Prevalence and Characterisation of Severe Left Ventricular Hypertrophy Diagnosed by Echocardiography in Hypertensive Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Documented Parameters
2.2.1. Echocardiography
2.2.2. Blood Pressure Measurements
2.2.3. Electrocardiogram
2.2.4. Medication
2.2.5. Comorbidities and Risk Factors
2.2.6. Blood Tests
2.3. Formation of Groups
2.4. Statistical Analysis
2.5. Ethical Approval
3. Results
3.1. Baseline Characteristics
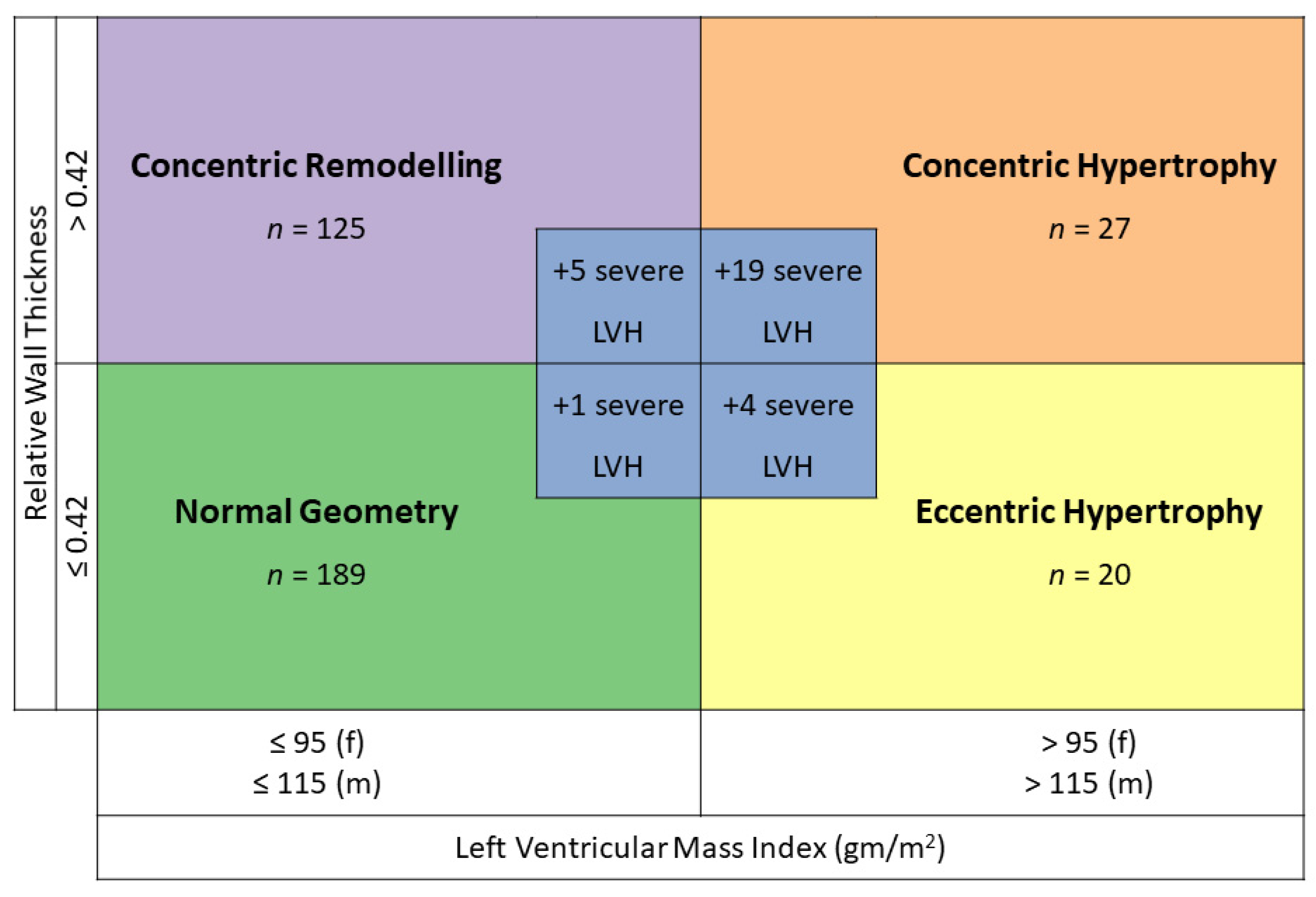
3.2. Prevalence of Left Ventricular Hypertrophy
3.3. Severe Left Ventricular Hypertrophy vs. No Left Ventricular Hypertrophy and vs. Left Ventricular Hypertrophy
3.4. Gender Differences Regarding Blood Pressure across All Geometries
3.5. Differences Regarding Blood Pressure across All Geometries
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mills, K.T.; Bundy, J.D.; Kelly, T.N.; Reed, J.; Kearney, P.M.; Reynolds, K.; Chen, J.; He, J. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-based Studies From 90 Countries. Circulation 2015, 132, 441–450. [Google Scholar] [CrossRef]
- de Simone, G.; Izzo, R.; Chinali, M.; De Marco, M.; Casalnuovo, G.; Rozza, F.; Girfoglio, D.; Iovino, G.L.; Trimarco, B.; De Luca, N. Does Information on Systolic and Diastolic Function Improve Prediction of a Cardiovascular Event by Left Ventricular Hypertrophy in Arterial Hypertension? Hypertension 2010, 56, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Salvetti, M.; Paini, A.; Bertacchini, F.; Stassaldi, D.; Aggiusti, C.; Agabiti Rosei, C.; Bassetti, D.; Agabiti-Rosei, E.; Muiesan, M.L. Changes in left ventricular geometry during antihypertensive treatment. Pharmacol. Res. 2018, 134, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Devereux, R.B.; Wachtell, K.; Gerdts, E.; Boman, K.; Nieminen, M.S.; Papademetriou, V.; Rokkedal, J.; Harris, K.; Aurup, P.; Dahlöf, B. Prognostic Significance of Left Ventricular Mass Change During Treatment of Hypertension. JAMA 2004, 292, 2350–2356. [Google Scholar] [CrossRef]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Edvardsen, T.; Goldstein, S.; Lancellotti, P.; LeFevre, M.; Miller, F., Jr.; Otto, C.M. Recommendations on the Echocardiographic Assessment of Aortic Valve Stenosis: A Focused Update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2017, 30, 372–392. [Google Scholar] [CrossRef]
- Elliott, P.M.; Anastasakis, A.; Borger, M.A.; Borggrefe, M.; Cecchi, F.; Charron, P.; Alain Hagege, A.; Lafont, A.; Limongelli, G.; Mahrholdt, H.; et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: The Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2733–2779. [Google Scholar]
- Levy, D.; Anderson, K.M.; Savage, D.D.; Kannel, W.B.; Christiansen, J.C.; Castelli, W.P. Echocardiographically Detected Left Ventricular Hypertrophy: Prevalence and Risk Factors: The Framingham Heart Study. Ann. Intern. Med. 1988, 108, 7–13. [Google Scholar] [CrossRef]
- Santos, M.; Shah, A.M. Alterations in cardiac structure and function in hypertension. Curr. Hypertens. Rep. 2014, 16, 428. [Google Scholar] [CrossRef]
- Fox, E.R.; Taylor, J.; Taylor, H.; Han, H.; Samdarshi, T.; Arnett, D.; Myerson, M. Left ventricular geometric patterns in the Jackson cohort of the Atherosclerotic Risk in Communities (ARIC) Study: Clinical correlates and influences on systolic and diastolic dysfunction. Am. Hear. J. 2007, 153, 238–244. [Google Scholar] [CrossRef]
- Abdalla, M.; Booth, J.N., 3rd; Diaz, K.M.; Sims, M.; Muntner, P.; Shimbo, D. Hypertension and alterations in left ventricular structure and geometry in African Americans: The Jackson Heart Study. J. Am. Soc. Hypertens. 2016, 10, 550–558.e10. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Huang, Y.; Zhong, Q.; Cai, A.; Feng, Y.Q. Prevalence of and risk factors for abnormal left ventricular geometrical patterns in hypertensive subjects administered irbesartan. J. Clin. Lab. Anal. 2021, 35, e23688. [Google Scholar] [CrossRef] [PubMed]
- Lehtonen, A.O.; Puukka, P.; Varis, J.; Porthan, K.; Tikkanen, J.T.; Nieminen, M.S.; Huikuri, H.V.; Anttila, I.; Nikus, K.; Kähönen, M.; et al. Prevalence and prognosis of ECG abnormalities in normotensive and hypertensive individuals. J. Hypertens. 2016, 34, 959–966. [Google Scholar] [CrossRef]
- Nardi, E.; Palermo, A.; Mulè, G.; Cusimano, P.; Cerasola, G.; Rini, G.B. Prevalence and predictors of left ventricular hypertrophy in patients with hypertension and normal electrocardiogram. Eur. J. Prev. Cardiol. 2013, 20, 854–861. [Google Scholar] [CrossRef][Green Version]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of, Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef]
- Williams, B.R.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar]
- Sokolow, M.; Lyon, T.P. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am. Heart J. 1949, 37, 161–186. [Google Scholar] [CrossRef]
- Parati, G.; Stergiou, G.; O’Brien, E.; Asmar, R.; Beilin, L.; Bilo, G.; Clement, D.; de la Sierra, A.; de Leeuw, P.; Dolan, E.; et al. European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. J. Hypertens. 2014, 32, 1359–1366. [Google Scholar] [CrossRef]
- Dolan, E.; Stanton, A.; Thijs, L.; Hinedi, K.; Atkins, N.; McClory, S.; Hond, E.D.; McCormack, P.; Staessen, J.A.; O’Brien, E. Superiority of Ambulatory Over Clinic Blood Pressure Measurement in Predicting Mortality: The Dublin Outcome Study. Hypertension 2005, 46, 156–161. [Google Scholar] [CrossRef]
- Oh, J.S.; Lee, C.H.; Park, J.I.; Park, H.K.; Hwang, J.K. Hypertension-Mediated Organ Damage and Long-term Cardiovascular Outcomes in Asian Hypertensive Patients without Prior Cardiovascular Disease. J. Korean Med. Sci. 2020, 35, e400. [Google Scholar] [CrossRef]
- Cuspidi, C.; Facchetti, R.; Quarti-Trevano, F.; Sala, C.; Tadic, M.; Grassi, G.; Mancia, G. Incident Left Ventricular Hypertrophy in Masked Hypertension. Hypertension 2019, 74, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Schröder, J.; Nuding, S.; Müller-Werdan, U.; Werdan, K.; Kluttig, A.; Russ, M.; Greiser, K.H.; Kors, J.A.; Haerting, J.; Medenwald, D. Performance of Sokolow-Lyon index in detection of echocardiographically diagnosed left ventricular hypertrophy in a normal Eastern German population—results of the CARLA study. BMC Cardiovasc. Disord. 2015, 15, 69. [Google Scholar] [CrossRef] [PubMed]
- Finocchiaro, G.; Sheikh, N.; Biagini, E.; Papadakis, M.; Maurizi, N.; Sinagra, G.; Pelliccia, A.; Rapezzi, C.; Sharma, S.; Olivotto, I. The electrocardiogram in the diagnosis and management of patients with hypertrophic cardiomyopathy. Heart Rhythm. 2020, 17, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Marwick, T.H.; Gillebert, T.C.; Aurigemma, G.; Chirinos, J.; Derumeaux, G.; Galderisi, M.; Gottdiener, J.; Haluska, B.; Ofili, E.; Segers, P.; et al. Recommendations on the Use of Echocardiography in Adult Hypertension: A Report from the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE). J. Am. Soc. Echocardiogr. 2015, 28, 727–754. [Google Scholar] [CrossRef] [PubMed]
- Neisius, U.; Myerson, L.; Fahmy, A.S.; Nakamori, S.; El-Rewaidy, H.; Joshi, G.; Duan, C.; Manning, W.J.; Nezafat, R. Cardiovascular magnetic resonance feature tracking strain analysis for discrimination between hypertensive heart disease and hypertrophic cardiomyopathy. PLoS ONE 2019, 14, e0221061. [Google Scholar] [CrossRef]




| Parameter | Total | Normal LV Geometry | Concentric Remodelling | Concentric Hypertrophy | Eccentric Hypertrophy | Severe LVH |
|---|---|---|---|---|---|---|
| n = 400 | n = 189 | n = 125 | n = 37 | n = 20 | n = 29 | |
| General characteristics: | ||||||
| Female gender; n (%) | 177 (44.3) | 97 (51.3) | 48 (38.4) | 18 (48.6) | 7 (35.0) | 7 (24.1) |
| Male gender; n (%) | 223 (55.7) | 92 (48.7) | 77 (61.6) | 19 (51.4) | 13 (65.0) | 22 (75.9) |
| Age (years); median (IQR) | 56 (43–70) | 50.4 (36.5–60.8) | 59.4 (47.9–72.1) | 62.6 (53.7–74.3) | 71.5 (61.5–77.6) | 64.9 (52.9–73.7) |
| BMI (kg/m2); median (IQR) | 27 (24–31) | 27 (25–31) | 26 (23–30) | 28 (26–31) | 27 (24–31) | 29 (27–36) |
| Hypertension: | ||||||
| Duration AHT (months); median (IQR) | 23 (2–95) | 13.1 (1.4–65.9) | 34.1 (3.0–106.1) | 27.7 (2.1–190.5) | 73.6 (9.2–196) | 34.8 (1.9– 115.4) |
| Secondary form of AHT; n (%) | 130/349 (37.2) | 49/166 (29.5) | 40/108 (37.0) | 19/33 (57.6) | 7/17 (41.2) | 15/25 (60.0) |
| Mean ABPM results: | ||||||
| Systolic 24 h BP (mmHg); median (IQR) | 133 (125–143) | 132 (124–140) | 131 (124–142) | 142 (129–149) | 136 (121–149) | 145 (131–162) |
| Diastolic 24 h BP (mmHg); median (IQR) | 81 (75–88) | 81 (75–88) | 81 (74–88) | 80 (74–90) | 76 (70–84) | 85 (80–99) |
| Systolic awake BP (mmHg); median (IQR) | 136 (127–146) | 136 (127–144) | 136 (126–144) | 143 (132–153) | 138 (124–147) | 150 (135–162) |
| Diastolic awake BP (mmHg); median (IQR) | 83 (77–91) | 84 (77–91) | 84 (76–91) | 81 (77–91) | 79 (71–87) | 88 (82–100) |
| Systolic asleep BP (mmHg); median (IQR) | 124 (115–138) | 122 (114–133) | 123 (115–134) | 137 (125–144) | 126 (111–146) | 140 (125–155) |
| Diastolic asleep BP (mmHg); median (IQR) | 73 (67–82) | 72 (67–81) | 73 (67–85) | 78 (69–82) | 71 (63–80) | 81 (75–93) |
| Systolic 24 h mean BP ≥ 130 mmHg; n (%) | 227 (56.8) | 102 (55.7) | 66 (54.1) | 26 (72.2) | 11 (61.1) | 22 (75.9) |
| Diastolic 24 h mean BP ≥ 80 mmHg; n (%) | 219 (54.8) | 101 (55.2) | 68 (55.7) | 20 (55.6) | 8 (44.4) | 22 (75.9) |
| Systolic awake BP ≥ 135 mmHg; n (%) | 219 (56.3) | 96 (52.2) | 66 (54.1) | 25 (69.4) | 10 (55.6) | 22 (75.9) |
| Diastolic awake BP ≥85 mmHg; n (%) | 177 (45.5) | 86 (46.7) | 54 (44.3) | 14 (38.9) | 5 (27.8) | 18 (62.1) |
| Systolic asleep BP ≥ 120 mmHg; n (%) | 244 (61.0) | 104 (57.5) | 73 (60.3) | 30 (83.3) | 12 (66.7) | 25 (89.3) |
| Diastolic asleep BP ≥ 70 mmHg; n (%) | 262 (65.5) | 117 (64.6) | 82 (67.8) | 26 (72.2) | 10 (55.6) | 27 (96.4) |
| Comorbidities: | ||||||
| Coronary artery disease; n (%) | 85 (21.4) | 36 (19.0) | 24 (19.5) | 10 (27.0) | 4 (20.0) | 11 (37.9) |
| Stroke/TIA; n (%) | 45 (11.3) | 19 (10.1) | 12 (9.6) | 6 (16.2) | 2 (10.0) | 6 (20.7) |
| Peripheral arterial disease; n (%) | 25 (6.3) | 9 (4.8) | 8 (6.5) | 3 (8.6) | 3 (15.0) | 2 (6.9) |
| Aortic aneurysm; n (%) | 12 (9.8) | 2/56 (3.6) | 2/38 (5.3) | 5/13 (38.5) | 1/4 (25.0) | 2/11 (18.2) |
| Diabetes; n (%) | 70 (17.5) | 25 (13.3) | 25 (20.0) | 9 (24.3) | 5 (25.0) | 6 (20.7) |
| HbA1c (%); median (IQR) | 5.6 (5.3–5.9) | 5.5 (5.2–5.8) | 5.6 (5.3–5.9) | 5.7 (5.4–5.9) | 5.7 (5.4–6.5) | 5.7 (5.2–6.0) |
| eGFR (ml/min/1.7); median (IQR) | 84 (64–101) | 93 (76–106) | 82 (63–97) | 64 (43.5–90) | 81 (52–94) | 59 (38–79) |
| Microalbuminuria ≥ 3.4 mg/mmol; n (%) | 47/221 (21.3) | 13/104 (12.5) | 13/65 (20.0) | 6/24 (25.0) | 5/11 (45.5) | 10/17 (58.8) |
| Active smokers; n (%) | 80 (20.0) | 38 (20.7) | 31 (25.4) | 4 (11.1) | 5 (26.3) | 2 (7.7) |
| History of smoking; n (%) | 106 (27.4) | 48 (26.1) | 28 (23.0) | 11 (30.6) | 8 (42.1) | 11 (42.3) |
| Family history HCM; n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Family history SCD; n (%) | 16/328 (4.9) | 3/164 (1.8) | 5/104 (4.8) | 4/29 (13.8) | 2/15.4 (15.4) | 2/18 (11.1) |
| Family history AHT; n (%) | 145/307 (44.9) | 77/157 (49.0) | 42/93 (45.2) | 12/26 (46.2) | 6/14 (42.9) | 8/17 (47.1) |
| Medication: | ||||||
| ACE/ARB; n (%) | 286 (71.9) | 124 (66.0) | 92 (73.6) | 28 (75.7) | 19 (100) | 23 (79.3) |
| CCB; n (%) | 228 (57.0) | 100 (52.9) | 69 (55.2) | 29 (78.4) | 11 (55.0) | 19 (65.5) |
| Diuretics; n (%) | 130 (32.7) | 37 (19.7) | 39 (31.5) | 24 (64.9) | 11 (55.0) | 19 (65.5) |
| Beta blockers; n (%) | 132 (33.0) | 50 (26.5) | 34 (27.2) | 20 (54.1) | 12 (60.0) | 16 (55.2) |
| Any antihypertensive; n (%) | 348 (87.7) | 156 (83.0) | 110 (88.7) | 35 (94.6) | 19 (100.0) | 28 (96.6) |
| Number of first line drugs (ACE/ARB/CCB/Diuretics); median (IQR) | 2 (1–2) | 1 (1–2) | 2 (1–2) | 2 (2–3) | 2 (2–3) | 2 (2–3) |
| Monotherapy (ACE/ARB/CCB/Diuretics); n (%) | 113 (28.2) | 60 (32.3) | 40 (32.3) | 5 (13.5) | 4 (21.1) | 4 (13.8) |
| Dual therapy (ACE/ARB/CCB/Diuretics); n (%) | 132 (33.0) | 59 (31.2) | 41 (32.8) | 14 (37.8) | 8 (40.0) | 9 (31.0) |
| Triple therapy (ACE/ARB/CCB/Diuretics); n (%) | 87 (21.8) | 27 (14.3) | 26 (20.8) | 16 (43.2) | 7 (35.0) | 13 (44.8) |
| ECG: | ||||||
| Positive Sokolow-Lyon index; n (%) | 16/360 (4.4) | 4 (2.4) | 4 (3.7) | 2 (5.6) | 3 (15.0) | 3 (11.1) |
| Any ST-Depression; n (%) | 55/359 (15.3) | 7 (4.1) | 10 (9.3) | 6 (16.7) | 3 (15.0) | 5 (18.5) |
| Negative T V4-V6 in at least 1 lead; n (%) | 31 (7.8) | 18 (10.7) | 14 (13.1) | 6 (16.7) | 10 (50.0) | 7 (25.9) |
| TTE: | ||||||
| Left ventricular ejection fraction (%); median (IQR) | 61 (58–65) | 61 (59–65) | 62 (59–67) | 63 (59–67) | 56 (46–60) | 56 (52–61) |
| Left atrial volume index (mL/m2); median (IQR) | 29 (24–36) | 27 (23–33) | 28 (24–33) | 32 (29–41) | 34 (27–48) | 37 (27–45) |
| Parameter | Severe LVH n = 29 | No LVH n = 189 | LVH n = 57 | Severe LVH vs. No LVH; p-Value | Severe LVH vs. LVH; p-Value |
|---|---|---|---|---|---|
| Female gender; n (%) | 7 (24.1) | 97 (51.3) | 25 (43.9) | 0.009 | 0.099 |
| Age (years); median (IQR) | 64.9 (52.9–73.7) | 50.4 (36.5–60.8) | 66.3 (55.1–75.4) | 0.001 | 0.308 |
| BMI (kg/m2); median (IQR) | 29.0 (27.0–36.0) | 27.0 (25.0–31.0) | 27.0 (35.0–31.0) | 0.005 | 0.044 |
| Hypertension: | |||||
| Duration AHT (months); median (IQR) | 34.8 (1.9–115.4) | 13.1 (1.4–65.9) | 39.9 (5.5–192.6) | 0.307 | 0.740 |
| Secondary form of hypertension | 15/25 (60.0) | 49/166 (29.5) | 26/50 (52.0) | 0.005 | 0.625 |
| ABPM results: | |||||
| Systolic 24 h mean BP (mmHg); median (IQR) | 145 (131–162) | 132 (124–140) | 141 (126–149) | <0.0005 | 0.056 |
| Diastolic 24 h mean BP (mmHg); median (IQR) | 85 (80–99) | 81 (75–88) | 80 (73–88) | 0.018 | 0.008 |
| Systolic awake BP (mmHg); median (IQR) | 150 (135–162) | 136 (127–144) | 141 (129–150) | <0.0005 | 0.035 |
| Diastolic awake BP (mmHg); median (IQR) | 88 (82–100) | 84 (77–91) | 81 (74–90) | 0.034 | 0.008 |
| Systolic asleep BP (mmHg); median (IQR) | 140 (125–155) | 122 (114–133) | 136 (120–144) | <0.0005 | 0.114 |
| Diastolic asleep BP (mmHg); median (IQR) | 81 (75–93) | 72.0 (66.5–81.0) | 76 (66–81) | <0.0005 | 0.007 |
| Systolic 24 h mean BP ≥ 130 mmHg; n (%) | 22 (75.9) | 102 (55.7) | 37 (68.5) | 0.044 | 0.614 |
| Diastolic 24 h mean BP ≥ 80 mmHg; n (%) | 22 (75.9) | 101 (55.2) | 28 (51.9) | 0.043 | 0.037 |
| Comorbidities: | |||||
| Coronary heart disease; n (%) | 11 (37.9) | 36 (19.0) | 14 (24.6) | 0.029 | 0.217 |
| Stroke/TIA; n (%) | 6 (20.7) | 19 (10.1) | 8 (14.0) | 0.116 | 0.539 |
| Peripheral arterial disease; n (%) | 2 (6.9) | 9 (4.8) | 6 (10.9) | 0.646 | 0.708 |
| Aortic aneurysm; n (%) | 2/11 (18.2) | 2/56 (3.6) | 6/17 (35.3) | 0.123 | 0.419 |
| Diabetes; n (%) | 6 (20.7) | 25 (13.3) | 14 (24.6) | 0.268 | 0.791 |
| eGFR (ml/min/1.7); median (IQR) | 59.0 (38.0–79.0) | 93.0 (75.8–106.0) | 67.0 (47.0–90.0) | <0.0005 | 0.123 |
| Microalbuminuria ≥ 3.4 mg/mmol; n (%) | 10/17 (58.8) | 13/104 (12.5) | 11 (31.4) | <0.0005 | 0.076 |
| Active or history of smoking; n (%) | 13/26 (50.0) | 86/184 (46.7) | 28 (50.9) | 0.835 | 1.000 |
| Family history SCD; n (%) | 2/18 (11.1) | 3/164 (1.8) | 6/42 (14.3) | 0.077 | 1.000 |
| Family history AHT; n (%) | 8/17 (47.1) | 77/157 (49.0) | 18/40 (45.0) | 1.000 | 1.000 |
| Medication: | |||||
| ACE/ARB; n (%) | 23 (79.3) | 124 (66.0) | 47 (83.9) | 0.201 | 0.765 |
| CCB; n (%) | 19 (65.5) | 100 (52.9) | 40 (70.2) | 0.234 | 0.806 |
| Diuretics; n (%) | 19 (65.5) | 37 (19.7) | 35 (61.4) | <0.0005 | 0.815 |
| Beta blocker; n (%) | 16 (55.2) | 50 (26.5) | 32 (56.1) | 0.004 | 1.000 |
| Any antihypertensive; n (%) | 28 (96.6) | 156 (83.0) | 54 (96.4) | 0.090 | 1.000 |
| Monotherapy (ACE/ARB/CCB/Diuretics); n (%) | 4 (13.8) | 60 (32.3) | 9 (16.1) | 0.050 | 1.000 |
| Dual therapy (ACE/ARB/CCB/Diuretics); n (%) | 9 (31.0) | 60 (32.3) | 22 (39.3) | 1.000 | 0.487 |
| Triple therapy (ACE/ARB/CCB/Diuretics); n (%) | 12 (41.4) | 27 (14.5) | 23 (41.1) | 0.004 | 1.000 |
| ECG: | |||||
| Positive Sokolow-Lyon index; n (%) | 3 (11.1) | 4 (2.4) | 5 (8.9) | 0.056 | 0.711 |
| Negative T V4-V6 in at least 1 lead; n (%) | 5 (18.5) | 7 (4.1) | 9 (16.1) | 0.014 | 0.764 |
| Any ST-Depression; n (%) | 7 (25.9) | 18 (10.7) | 16 (28.6) | 0.055 | 1.000 |
| TTE: | |||||
| LVEF (%); median (IQR) | 56 (52–61) | 61 (59–65) | 61 (56–66) | <0.0005 | 0.037 |
| LAVI (mL/m2); median (IQR) | 37 (27–45) | 27 (23–33) | 32 (28–46) | 0.002 | 0.996 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apitz, A.; Socrates, T.; Burkard, T.; Mayr, M.; Vischer, A.S. Prevalence and Characterisation of Severe Left Ventricular Hypertrophy Diagnosed by Echocardiography in Hypertensive Patients. J. Clin. Med. 2023, 12, 228. https://doi.org/10.3390/jcm12010228
Apitz A, Socrates T, Burkard T, Mayr M, Vischer AS. Prevalence and Characterisation of Severe Left Ventricular Hypertrophy Diagnosed by Echocardiography in Hypertensive Patients. Journal of Clinical Medicine. 2023; 12(1):228. https://doi.org/10.3390/jcm12010228
Chicago/Turabian StyleApitz, Anett, Thenral Socrates, Thilo Burkard, Michael Mayr, and Annina S. Vischer. 2023. "Prevalence and Characterisation of Severe Left Ventricular Hypertrophy Diagnosed by Echocardiography in Hypertensive Patients" Journal of Clinical Medicine 12, no. 1: 228. https://doi.org/10.3390/jcm12010228
APA StyleApitz, A., Socrates, T., Burkard, T., Mayr, M., & Vischer, A. S. (2023). Prevalence and Characterisation of Severe Left Ventricular Hypertrophy Diagnosed by Echocardiography in Hypertensive Patients. Journal of Clinical Medicine, 12(1), 228. https://doi.org/10.3390/jcm12010228






