Utility of Simultaneous Biatrial Atrial Anti-Tachycardia Pacing for the Termination of Atrial Fibrillation during Catheter Ablation of Atrial Fibrillation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. AF Ablation Protocol
2.3. Pacing Protocol
- A.
- Th1. Decremental pacing: 91% of AF CL × 13 beats, reduce by 10 ms (down to 150 ms)
- B.
- Th2. Straight pacing: 84% of AF CL × 13 beats, reduce by 10 ms (down to 150 ms)
- C.
- Th3. Decremental pacing: 81% of AF CL × 13 beats, reduce by 10 ms (down to 150 ms)
- D.
- Th4. Straight pacing: 84% of AF CL × 20 beats, reduce by 10 ms (down to 150 ms)
- E.
- Th5. Decremental pacing: 81% of AF CL × 20 beats, reduce by 10 ms (down to 150 ms)
2.4. Electrocardiogram Acquisition
2.5. Continuous Wavelet Transform (CWT) Analysis
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
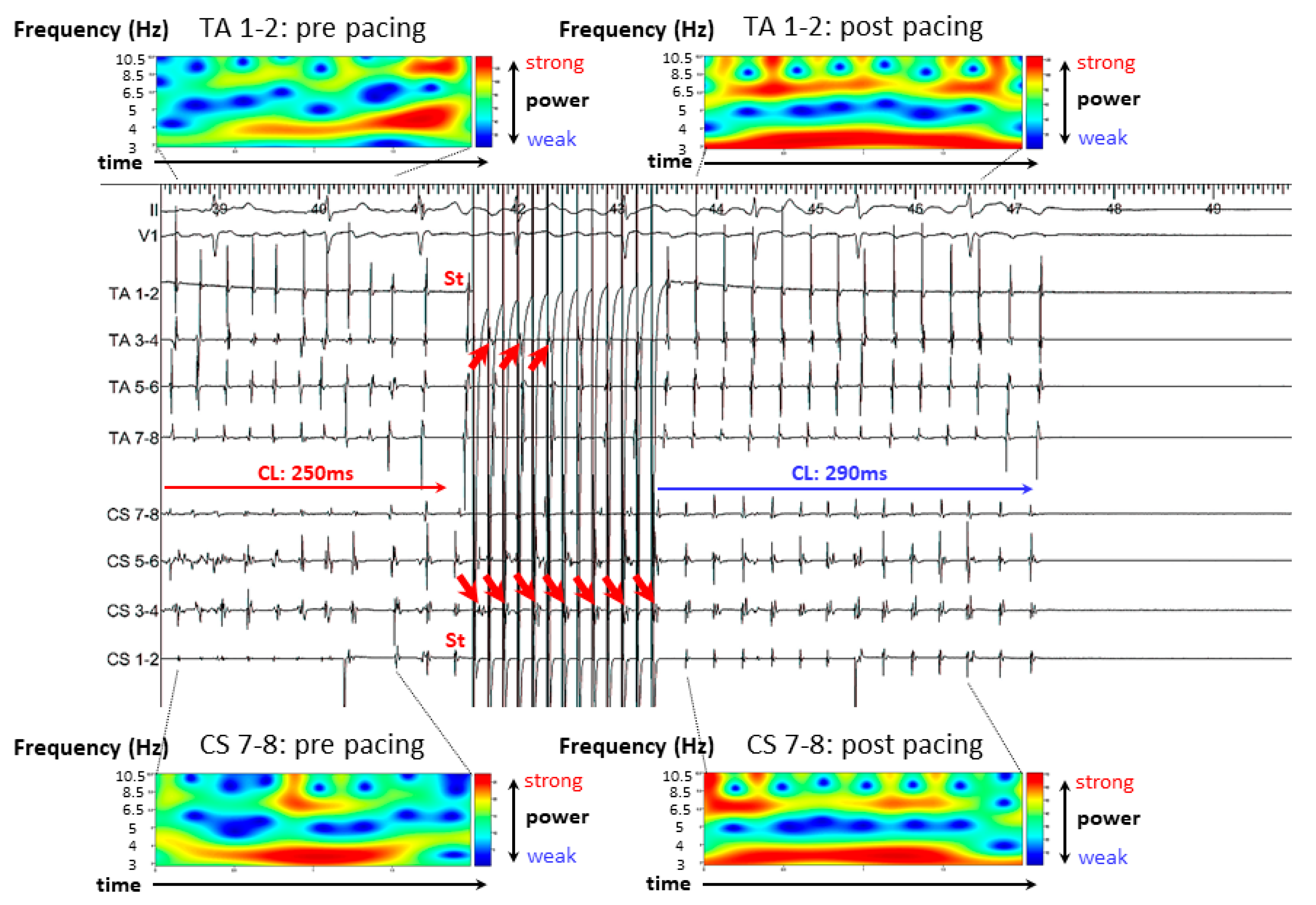
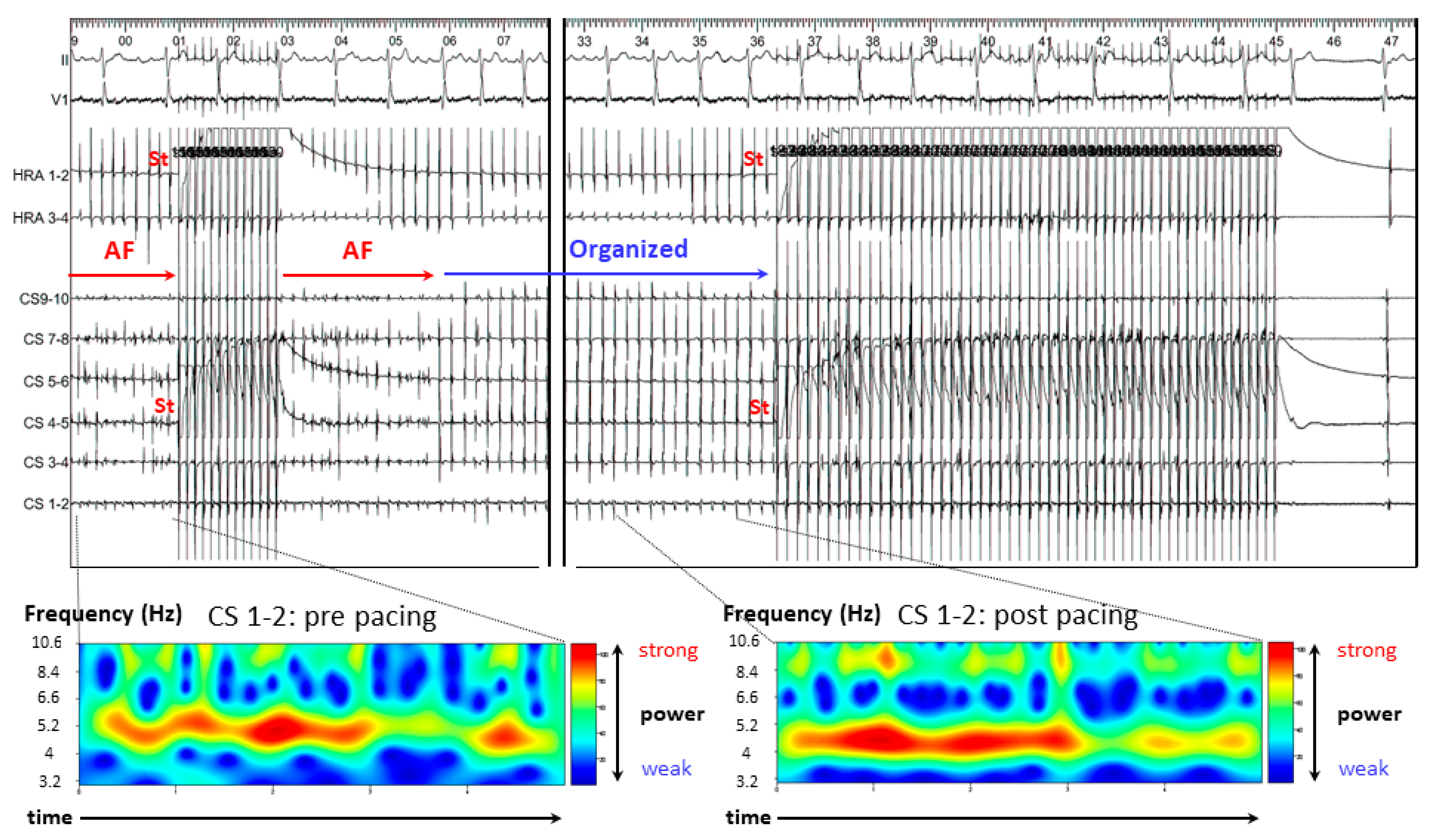
3.2. Representative Cases
3.2.1. Case 1: 42-Year-Old Man with Symptomatic PAF
3.2.2. Case 2: 72-Year-Old Man with Symptomatic PAF
3.2.3. Case 3: 67-Year-Old Man with Symptomatic PAF
3.3. Results of A-ATP
3.4. Mean AF CL
3.5. CoV of the DFs
4. Discussion
4.1. Mechanism of AF Termination
- (A)
- (B)
- (C)
- Leading circle theory [14]; The presence of an excitable gap is well known for the maintenance of AF. Pacing stimuli entrain and fill the excitable gaps of tachycardia, affecting the refractory period. Eventually, AF is unable to sustain itself and thus terminates slowly.


4.2. Clinical Implications
4.3. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boriani, G.; Glotzer, T.V.; Santini, M.; West, T.M.; Melis, M.D.; Sepsi, M.; Gasparini, M.; Lewalter, T.; Camm, J.A.; Singer, D.E. Device-detected atrial fibrillation and risk for stroke: An analysis of >10,000 patients from the SOS AF project (Stroke preventiOn Strategies based on Atrial Fibrillation information from implanted devices). Eur. Heart J. 2014, 35, 508–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boriani, G.; Tukkie, R.; Manolis, A.S.; Mont, L.; Pürerfellner, H.; Santini, M.; Inama, G.; Serra, P.; de Sousa, J.; Botto, G.L.; et al. Atrial antitachycardia pacing and managed ventricular pacing in bradycardia patients with paroxysmal or persistent atrial tachyarrhythmias: The MINERVA randomized multicentre international trial. Eur. Heart J. 2014, 35, 2352–2562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camm, A.J.; Lip, G.Y.; De Caterina, R.; Savelieva, I.; Atar, D.; Hohnloser, S.H.; Hindricks, G.; Kirchhof, P. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: An update of the 2010 ESC Guidelines for the management of atrial fibrillation—Developed with the special contribution of the European Heart Rhythm Association. Europace 2012, 14, 1385–1413. [Google Scholar] [PubMed]
- January, C.T.; Wann, L.S.; Alpert, J.S.; Calkins, H.; Cleveland, J.C., Jr.; Cigarroa, J.E.; Conti, J.B.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014, 130, e199–e267. [Google Scholar] [CrossRef] [PubMed]
- Kimata, A.; Yokoyama, Y.; Aita, S.; Nakamura, H.; Higuchi, K.; Tanaka, Y.; Nogami, A.; Hirao, K.; Aonuma, K. Temporally stable frequency mapping using continuous wavelet transform analysis in patients with persistent atrial fibrillation. J. Cardiovasc. Electrophysiol. 2018, 29, 514–522. [Google Scholar] [CrossRef]
- Higuchi, K.; Iwai, S.; Yokoyama, Y.; Hirao, K. Persistent left superior vena cava as a perpetuator of atrial fibrillation: Frequency analysis using continuous wavelet transform analysis. J. Cardiovasc. Electrophysiol. 2019, 30, 1701–1705. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Goya, M.; Yagishita, A.; Takahashi, Y.; Kawabata, M.; Casado Arroyo, R.; Hirao, K. Atrial anti-tachycardia pacing resulting in termination of atrial flutter: Intracardiac electrograms providing insight into the mechanism of arrhythmia termination. J. Int. Med. Res. 2019, 47, 3389–3393. [Google Scholar] [CrossRef] [PubMed]
- Jonathan, W.W.; Mark, E.J. Mechanisms of Atrial Fibrillation—Reentry, Rotors and Reality. Arrhythm. Electrophysiol. Rev. 2014, 3, 90–100. [Google Scholar]
- Moe, G.K. On the multiple wavelet hypothesis of atrial fibrillation. Arch. Int. Pharmacodyn. Ther. 1962, 140, 183–188. [Google Scholar]
- Carrick, R.T.; Benson, B.E.; Habel, N.; Bates, O.R.J.; Bates, J.H.T.; Spector, P.S. Ablation of multi wavelet reentry guided by circuit-density and distribution: Maximizing the probability of circuit annihilation. Circ. Arrhythm. Electrophysiol. 2013, 6, 1229–1235. [Google Scholar] [CrossRef] [Green Version]
- Benson, B.E.; Carrick, R.; Habel, N.; Bates, O.; Bates, J.H.T.; Bielau, P.; Spector, P. Mapping multi-wavelet reentry without isochrones: An electrogram-guided approach to define substrate distribution. Europace 2014, 16, iv102–iv109. [Google Scholar] [CrossRef] [Green Version]
- Jalife, J.; Berenfeld, O.; Mansour, M. Mother rotors and fibrillatory conduction: A mechanism of atrial fibrillation. Cardiovasc. Res. 2002, 54, 204–216. [Google Scholar] [CrossRef] [Green Version]
- Narayan, S.M.; Krummen, D.E.; Rappel, W.J. Clinical mapping approach to diagnose electrical rotors and focal impulse sources for human atrial fibrillation. J. Cardiovasc. Electrophysiol. 2012, 23, 447–454. [Google Scholar] [CrossRef] [Green Version]
- Allessie, M.A.; Bonke, F.I.; Schopman, F.J. Circus movement in rabbit atrial muscle as a mechanism of tachycardia. III. The “leading circle” concept: A new model of circus movement in cardiac tissue without the involvement of an anatomical obstacle. Circ. Res. 1977, 41, 9–18. [Google Scholar] [CrossRef] [Green Version]





| PAF (n = 21) | PEF (n = 20) | p Value | |
|---|---|---|---|
| Age | 65 ± 12 | 60 ± 11 | 0.26 |
| Male, n (%) | 19 (90) | 15 (75) | 0.19 |
| Diabetes mellitus, n (%) | 2 (14) | 6 (30) | 0.10 |
| Hypertension, n (%) | 13 (62) | 8 (40) | 0.16 |
| Congestive heart failure, n (%) | 0 (0) | 3 (15) | 0.07 |
| Stroke, n (%) | 3 (14) | 3 (15) | 0.95 |
| Age > 75 y.o., n (%) | 5 (24) | 1 (5) | 0.09 |
| Chronic kidney disease, n (%) | 3 (14) | 3 (15) | 0.95 |
| Coronary artery disease, n (%) | 4 (19) | 3 (15) | 0.73 |
| CHADS2 score | 1.3 ± 1.0 | 1.3 ± 1.4 | 0.90 |
| Beta-blocker | 8 | 13 | 0.08 |
| Anti-arrhythmic drug | 13 | 7 | 0.08 |
| Pilsicainide | 5 | 1 | 0.09 |
| Flecainide | 5 | 2 | 0.24 |
| Bepridil | 3 | 4 | 0.63 |
| LA diameter, (mm) | 40 ± 5 | 46 ± 7 | 0.02 |
| Ejection Fraction, (%) | 65 ± 6 | 63 ± 6 | 0.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maeda, S.; Goya, M.; Shirai, Y.; Yagishita, A.; Tao, S.; Liang, J.J.; Casado Arroyo, R.; Takahashi, Y.; Kawabata, M.; Sasano, T.; et al. Utility of Simultaneous Biatrial Atrial Anti-Tachycardia Pacing for the Termination of Atrial Fibrillation during Catheter Ablation of Atrial Fibrillation. J. Clin. Med. 2022, 11, 575. https://doi.org/10.3390/jcm11030575
Maeda S, Goya M, Shirai Y, Yagishita A, Tao S, Liang JJ, Casado Arroyo R, Takahashi Y, Kawabata M, Sasano T, et al. Utility of Simultaneous Biatrial Atrial Anti-Tachycardia Pacing for the Termination of Atrial Fibrillation during Catheter Ablation of Atrial Fibrillation. Journal of Clinical Medicine. 2022; 11(3):575. https://doi.org/10.3390/jcm11030575
Chicago/Turabian StyleMaeda, Shingo, Masahiko Goya, Yasuhiro Shirai, Atsuhiko Yagishita, Susumu Tao, Jackson Jeikai Liang, Ruben Casado Arroyo, Yoshihide Takahashi, Mihoko Kawabata, Tetsuo Sasano, and et al. 2022. "Utility of Simultaneous Biatrial Atrial Anti-Tachycardia Pacing for the Termination of Atrial Fibrillation during Catheter Ablation of Atrial Fibrillation" Journal of Clinical Medicine 11, no. 3: 575. https://doi.org/10.3390/jcm11030575






