Metal Allergy Mediates the Development of Oral Lichen Planus via TSLP-TSLPR Signaling
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Antibodies
2.3. Histology and Immunohistochemistry
2.4. Analysis of Immunohistochemistry Staining
2.5. Cell Culture
2.6. Quantitative Real-Time PCR Reactions and Primers
2.7. ELISA
2.8. Statistical Analysis
3. Results
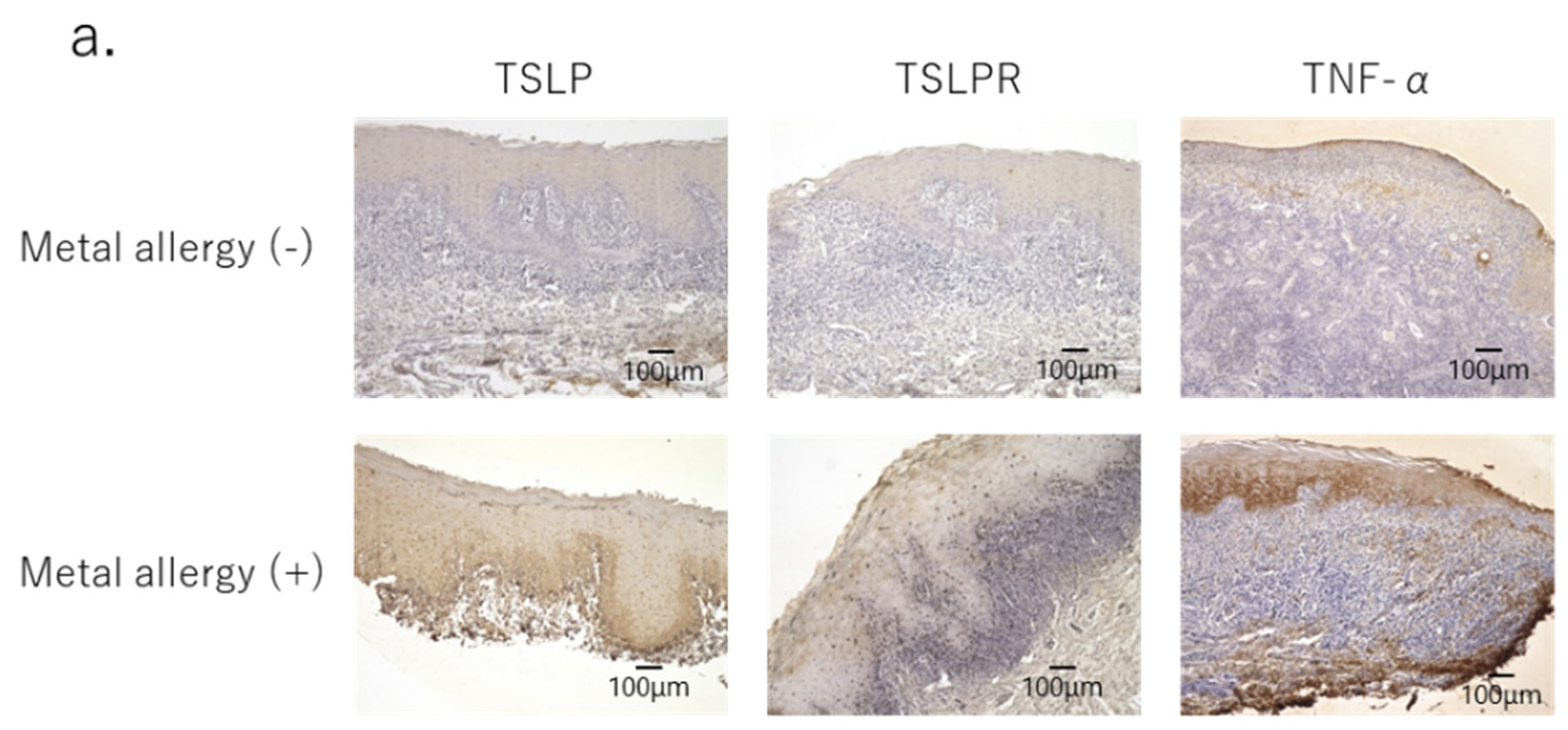
3.1. Profiles of Infiltrated Cells in Mucosa of OLP Lesions
3.2. Pro-Inflammatory Cytokines Produced by Cultured HaCaT Cells Stimulated with NiCl2
3.3. Secretion of Pro-Inflammatory Cytokines in OLP Lesions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Büdinger, L.; Hertl, M. Immunologic mechanisms in hypersensitivity reactions to metal ions: An overview. Allergy 2000, 55, 108–115. [Google Scholar] [CrossRef]
- Martin, S.F.; Jakob, T. From innate to adaptive immune responses in contact hypersensitivity. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 289–293. [Google Scholar] [CrossRef]
- Riedel, F.; Aparicio-Soto, M.; Curato, C.; Thierse, H.J.; Siewert, K.; Luch, A. Immunological Mechanisms of Metal Allergies and the Nickel-Specific TCR-pMHC Interface. Int. J. Environ. Res. Public Health 2021, 18, 10867. [Google Scholar] [CrossRef]
- Rasool, F.; Akhtar, S.; Hassan, I.; Zeerak, S.; Mubashir, S.; Sheikh, G. Common Contact Allergens in Patients with Palmoplantar and Scalp Psoriasis and Impact of their Avoidance on Dermatology Life Quality Index: A Hospital-Based Study. Indian J. Dermatol. 2018, 63, 160–164. [Google Scholar]
- Quarant, M.; Eyeric, S.; Knapp, B.; Nasorri, F.; Scarponim, C.; Mattii, M.; Garzorz, N.; Harlfinger, A.T.; Jaeger, T.; Grosber, G.; et al. Allergic Contact Dermatitis in Psoriasis Patients: Typical, Delayed, and Non-Interacting. PLoS ONE 2014, 9, e101814. [Google Scholar] [CrossRef]
- Vernetti, A.M.B.; Puntoni, M.; Massone, C. Palmoplantar Pustulosis and Allergies: A Systematic Review. Dermatol. Pr. Concept. 2019, 9, 105–110. [Google Scholar] [CrossRef]
- Scalf, L.A.; Fowler, J.F., Jr.; Morgan, K.W.; Looney, S.W. Dental metal allergy in patients with oral, cutaneous, and genital lichenoid reactions. Am. J. Contact Dermat. 2001, 12, 146–150. [Google Scholar] [PubMed]
- Sugerman, P.B.; Savage, N.W.; Walsh, L.J.; Zhao, Z.Z.; Zhou, X.J.; Khan, A.; Seymour, G.J.; Bigby, M. The pathogenesis of oral lichen planus. Crit. Rev. Oral Biol. Med. 2002, 13, 350–365. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.J.; Sugerman, P.B.; Savage, N.W.; Walsh, L.J.; Seymour, G.J. Intra-epithelial CD8+ T cells and basement membrane dis-ruption in oral lichen planus. J. Oral Pathol. Med. 2002, 31, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, J.; Fu, S.; Wang, C.; Zhou, B. A Study of Association between Oral Lichen Planus and Immune Balance of Th1/Th2 Cells. Inflammation 2015, 38, 1874–1879. [Google Scholar] [CrossRef]
- Lee, H.-C.; Ziegler, S.F. Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFκB. Proc. Natl. Acad. Sci. USA 2007, 104, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Favoreto, S., Jr.; Avilla, P.C.; Schleimer, R.P. TLR3- and Th2 Cytokine-Dependent Production of Thymic Stromal Lymphopoietin in Human Airway Epithelial Cells. J. Immunol. 2007, 179, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Soumelis, V.; Reche, P.A.; Kanzler, H.; Yuan, W.; Edward, G.; Homey, B.; Gilliet, M.; Ho, S.; Antonenko, S.; Lauerma, A.; et al. Human epithelial cells trigger dendritic cell–mediated allergic inflammation by producing TSLP. Nat. Immunol. 2002, 3, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-J.; Soumelis, V.; Watanabe, N.; Ito, T.; Wang, Y.-H.; de Waal Malefyt, R.; Omori, M.; Zhou, B.; Ziegler, S.F. TSLP: An Epithelial Cell Cytokine that Regulates T Cell Differentiation by Conditioning Dendritic Cell Maturation. Annu. Rev. Immunol. 2007, 25, 193–219. [Google Scholar] [CrossRef]
- Ziegler, S.F.; Artis, D. Sensing the outside world: TSLP regulates barrier immunity. Nat. Immunol. 2010, 11, 289–293. [Google Scholar] [CrossRef]
- Liu, Y.-J. Thymic stromal lymphopoietin: Master switch for allergic inflammation. J. Exp. Med. 2006, 203, 269–273. [Google Scholar] [CrossRef]
- Holgate, S.T.; Arshad, H.S.; Roberts, G.C.; Howarth, P.H.; Thurner, P.; Davies, D.E. A new look at the pathogenesis of asthma. Clin. Sci. 2009, 118, 439–450. [Google Scholar] [CrossRef]
- Yoo, J.; Omori, M.; Gyarmati, D.; Zhou, B.; Aye, T.; Brewer, A.; Comeau, M.R.; Campbell, D.J.; Ziegler, S.F. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J. Exp. Med. 2005, 202, 541–549. [Google Scholar] [CrossRef]
- Ito, T.; Wang, Y.H.; Duramad, O.; Hori, T.; Delespesse, G.J.; Watanabe, N.; Qin, F.X.; Yao, Z.; Cao, W.; Liu, Y.J. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J. Exp. Med. 2005, 202, 1213–1223. [Google Scholar] [CrossRef]
- Al-Shami, A.; Spolski, R.; Kelly, J.; Keane-Myers, A.; Leonard, W.J. A role for TSLP in the development of inflammation in an asthma model. J. Exp. Med. 2005, 202, 829–839. [Google Scholar] [CrossRef]
- Omori, M.; Ziegler, S. Induction of IL-4 expression in CD4(+) T cells by thymic stromal lymphopoietin. J. Immunol. 2007, 178, 1396–1404. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Ishimaru, N.; Ashrin, M.N.; Arakaki, R.; Yamada, A.; Ichikawa, T.; Hayashi, Y. A novel DC therapy with manipulation of MKK6 gene on nickel allergy in mice. PLoS ONE 2011, 6, e19017. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Arakaki, R.; Yamada, A.; Tsunematsu, T.; Kudo, Y.; Ishimaru, N. Molecular Mechanisms of Nickel Allergy. Int. J. Mol. Sci. 2016, 17, 202. [Google Scholar] [CrossRef]
- Ashrin, M.N.; Arakaki, R.; Yamada, A.; Kondo, T.; Kurosawa, M.; Kudo, Y.; Watanabe, M.; Ichikawa, T.; Hayashi, Y.; Ishimaru, N. A Critical Role for Thymic Stromal Lymphopoietin in Nickel-Induced Allergy in Mice. J. Immunol. 2014, 192, 4025–4031. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Z.; Savage, N.W.; Pujic, Z.; Walsh, L. Immunohistochemical localization of mast cells and mast cell-nerve interactions in oral lichen planus. Oral Dis. 2008, 3, 71–76. [Google Scholar] [CrossRef]
- Zhou, X.J.; Sugerman, P.B.; Savage, N.W.; Walsh, L. Matrix metalloproteinases and their inhibitors in oral lichen planus. J. Cutan. Pathol. 2001, 28, 72–82. [Google Scholar] [CrossRef]
- Juneja, M.; Mahajan, S.; Rao, N.N.; George, T.; Boaz, K. Histochemical analysis of pathological alterations in oral lichen planus and oral lichenoid reactions. J. Oral Sci. 2006, 48, 185–193. [Google Scholar] [CrossRef]
- Jontell, M.; Hansson, H.-A.; Nygren, H. Mast cells in oral lichen planus. J. Oral Pathol. Med. 1986, 15, 273–275. [Google Scholar] [CrossRef]
- Hosoki, M.; Tanoue, N.; Watanabe, M.; Yamase, M.; Iida, S.; Maida, T.; Oodaira, C.; Ookubo, C.; Akiba, Y.; Hattori, M. A Multi-Institutional Survey of Clinical Symptoms and Prosthodontic Treatment for Metal Allergy to Dental Materials. J. Jpn. Assoc. Dent. Sci. 2015, 34, 44–48. (In Japanese) [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yunizar, M.F.; Watanabe, M.; Liu, L.; Minami, N.; Ichikawa, T. Metal Allergy Mediates the Development of Oral Lichen Planus via TSLP-TSLPR Signaling. J. Clin. Med. 2022, 11, 519. https://doi.org/10.3390/jcm11030519
Yunizar MF, Watanabe M, Liu L, Minami N, Ichikawa T. Metal Allergy Mediates the Development of Oral Lichen Planus via TSLP-TSLPR Signaling. Journal of Clinical Medicine. 2022; 11(3):519. https://doi.org/10.3390/jcm11030519
Chicago/Turabian StyleYunizar, Mohammad Fadyl, Megumi Watanabe, Lipei Liu, Norikazu Minami, and Tetsuo Ichikawa. 2022. "Metal Allergy Mediates the Development of Oral Lichen Planus via TSLP-TSLPR Signaling" Journal of Clinical Medicine 11, no. 3: 519. https://doi.org/10.3390/jcm11030519
APA StyleYunizar, M. F., Watanabe, M., Liu, L., Minami, N., & Ichikawa, T. (2022). Metal Allergy Mediates the Development of Oral Lichen Planus via TSLP-TSLPR Signaling. Journal of Clinical Medicine, 11(3), 519. https://doi.org/10.3390/jcm11030519






