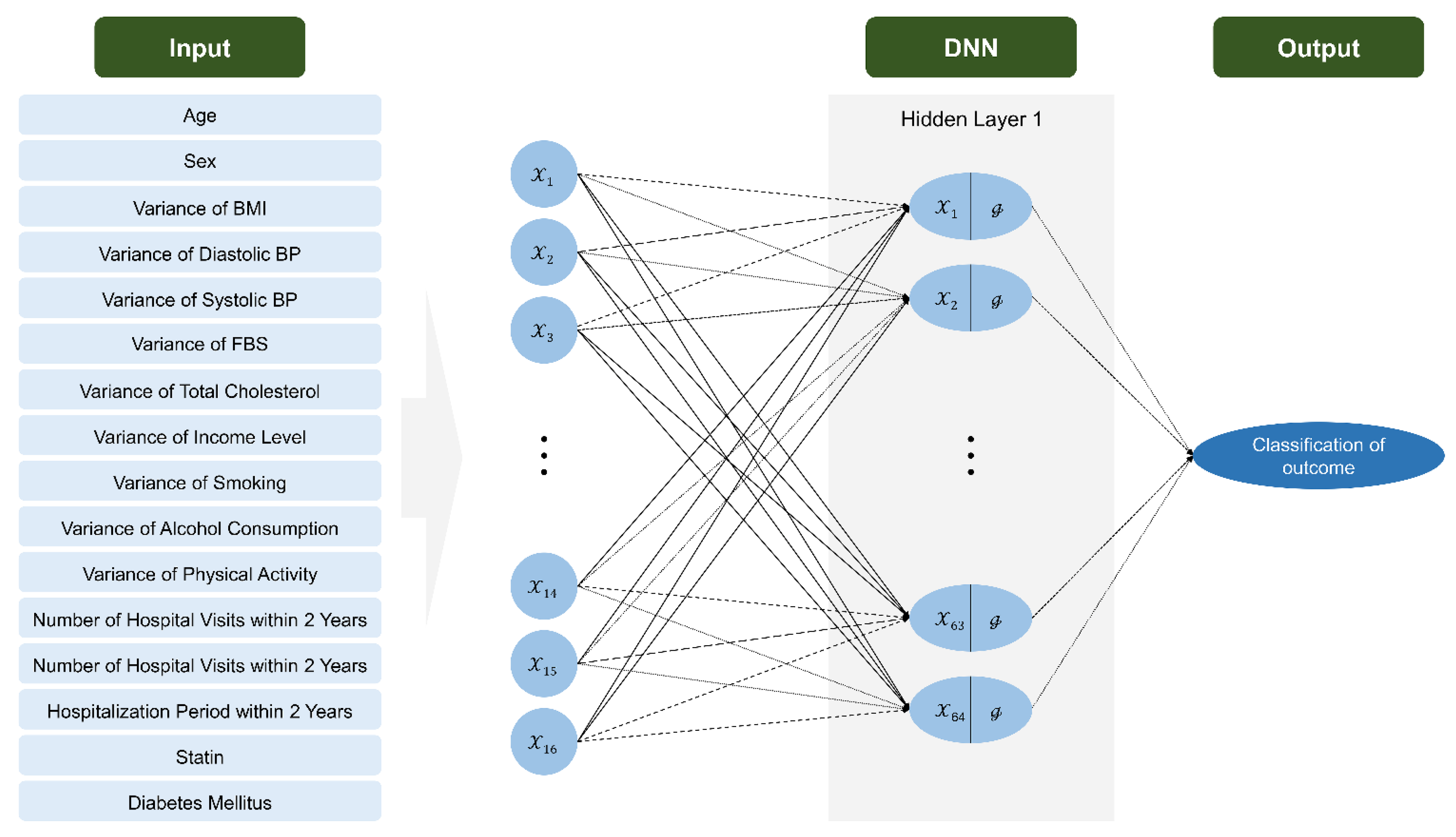
2.4. Algorithm Development and Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Variable Significance in Logistic Regression Model
3.3. Comparison of Model for Outcome Prediction
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2018, 71, e127–e248. [Google Scholar] [CrossRef] [PubMed]
- Collaboration, N.C.D.R.F. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef]
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef]
- Zhou, B.; Perel, P.; Mensah, G.A.; Ezzati, M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat. Rev. Cardiol. 2021, 18, 785–802. [Google Scholar] [CrossRef] [PubMed]
- Bundy, J.D.; Li, C.; Stuchlik, P.; Bu, X.; Kelly, T.N.; Mills, K.T.; He, H.; Chen, J.; Whelton, P.K.; He, J. Systolic Blood Pressure Reduction and Risk of Cardiovascular Disease and Mortality: A Systematic Review and Network Meta-analysis. JAMA Cardiol. 2017, 2, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Ettehad, D.; Emdin, C.A.; Kiran, A.; Anderson, S.G.; Callender, T.; Emberson, J.; Chalmers, J.; Rodgers, A.; Rahimi, K. Blood pressure lowering for prevention of cardiovascular disease and death: A systematic review and meta-analysis. Lancet 2016, 387, 957–967. [Google Scholar] [CrossRef]
- Mahmood, S.S.; Levy, D.; Vasan, R.S.; Wang, T.J. The Framingham Heart Study and the epidemiology of cardiovascular disease: A historical perspective. Lancet 2014, 383, 999–1008. [Google Scholar] [CrossRef]
- Morrow, D.A.; Antman, E.M.; Charlesworth, A.; Cairns, R.; Murphy, S.A.; de Lemos, J.A.; Giugliano, R.P.; McCabe, C.H.; Braunwald, E. TIMI risk score for ST-elevation myocardial infarction: A convenient, bedside, clinical score for risk assessment at presentation: An intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation 2000, 102, 2031–2037. [Google Scholar] [CrossRef]
- Conroy, R.M.; Pyorala, K.; Fitzgerald, A.P.; Sans, S.; Menotti, A.; De Backer, G.; De Bacquer, D.; Ducimetiere, P.; Jousilahti, P.; Keil, U.; et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: The SCORE project. Eur. Heart J. 2003, 24, 987–1003. [Google Scholar] [CrossRef]
- Hippisley-Cox, J.; Coupland, C.; Brindle, P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: Prospective cohort study. BMJ 2017, 357, j2099. [Google Scholar] [CrossRef] [PubMed]
- Wilsgaard, T.; Mathiesen, E.B.; Patwardhan, A.; Rowe, M.W.; Schirmer, H.; Lochen, M.L.; Sudduth-Klinger, J.; Hamren, S.; Bonaa, K.H.; Njolstad, I. Clinically significant novel biomarkers for prediction of first ever myocardial infarction: The Tromso Study. Circ. Cardiovasc. Genet. 2015, 8, 363–371. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goldstein, B.A.; Navar, A.M.; Pencina, M.J.; Ioannidis, J.P. Opportunities and challenges in developing risk prediction models with electronic health records data: A systematic review. J. Am. Med. Inform. Assoc. 2017, 24, 198–208. [Google Scholar] [CrossRef]
- Krittanawong, C.; Johnson, K.W.; Rosenson, R.S.; Wang, Z.; Aydar, M.; Baber, U.; Min, J.K.; Tang, W.H.W.; Halperin, J.L.; Narayan, S.M. Deep learning for cardiovascular medicine: A practical primer. Eur. Heart J. 2019, 40, 2058–2073. [Google Scholar] [CrossRef] [PubMed]
- Quer, G.; Arnaout, R.; Henne, M.; Arnaout, R. Machine Learning and the Future of Cardiovascular Care: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 77, 300–313. [Google Scholar] [CrossRef]
- Choi, E.K. Cardiovascular Research Using the Korean National Health Information Database. Korean Circ. J. 2020, 50, 754–772. [Google Scholar] [CrossRef]
- Harrison, D.G.; Coffman, T.M.; Wilcox, C.S. Pathophysiology of Hypertension: The Mosaic Theory and Beyond. Circ. Res. 2021, 128, 847–863. [Google Scholar] [CrossRef]
- Mills, K.T.; Bundy, J.D.; Kelly, T.N.; Reed, J.E.; Kearney, P.M.; Reynolds, K.; Chen, J.; He, J. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies From 90 Countries. Circulation 2016, 134, 441–450. [Google Scholar] [CrossRef]
- Liau, S.Y.; Mohamed Izham, M.I.; Hassali, M.A.; Shafie, A.A. A literature review of the cardiovascular risk-assessment tools: Applicability among Asian population. Heart Asia 2010, 2, 15–18. [Google Scholar] [CrossRef]
- Cai, R.; Wu, X.; Li, C.; Chao, J. Prediction models for cardiovascular disease risk in the hypertensive population: A systematic review. J. Hypertens. 2020, 38, 1632–1639. [Google Scholar] [CrossRef] [PubMed]
- Giles, T.D. Assessment of global risk: A foundation for a new, better definition of hypertension. J. Clin. Hypertens. 2006, 8, 5–14, quiz 39. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.R.; Lip, G.Y. Novel Risk Markers and Risk Assessments for Cardiovascular Disease. Circ. Res. 2017, 120, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.M. Cardiovascular risk prediction: Basic concepts, current status, and future directions. Circulation 2010, 121, 1768–1777. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.A.; Navar, A.M.; Carter, R.E. Moving beyond regression techniques in cardiovascular risk prediction: Applying machine learning to address analytic challenges. Eur. Heart J. 2017, 38, 1805–1814. [Google Scholar] [CrossRef]
- Sundstrom, J.; Hedberg, J.; Thuresson, M.; Aarskog, P.; Johannesen, K.M.; Oldgren, J. Low-Dose Aspirin Discontinuation and Risk of Cardiovascular Events: A Swedish Nationwide, Population-Based Cohort Study. Circulation 2017, 136, 1183–1192. [Google Scholar] [CrossRef]
- Loft, N.; Skov, L.; Richardson, C.; Trivedi, V.; Alarcon, I.; Egeberg, A. A nationwide population-based cohort study of the incidence of severe and rare infections among adults with psoriasis in Denmark. Br. J. Dermatol. 2022, 187, 353–363. [Google Scholar] [CrossRef]
- Zugna, D.; Richiardi, L.; Akre, O.; Stephansson, O.; Ludvigsson, J.F. A nationwide population-based study to determine whether coeliac disease is associated with infertility. Gut 2010, 59, 1471–1475. [Google Scholar] [CrossRef]
- Sung, J.M.; Cho, I.J.; Sung, D.; Kim, S.; Kim, H.C.; Chae, M.H.; Kavousi, M.; Rueda-Ochoa, O.L.; Ikram, M.A.; Franco, O.H.; et al. Development and verification of prediction models for preventing cardiovascular diseases. PLoS ONE 2019, 14, e0222809. [Google Scholar] [CrossRef]
- Choi, Y.Y.; Jeong, H.; Lee, J.H.; Sung, K.C.; Shin, J.-H.; Kim, H.C.; Kim, J.Y.; Kang, D.R. Cardiovascular disease prediction model in patients with hypertension using deep learning: Analysis of the National Health Insurance Service Database from Republic of Korea. Cardiometab. Syndr. J. 2021, 1, 145–154. [Google Scholar] [CrossRef]
- Gulshan, V.; Peng, L.; Coram, M.; Stumpe, M.C.; Wu, D.; Narayanaswamy, A.; Venugopalan, S.; Widner, K.; Madams, T.; Cuadros, J.; et al. Development and Validation of a Deep Learning Algorithm for Detection of Diabetic Retinopathy in Retinal Fundus Photographs. JAMA 2016, 316, 2402–2410. [Google Scholar] [CrossRef] [PubMed]
- Suh, Y.; Lee, C.J.; Cho, D.K.; Cho, Y.H.; Shin, D.H.; Ahn, C.M.; Kim, J.S.; Kim, B.K.; Ko, Y.G.; Choi, D.; et al. Impact of National Health Checkup Service on Hard Atherosclerotic Cardiovascular Disease Events and All-Cause Mortality in the General Population. Am. J. Cardiol. 2017, 120, 1804–1812. [Google Scholar] [CrossRef] [PubMed]



| Variables | CVD Hospitalization | CVD Death | ||||
|---|---|---|---|---|---|---|
| No (n = 1,873,341) | Yes (n = 163,686) | p Value | No (n = 2,005,393) | Yes (n = 31,634) | p Value | |
| Age, years | 62.8 ± 12.9 | 66.3 ± 13.4 | <0.0001 | 62.9 ± 12.9 | 75.9 ± 10.6 | <0.0001 |
| Sex | <0.0001 | <0.0001 | ||||
| Male | 966,057 (51.57) | 81,654 (49.88) | 1,030,193 (51.37) | 17,518 (55.38) | ||
| Female | 907,284 (48.43) | 82,032 (50.12) | 975,200 (48.63) | 14,116 (44.62) | ||
| Variance of BMI | −0.17 ± 8.69 | 0.12 ± 7.27 | <0.0001 | −0.16 ± 8.61 | 0.64 ± 6.85 | <0.0001 |
| Variance of Diastolic BP | 2.39 ± 13.72 | 1.57 ± 13.16 | <0.0001 | 2.31 ± 13.65 | 2.90 (15.26) | <0.0001 |
| Variance of Systolic BP | 1.11 ± 20.20 | 0.16 ± 19.63 | <0.0001 | 0.99 ± 20.09 | 4.04 (24.07) | <0.0001 |
| Variance of FBS | −6.82 ± 35.93 | −4.78 ± 37.63 | <0.0001 | −6.73 ± 35.87 | −1.83 (46.87) | <0.0001 |
| Variance of total cholesterol | 9.96 ± 56.37 | 5.83 ± 54.77 | <0.0001 | 9.61 ± 56.16 | 10.78 (61.97) | 0.0009 |
| Variance of income level | −82,776.98 ± 104,588.08 | −82,947.07 ± 105,521.46 | 0.5314 | −82,975.97 ± 104,855.49 | −71,041.90 ± 90,900.10 | <0.0001 |
| Variance of smoking | 1.32 ± 6.89 | 1.21 ± 6.66 | <0.0001 | 1.32 ± 6.87 | 0.91 ± 6.47 | <0.0001 |
| Variance of alcohol consumption | −25.86 ± 105.35 | −17.19 ± 98.33 | <0.0001 | −25.38 ± 105.15 | −11.87 ± 80.59 | <0.0001 |
| Variance of physical activity | −0.62 ± 3.01 | −0.42 ± 3.00 | <0.0001 | −0.61 ± 3.01 | 0.08 ± 2.80 | <0.0001 |
| Number of hospital visits within 2 years | 13.73 ± 15.22 | 10.11 (17.29) | <0.0001 | 13.34 ± 15.31 | 19.42 ± 20.67 | <0.0001 |
| Number of prescriptions within 2 years | 462.48 ± 343.64 | 290.77 ± 346.72 | <0.0001 | 449.01 ± 347.20 | 427.84 ± 335.88 | <0.0001 |
| Hospitalization period within 2 years, days | 25.90 ± 69.61 | 32.22 ± 90.66 | <0.0001 | 24.81 ± 66.54 | 127.71 ± 196.46 | <0.0001 |
| Statin | <0.0001 | <0.0001 | ||||
| No | 1,156,157 (61.72) | 117,151 (71.57) | 1,248,568 (62.26) | 24,740 (78.21) | ||
| Yes | 717,184 (38.28) | 46,535 (28.43) | 756,825 (37.74) | 6,894 (21.79) | ||
| Diabetes | <0.0001 | <0.0001 | ||||
| No | 1,244,737 (66.44) | 92,650 (56.60) | 1,321,387 (65.89) | 16,000 (50.58) | ||
| Yes | 628,604 (33.56) | 71,036 (43.40) | 684,006 (34.11) | 15,634 (49.42) | ||
| MI | <0.0001 | <0.0001 | ||||
| No | 1,857,062 (99.13) | 160,155 (97.84) | 1,988,626 (99.16) | 28,592(90.38) | ||
| Yes | 16,279 (0.87) | 3,531 (2.16) | 16,767 (0.84) | 3,043(9.62) | ||
| Stroke | <0.0001 | <0.0001 | ||||
| No | 1,798,818 (96.02) | 143,190 (87.48) | 1,921,488 (95.82) | 20,520 (64.87) | ||
| yes | 74,523 (3.98) | 20,496 (12.52) | 83,905 (4.18) | 11,114 (35.13) | ||
| Variable | CVD Hospital | CVD Death | CVD Total (Hospital + Death) | |||
|---|---|---|---|---|---|---|
| Missing Value (+) OR (95% CI) | Missing Value (−) OR (95% CI) | Missing Value (+) OR (95% CI) | Missing Value (−) OR (95% CI) | Missing Value (+) or (95% CI) | Missing Value (−) or (95% CI) | |
| Age, years | 1.028 (1.027-1.028) | 1.021 (1.021–1.021) | 1.094 (1.092–1.095) | 1.074 (1.074–1.075) | 1.035 (1.035–1.036) | 1.035 (1.035–1.036) |
| Sex | ||||||
| Male | Reference | Reference | Reference | Reference | Reference | Reference |
| Female | 1.016 (1.005–1.027) | 0.973 (0.965–0.981) | 0.640 (0.625–0.655) | 0.815 (0.806–0.825) | 0.961 (0.951–0.971) | 0.947 (0.940–0.954) |
| Variance of BMI | 1.001 (1.000–1.000) | 1.001 (1.001–1.001) | 1.001 (1.001–1.002) | 1.001 (1.000–1.001) | 1.001 (1.001–1.001) | 1.001 (1.001–1.001) |
| Variance of Diastolic BP | 0.998 (0.998–0.999) | 0.997 (0.997–0.998) | 0.989 (0.988–0.990) | 0.985 (0.985–0.986) | 0.996 (0.996–0.997) | 0.994 (0.993–0.994) |
| Variance of Systolic BP | 1.001 (1.001–1.001) | 1.001 (1.001–1.002) | 1.009 (1.008–1.010) | 1.008 (1.007–1.008) | 1.003 (1.002–1.003) | 1.003 (1.003–1.004) |
| Variance of FBS | 1.001 (1.001–1.001) | 1.001 (1.001–1.002) | 1.002 (1.001–1.002) | 1.003 (1.002–1.003) | 1.001 (1.001–1.001) | 1.002 (1.002–1.002) |
| Variance of Total Cholesterol | 0.999 (0.999–1.000) | 0.999 (0.999–0.999) | 1.000 (0.999–1.000) | 0.998 (0.998–0.999) | 0.999 (0.999–0.999) | 0.999 (0.999–0.999) |
| Variance of Income level | 1.000 (1.000–1.000) | 1.000 (1.000–1.000) | 1.000 (1.000–1.000) | 1.000 (1.000–1.000) | 1.000 (1.000–1.000) | 1.000 (1.000–1.000) |
| Variance of smoking | 1.000 (0.999–1.001) | 0.997 (0.997–0.998) | 0.992 (0.990–0.994) | 0.979 (0.977–0.980) | 0.999 (0.998–1.000) | 0.993 (0.993–0.994) |
| Variance of alcohol consumption | 1.001 (1.000–1.001) | 1.001 (1.001–1.001) | 1.001 (1.000–1.001) | 1.001 (1.001–1.001) | 1.001 (1.001–1.001) | 1.001 (1.001–1.001) |
| Variance of Physical activity | 1.009 (1.007–1.011) | 1.013 (1.011–1.015) | 1.030 (1.026–1.034) | 1.035 (1.032–1.037) | 1.012 (1.011–1.014) | 1.019 (1.017–1.020) |
| Number of hospital visits within 2 years | 0.991 (0.991–0.992) | 0.995 (0.995–0.995) | 1.007 (1.006–1.007) | 1.005 (1.005–1.005) | 0.997 (0.997–0.998) | 1.000 (1.000–1.000) |
| Number of prescriptions within 2 years | 0.998 (0.998–0.998) | 0.999 (0.999–0.999) | 0.999 (0.999–0.999) | 0.999 (0.999–0.999) | 0.998 (0.998–0.998) | 0.999 (0.999–0.999) |
| Hospitalization period within 2 years, days | 1.000 (1.000–1.000) | 1.000 (1.000–1.000) | 1.003 (1.003–1.003) | 1.002 (1.002–1.002) | 1.001 (1.001–1.001) | 1.001 (1.00–1.001) |
| Statin | ||||||
| No | Reference | Reference | Reference | Reference | Reference | Reference |
| Yes | 0.640 (0.632–0.647) | 0.648 (0.642–0.654) | 0.534 (0.519–0.549) | 0.489 (0.481–0.497) | 0.617 (0.610–0.624) | 0.584 (0.579–0.589) |
| Diabetes | ||||||
| No | Reference | Reference | Reference | Reference | Reference | Reference |
| Yes | 1.749 (1.730–1.768) | 1.880 (1.866–1.896) | 1.657 (1.619–1.696) | 1.384 (1.368–1.401) | 1.715 (1.698–1.733) | 1.708 (1.696–1.721) |
| Variable | CVD Hospital | CVD Death | CVD Total (Hospital + Death) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Missing Value (+) | Missing Value (−) | Missing Value (+) | Missing Value (−) | Missing Value (+) | Missing Value (−) | |||||||
| LR | DNN | LR | DNN | LR | DNN | LR | DNN | LR | DNN | LR | DNN | |
| Accuracy | 0.646 | 0.824 | 0.655 | 0.863 | 0.777 | 0.886 | 0.780 | 0.925 | 0.673 | 0.843 | 0.659 | 0.860 |
| F1 score | 0.648 | 0.811 | 0.656 | 0.854 | 0.782 | 0.889 | 0.783 | 0.924 | 0.672 | 0.834 | 0.658 | 0.852 |
| Precision | 0.645 | 0.877 | 0.654 | 0.912 | 0.764 | 0.870 | 0.773 | 0.935 | 0.675 | 0.879 | 0.662 | 0.903 |
| Recall | 0.650 | 0.754 | 0.658 | 0.803 | 0.800 | 0.908 | 0.793 | 0.912 | 0.669 | 0.794 | 0.654 | 0.807 |
| AUC | 0.646 | 0.907 | 0.655 | 0.932 | 0.777 | 0.959 | 0.780 | 0.979 | 0.673 | 0.923 | 0.660 | 0.933 |
| Variable | MI | Stroke | ||||||
|---|---|---|---|---|---|---|---|---|
| Missing Value (+) | Missing Value (−) | Missing Value (+) | Missing Value (−) | |||||
| LR | DNN | LR | DNN | LR | DNN | LR | DNN | |
| Accuracy | 0.686 | 0.911 | 0.727 | 0.917 | 0.681 | 0.883 | 0.682 | 0.897 |
| F1 score | 0.696 | 0.908 | 0.734 | 0.916 | 0.687 | 0.874 | 0.690 | 0.889 |
| Precision | 0.675 | 0.939 | 0.715 | 0.927 | 0.674 | 0.935 | 0.674 | 0.956 |
| Recall | 0.718 | 0.879 | 0.754 | 0.907 | 0.700 | 0.824 | 0.707 | 0.832 |
| AUC | 0.686 | 0.968 | 0.727 | 0.972 | 0.681 | 0.948 | 0.682 | 0.951 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-J.; Lee, S.-H.; Choi, H.-I.; Lee, J.-Y.; Jeong, Y.-W.; Kang, D.-R.; Sung, K.-C. Deep Learning Improves Prediction of Cardiovascular Disease-Related Mortality and Admission in Patients with Hypertension: Analysis of the Korean National Health Information Database. J. Clin. Med. 2022, 11, 6677. https://doi.org/10.3390/jcm11226677
Lee S-J, Lee S-H, Choi H-I, Lee J-Y, Jeong Y-W, Kang D-R, Sung K-C. Deep Learning Improves Prediction of Cardiovascular Disease-Related Mortality and Admission in Patients with Hypertension: Analysis of the Korean National Health Information Database. Journal of Clinical Medicine. 2022; 11(22):6677. https://doi.org/10.3390/jcm11226677
Chicago/Turabian StyleLee, Seung-Jae, Sung-Ho Lee, Hyo-In Choi, Jong-Young Lee, Yong-Whi Jeong, Dae-Ryong Kang, and Ki-Chul Sung. 2022. "Deep Learning Improves Prediction of Cardiovascular Disease-Related Mortality and Admission in Patients with Hypertension: Analysis of the Korean National Health Information Database" Journal of Clinical Medicine 11, no. 22: 6677. https://doi.org/10.3390/jcm11226677
APA StyleLee, S.-J., Lee, S.-H., Choi, H.-I., Lee, J.-Y., Jeong, Y.-W., Kang, D.-R., & Sung, K.-C. (2022). Deep Learning Improves Prediction of Cardiovascular Disease-Related Mortality and Admission in Patients with Hypertension: Analysis of the Korean National Health Information Database. Journal of Clinical Medicine, 11(22), 6677. https://doi.org/10.3390/jcm11226677







