New Approach to Hyponatremia: High Prevalence of Cerebral/Renal Salt Wasting, Identification of Natriuretic Protein That Causes Salt Wasting
Abstract
:1. Introduction
2. It Is Time to Abandon the Outmoded Volume Approach
3. Pathophysiologic Approach to Evaluate Hyponatremic Patients
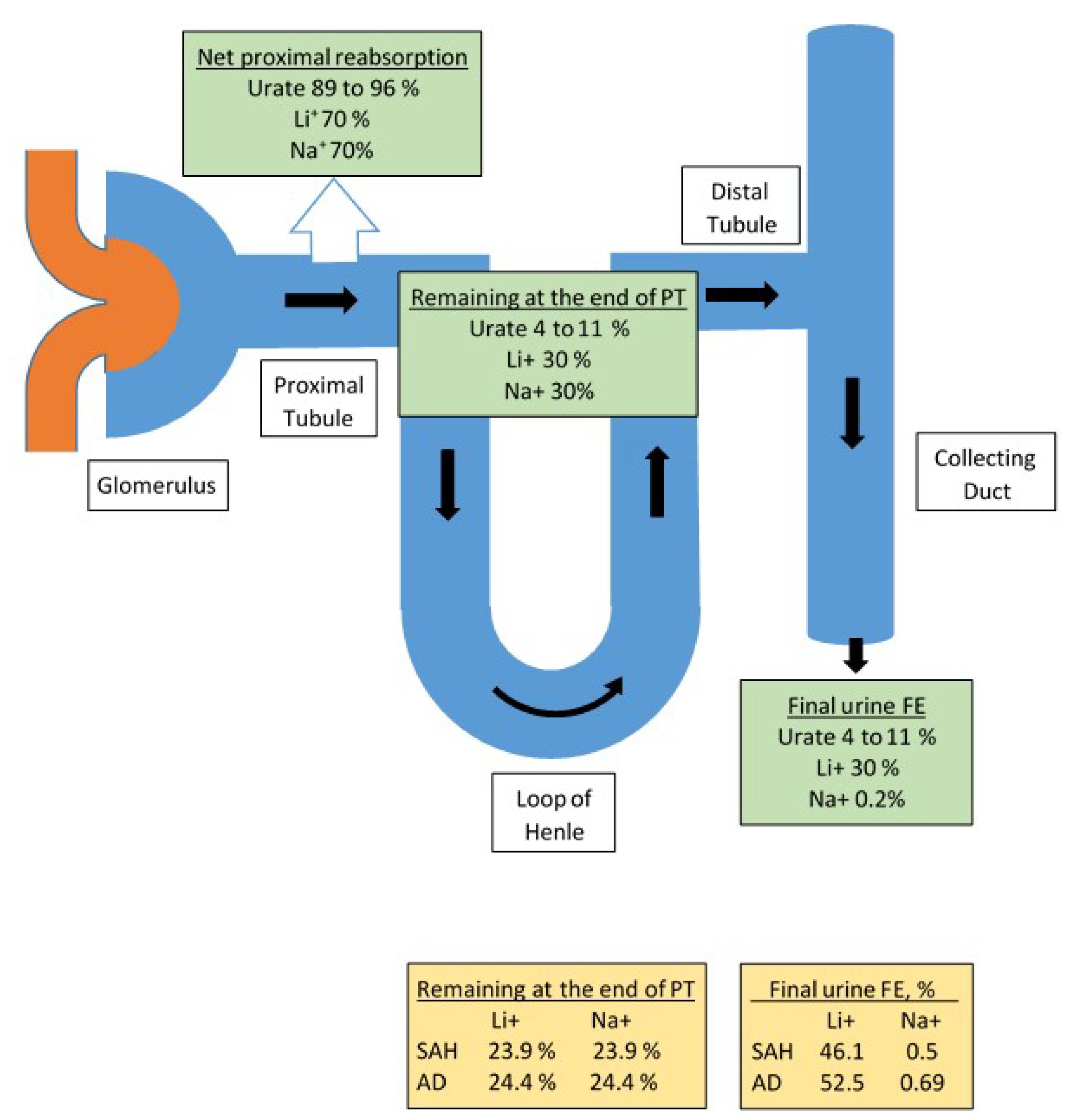
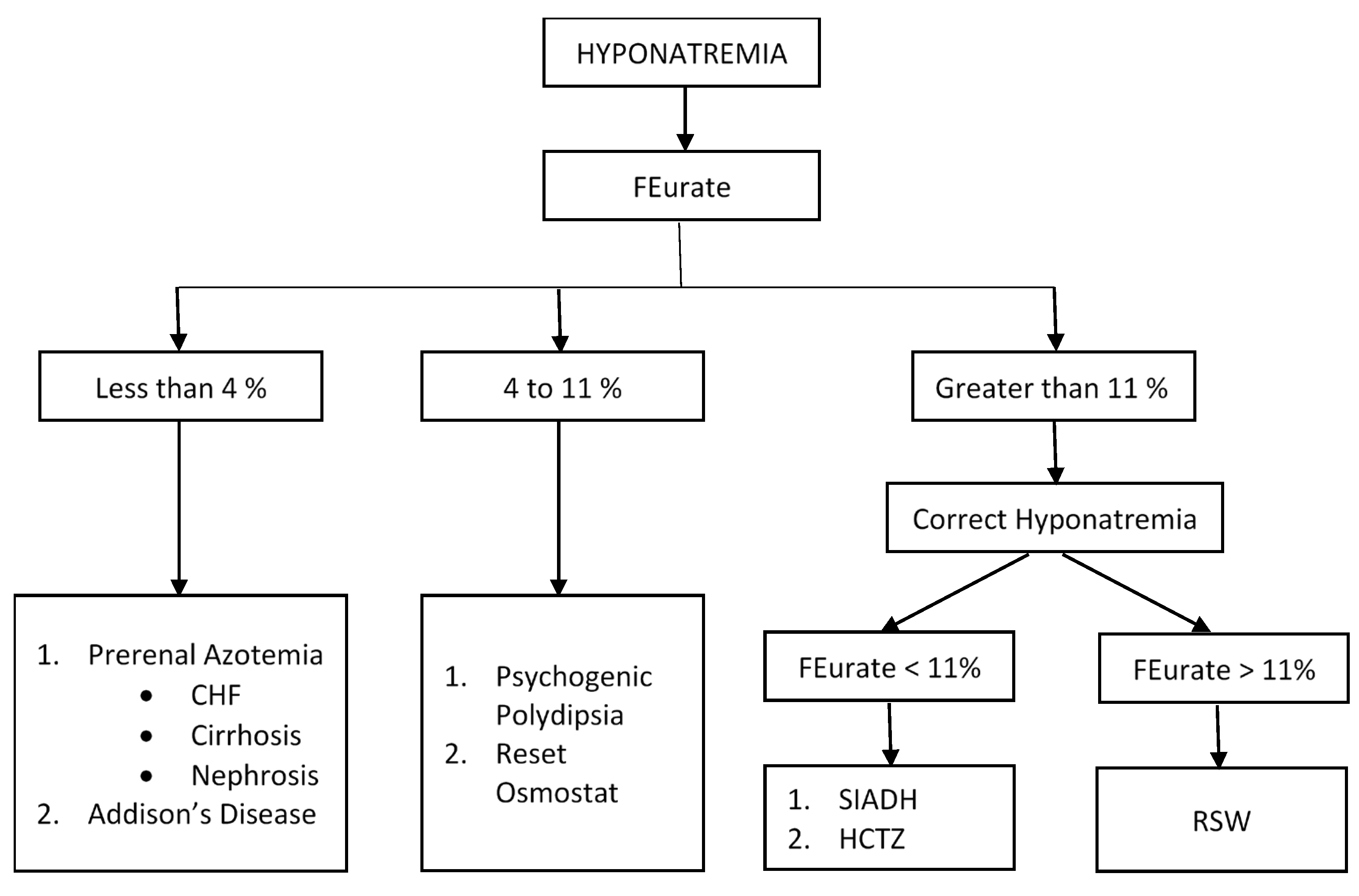
3.1. Determination of FEurate
3.1.1. Increased FEurate > 11%
3.1.2. Normal FEurate of 4–11%
3.1.3. Decreased FEurate < 4%
3.2. Response to Isotonic Infusions
4. Utilization of FEurate and Response to Isotonic Saline in Evaluation of Hyponatremic Patients
5. Pathophysiology of RSW
5.1. Initiation of Nonequilibrated Phase
5.2. Equilibrated State
6. Identification of Haptoglobin Related Protein without Signal Peptide as Natriuretic Factor in RSW
7. RSW Occurring without Hyponatremia, Especially Alzheimer’s Disease and Subarachnoid Hemorrhage
8. Conclusions
- RSW is common in the general medical wards of the hospital
- Change cerebral salt wasting to RSW. Twenty one of the 24 patients with RSW did not have clinical evidence of cerebral disease. RSW would not be considered without the presence of cerebral disease. We must incorporate this important change in nomenclature.
- RSW can occur in hyponatremic and in a potentially large number of normonatremic patients.
- We have introduced and support the conclusion that RSW is not only a new syndrome in AD but is probably common.
- Determining urine sodium concentrations in work up of hyponatremia is not as informative as professed to be. It should be viewed as being less informative.
- The identification of HPRWSP as the natriuretic protein that probably causes RSW can have the following clinical applications:
- a.
- Serve as a biomarker of RSW to simplify the diagnosis of RSW in a wide variety of comorbidities, including a new syndrome of RSW in AD, on first encounter with the patients and to deliver the appropriate management and improve clinical outcomes.
- b.
- Because increasing salt and water intake in patients with RSW will increase excretion of large urine volumes that includes a distressing nocturia, there is a need to develop an inhibitor to HPRWSP to improve outcomes.
- c.
- HPRWSP satisfies our long search for a potent proximal diuretic, which can be combined with a distal diuretic to effectively eliminate the fluid overload of congestive heart failure and improve clinical outcomes.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Corona, G.; Giuliani, C.; Parenti, G.; Norello, D.; Verbalis, J.G.; Forti, G.; Maggi, M.; Peri, A. Moderate Hyponatremia Is Associated with Increased Risk of Mortality: Evidence from a Meta-Analysis. PLOS ONE 2013, 8, e80451. [Google Scholar] [CrossRef] [PubMed]
- Arieff, A.I.; Llach, F.; Massry, S.G. Neurological Manifestations and Morbidity of Hyponatremia: Correlation with Brain Water and Electrolytes. Medicine 1976, 55, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Kengne, F.G.; Andres, C.; Sattar, L.; Melot, C.; Decaux, G. Mild hyponatremia and risk of fracture in the ambulatory elderly. QJM 2008, 101, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Renneboog, B.; Musch, W.; Vandemergel, X.; Manto, M.U.; Decaux, G. Mild Chronic Hyponatremia Is Associated With Falls, Unsteadiness, and Attention Deficits. Am. J. Med. 2006, 119, 71.e1–71.e8. [Google Scholar] [CrossRef]
- Decaux, G. Is Asymptomatic Hyponatremia Really Asymptomatic? Am. J. Med. 2006, 119, S79–S82. [Google Scholar] [CrossRef]
- Verbalis, J.G.; Barsony, J.; Sugimura, Y.; Tian, Y.; Adams, D.J.; A Carter, E.; E Resnick, H. Hyponatremia-induced osteoporosis. J. Bone Miner. Res. 2009, 25, 554–563. [Google Scholar] [CrossRef] [Green Version]
- Chung, H.-M.; Kluge, R.; Schrier, R.W.; Anderson, R.J. Clinical assessment of extracellular fluid volume in hyponatremia. Am. J. Med. 1987, 83, 905–908. [Google Scholar] [CrossRef]
- Singh, S.; Bohn, D.; Carlotti, A.P.C.P.; Cusimano, M.; Rutka, J.T.; Halperin, M.L. Cerebral salt wasting: Truths, fallacies, theories, and challenges. Crit. Care Med. 2002, 30, 2575–2579. [Google Scholar] [CrossRef]
- Schwartz, W.B.; Bennett, W.; Curelop, S.; Bartter, F.C. A syndrome of renal sodium loss and hyponatremia probably resulting from inappropriate secretion of antidiuretic hormone. Am. J. Med. 1957, 23, 529–542. [Google Scholar] [CrossRef]
- Bitew, S.; Imbriano, L.; Miyawaki, N.; Fishbane, S.; Maesaka, J.K. More on Renal Salt Wasting Without Cerebral Disease: Response to Saline Infusion. Clin. J. Am. Soc. Nephrol. 2009, 4, 309–315. [Google Scholar] [CrossRef]
- Nelson, P.B.; Seif, S.M.; Maroon, J.C.; Robinson, A.G. Hyponatremia in intracranial disease: Perhaps not the syndrome of inappropriate secretion of antidiuretic hormone (SIADH). J. Neurosurg. 1981, 55, 938–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wijdicks, E.F.M.; Vermeulen, M.; Haaf, J.A.T.; Hijidra, A.; Bakker, W.H.; van Gijn, J. Volume depletion and natriuresis in patients with a ruptured intracranial aneurysm. Ann. Neurol. 1985, 18, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Verbalis, J.G. Hyponatremia With Intracranial Disease: Not Often Cerebral Salt Wasting. J. Clin. Endocrinol. Metab. 2014, 99, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Verbalis, J.G. The Curious Story of Cerebral Salt Wasting: Fact or Fiction? Clin. J. Am. Soc. Nephrol. 2020, 15. [Google Scholar] [CrossRef]
- Peters, J.P.; Welt, L.G.; Sims, E.A.H.; Orloff, J.; Needham, J. A salt-wasting syndrome associated with cerebral disease. Trans. Assoc. Am. Physicians 1950, 63, 57–64. [Google Scholar]
- Maesaka, J.K.; Imbriano, L.; Shirazian, S.; Miyawaki, N. Complexity of differentiating cerebral-renal salt wasting from SIADH, emerging importance of determining fractional urate excretion. In Novel Insights on Chronic Kidney Disease, Acute Kidney Injury and Polycystic Kidney Disease; Vijayakumar, S., Ed.; InTech: Rijeka, Croatia, 2012; pp. 41–66. [Google Scholar]
- Maesaka, J.K.; Imbriano, L.J.; Miyawaki, N. High Prevalence of Renal Salt Wasting Without Cerebral Disease as Cause of Hyponatremia in General Medical Wards. Am. J. Med Sci. 2018, 356, 15–22. [Google Scholar] [CrossRef]
- Maesaka, J.K.; Imbriano, L.J.; Pinkhasov, A.; Muralidharan, R.; Song, X.; Russo, L.M.; Comper, W.D. Identification of a Novel Natriuretic Protein in Patients With Cerebral-Renal Salt Wasting—Implications for Enhanced Diagnosis. Am. J. Med Sci. 2021, 361, 261–268. [Google Scholar] [CrossRef]
- Beck, L.H. Hypouricemia in the Syndrome of Inappropriate Secretion of Antidiuretic Hormone. N. Engl. J. Med. 1979, 301, 528–530. [Google Scholar] [CrossRef]
- Assadi, F.K.; John, E.G. Hypouricemia in Neonates with Syndrome of Inappropriate Secretion of Antidiuretic Hormone. Pediatr. Res. 1985, 19, 424–427. [Google Scholar] [CrossRef] [Green Version]
- Mees, E.J.D.; Van Assendelft, P.B.; Nieuwenhuis, M.G. Elevation of uric acid clearance caused by inappropriate antidiuretic hormone secretion. Acta Medica Scand. 1971, 189, 69–72. [Google Scholar] [CrossRef]
- Sonnenblick, M.; Rosin, A. Increased uric acid clearance in the syndrome of inappropriate secretion of antidiuretic hormone. Isr. J. Med Sci. 1988, 24, 20–23. [Google Scholar] [PubMed]
- Imbriano, L.J.; Mattana, J.; Drakakis, J.; Maesaka, J.K. Identifying Different Causes of Hyponatremia With Fractional Excretion of Uric Acid. Am. J. Med Sci. 2016, 352, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Maesaka, J.K.; Batuman, V.; Yudd, M.; Salem, M.; Sved, A.F.; Venkatesan, J. Hyponatremia and hypouricemia: Differentiation from SIADH. Clin. Nephrol. 1990, 33, 174–178. [Google Scholar] [PubMed]
- Maesaka, J.; Miyawaki, N.; Palaia, T.; Fishbane, S.; Durham, J. Renal salt wasting without cerebral disease: Diagnostic value of urate determinations in hyponatremia. Kidney Int. 2007, 71, 822–826. [Google Scholar] [CrossRef] [Green Version]
- Bassi, V.; Fattoruso, O. The Role of Fractional Excretion of Uric Acid in the Differential Diagnosis of Hypotonic Hyponatraemia in Patients with Diuretic Therapy. Cureus 2020, 12, e7762. [Google Scholar] [CrossRef] [Green Version]
- Imbriano, L.J.; Ilamathi, E.; Ali, N.M.; Miyawaki, N.; Maesaka, J.K. Normal fractional urate excretion identifies hyponatremic patients with reset osmostat. J. Nephrol. 2012, 25, 833–838. [Google Scholar] [CrossRef]
- Robertson, G.L. Regulation of Arginine Vasopressin in the Syndrome of Inappropriate Antidiuresis. Am. J. Med. 2006, 119, S36–S42. [Google Scholar] [CrossRef]
- Ali, N.; Imbriano, L.J.; Maesaka, J.K. The Case ∣ A 66-year-old male with hyponatremia. Psychogenic Polydipsia Kidney Int. 2009, 76, 233–234. [Google Scholar] [CrossRef] [Green Version]
- Weinman, E.J.; Steplock, D.; Suki, W.N.; Eknoyan, G. Urate reabsorption in proximal convoluted tubule of the rat kidney. Am. J. Physiol. 1976, 231, 509–515. [Google Scholar] [CrossRef] [Green Version]
- Stricker, E.M.; Verbalis, J. Water intake and body fluids. In Fundamental Neuroscience, 2nd ed.; Squire, L.E., Roberts, J.L., Spitzer, N.C., Zigmond, M.J., McConnell, S.K., Bloom, F.E., Eds.; Elsevier: La Jolla, CA, USA, 2003; pp. 1011–1029. [Google Scholar]
- Abuelo, J.G. Normotensive Ischemic Acute Renal Failure. N. Engl. J. Med. 2007, 357, 797–805. [Google Scholar] [CrossRef] [Green Version]
- Papierska, L.; Rabijewski, M. Delay in Diagnosis of Adrenal Insufficiency Is a Frequent Cause of Adrenal Crisis. Int. J. Endocrinol. 2013, 2013, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Saevik, B.; Åkerman, A.-K.; Grønning, K.; Nermoen, I.; Valland, S.F.; Finnes, T.E.; Isaksson, M.; Dahlqvist, P.; Bergthorsdottir, R.; Ekwall, O.; et al. Clues for early detection of autoimmune Addison’s disease-myths and realities. J. Intern. Med. 2018, 283, 190–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartter, F.C.; Schwartz, W.B. The syndrome of inappropriate secretion of antidiuretic hormone. Am. J. Med. 1967, 42, 790–806. [Google Scholar] [CrossRef] [PubMed]
- Sterns, R.H. Disorders of Plasma Sodium — Causes, Consequences, and Correction. New Engl. J. Med. 2015, 372, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Maesaka, J.K.; Imbriano, L.J.; Ali, N.M.; Ilamathi, E. Is it cerebral or renal salt wasting? Kidney Int. 2009, 76, 934–938. [Google Scholar] [CrossRef] [Green Version]
- Milionis, H.J.; Liamis, G.L.; Elisaf, M.S. The hyponatremic patient: A systematic approach to laboratory diagnosis. CMAJ 2002, 166, 1056–1118. [Google Scholar]
- Musch, W.; Decaux, G. Utility and limitations of biochemical parameters in the evaluation of hyponatremia in the elderly. Int. Urol. Nephrol. 2001, 32, 475–493. [Google Scholar] [CrossRef]
- Wijdicks, E.F.M.; Vermeulen, M.; Hijdra, A.; van Gijn, J. Hyponatremia and cerebral infarction in patients with ruptured intracranial aneurysms: Is fluid restriction harmful? Ann. Neurol. 1985, 17, 137–140. [Google Scholar] [CrossRef]
- Leaf, A.; Bartter, F.C.; Santos, R.F.; Wrong, O. Evidence in man that urinary electrolyte loss induced by pitressin is a function of water retention. J. Clin. Investig. 1953, 32, 868–878. [Google Scholar] [CrossRef] [Green Version]
- Levinsky, N.G.; Davidson, D.G.; Berliner, R.W. Changes in urine concentration during prolonged administration of vasopressin and water. Am. J. Physiol. 1959, 196, 451–456. [Google Scholar] [CrossRef]
- Maesaka, J.K.; Venkatesan, J.; Piccione, J.M.; Decker, R.; Dreisbach, A.W.; Wetherington, J. Plasma natriuretic factor(s) in patients with intracranial disease, renal salt wasting and hyperuricosuria. Life Sci. 1993, 52, 1875–1882. [Google Scholar] [CrossRef] [PubMed]
- Maesaka, J.K.; Wolf-Klein, G.; Piccione, J.M.; Ma, C.M. Hypouricemia, Abnormal Renal Tubular Urate Transport, and Plasma Natriuretic Factor(s) in Patients with Alzheimer’s Disease. J. Am. Geriatr. Soc. 1993, 41, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Hayslett, J.P.; Kashgarian, M. A micropuncture study of the renal handling of lithium. Pflugers Arch. 1979, 380, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Mullens, W.; Dauw, J.; Martens, P.; Verbrugge, F.H.; Nijst, P.; Meekers, E.; Tartaglia, K.; Chenot, F.; Moubayed, S.; Dierckx, R.; et al. Acetazolamide in Acute Decompensated Heart Failure with Volume Overload. N. Engl. J. Med. 2022, 387, 1185–1195. [Google Scholar] [CrossRef]
- Maesaka, J.K.; Imbriano, L.J. Cerebral Salt Wasting is a real cause of hyponatremia: PRO. Kidney360 2022. ahead of print. [Google Scholar] [CrossRef]




|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maesaka, J.K.; Imbriano, L.J.; Grant, C.; Miyawaki, N. New Approach to Hyponatremia: High Prevalence of Cerebral/Renal Salt Wasting, Identification of Natriuretic Protein That Causes Salt Wasting. J. Clin. Med. 2022, 11, 7445. https://doi.org/10.3390/jcm11247445
Maesaka JK, Imbriano LJ, Grant C, Miyawaki N. New Approach to Hyponatremia: High Prevalence of Cerebral/Renal Salt Wasting, Identification of Natriuretic Protein That Causes Salt Wasting. Journal of Clinical Medicine. 2022; 11(24):7445. https://doi.org/10.3390/jcm11247445
Chicago/Turabian StyleMaesaka, John K., Louis J. Imbriano, Candace Grant, and Nobuyuki Miyawaki. 2022. "New Approach to Hyponatremia: High Prevalence of Cerebral/Renal Salt Wasting, Identification of Natriuretic Protein That Causes Salt Wasting" Journal of Clinical Medicine 11, no. 24: 7445. https://doi.org/10.3390/jcm11247445
APA StyleMaesaka, J. K., Imbriano, L. J., Grant, C., & Miyawaki, N. (2022). New Approach to Hyponatremia: High Prevalence of Cerebral/Renal Salt Wasting, Identification of Natriuretic Protein That Causes Salt Wasting. Journal of Clinical Medicine, 11(24), 7445. https://doi.org/10.3390/jcm11247445







