The Effect of Pressotherapy on Performance and Recovery in the Management of Delayed Onset Muscle Soreness: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy and Screening Procedures
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Selection Process, Data Collection, Data Extraction, and Management
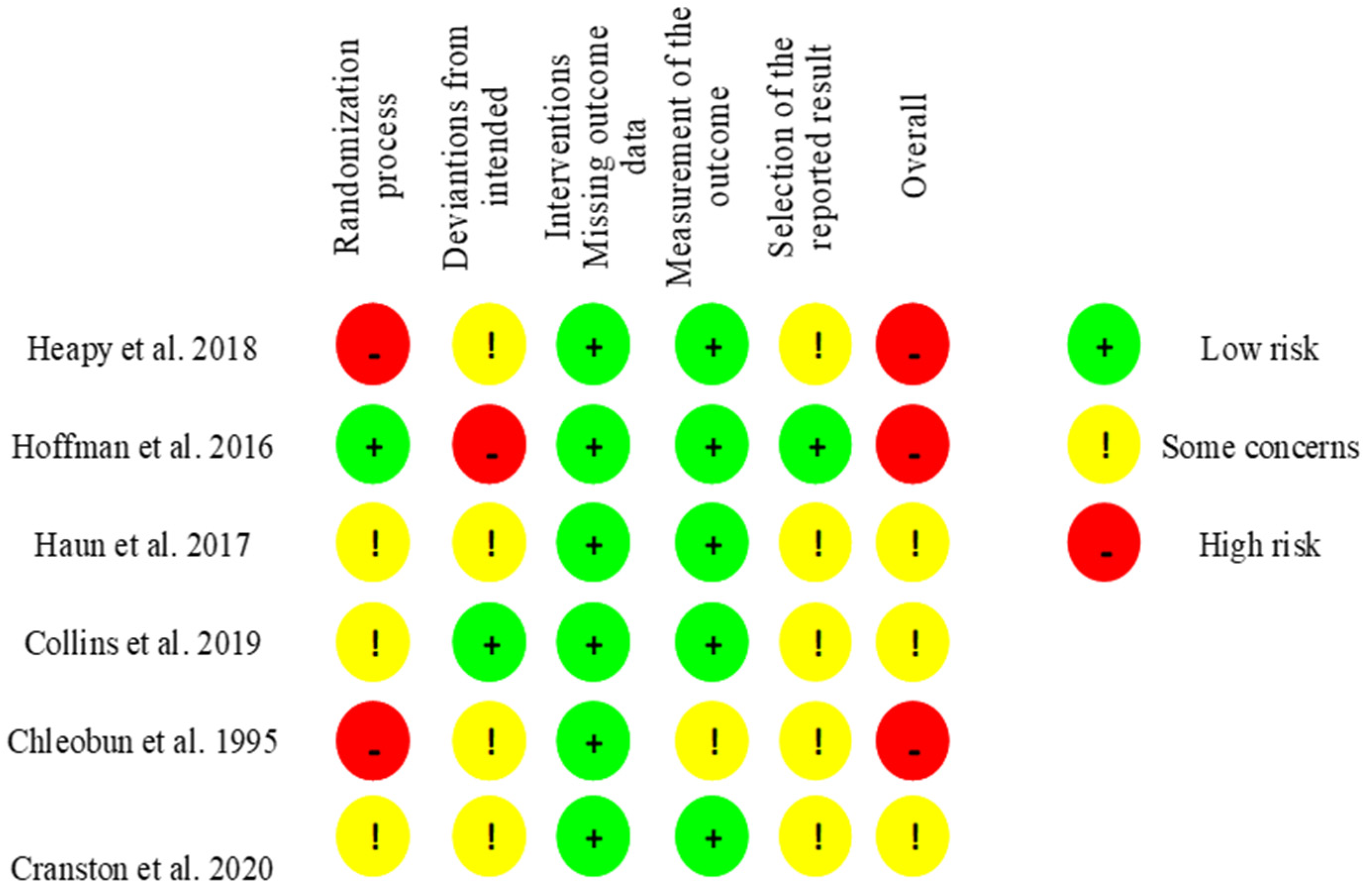
2.5. Risk of Bias Assessment
2.6. Outcome Measures
2.7. Primary Outcomes
2.8. Secondary Outcomes
2.9. Statistical Considerations
3. Results
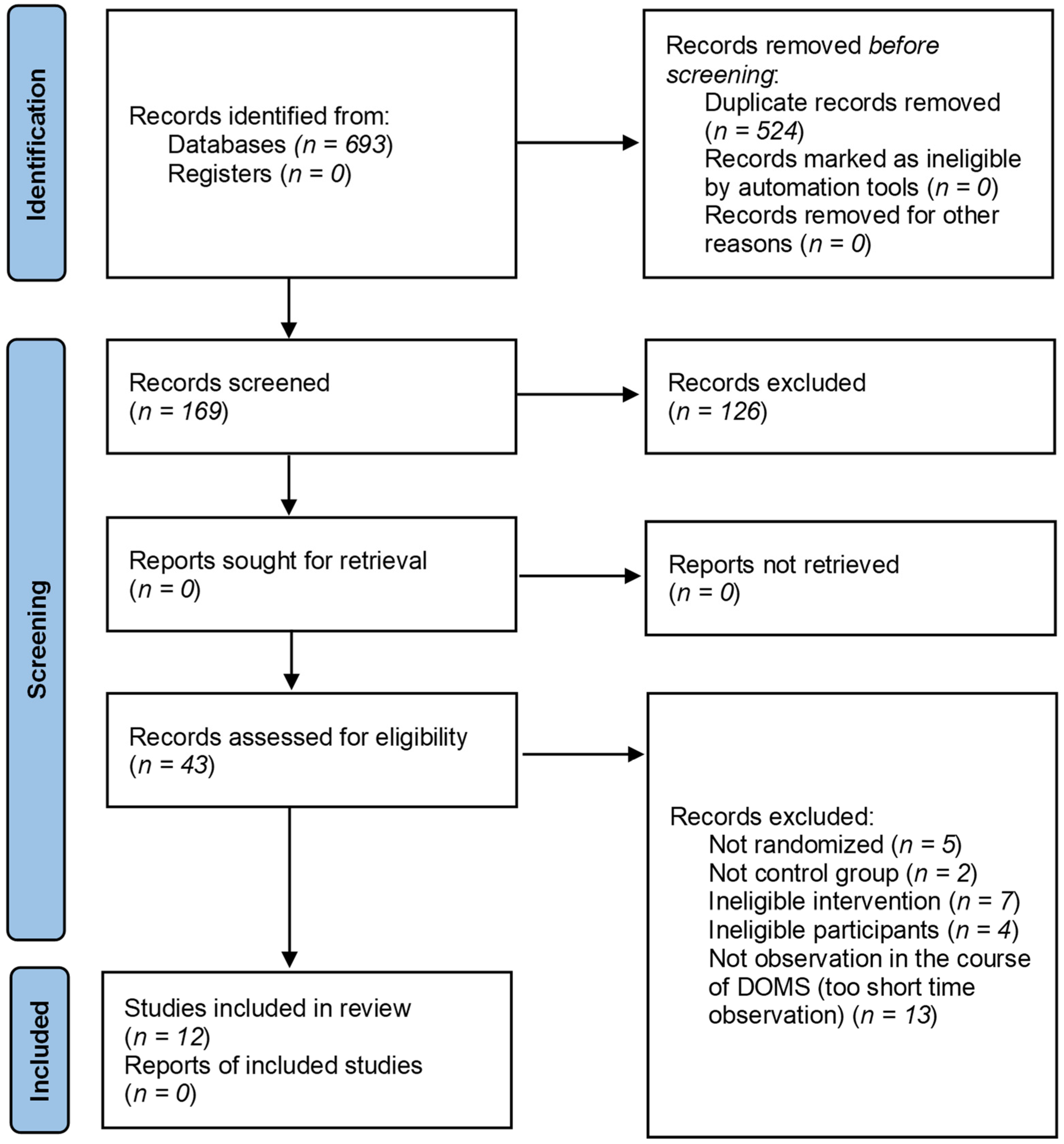
3.1. Results of the Search
3.2. Details of the Intervention Groups in the Included Studies
3.3. Characteristics of the Exercise Protocols, Therapies and Outcomes
3.4. Subgroup Analysis
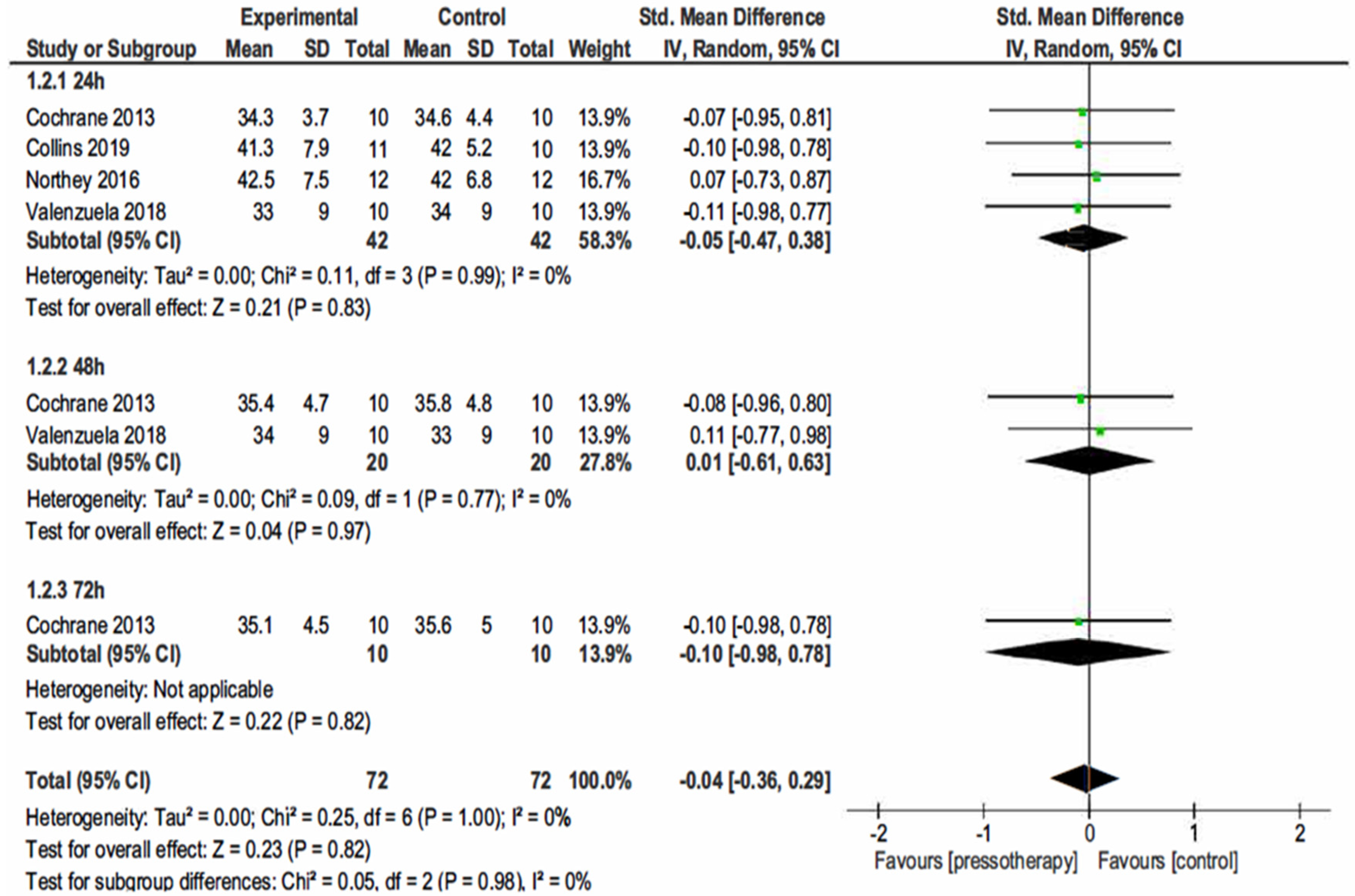
3.4.1. Muscle Soreness
3.4.2. Jump Performance
3.4.3. Creatine Kinase
4. Discussion
4.1. Brief Study Informations: Purposes, Direction, and Possible Main Outcomes
4.2. Serum CK Level
4.3. DOMS
4.4. Jump Performance
4.5. Practical Implications
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Howatson, G.; van Someren, K.A. The Prevention and Treatment of Exercise-Induced Muscle Damage. Sport. Med. 2008, 38, 483–503. [Google Scholar] [CrossRef]
- McAnulty, S.; McAnulty, L.; Nieman, D.; Morrow, J.; Dumke, C.; Henson, D. Effect of NSAID on Muscle Injury and Oxidative Stress. Int. J. Sports Med. 2007, 28, 909–915. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J. A review of nutritional intervention on delayed onset muscle soreness. Part I. J. Exerc. Rehabil. 2014, 10, 349–356. [Google Scholar] [CrossRef]
- Monedero, J.; Donne, B. Effect of Recovery Interventions on Lactate Removal and Subsequent Performance. Int. J. Sports Med. 2000, 21, 593–597. [Google Scholar] [CrossRef] [Green Version]
- Tanner, R.K.; Fuller, K.L.; Ross, M.L.R. Evaluation of three portable blood lactate analysers: Lactate Pro, Lactate Scout and Lactate Plus. Eur. J. Appl. Physiol. 2010, 109, 551–559. [Google Scholar] [CrossRef]
- Mair, J.; Mayr, M.; Mullet, E.; Koller, A.; Haid, C.; Artner-Dworzak, E.; Calzolari, C.; Larue, C.; Puschendorf, B. Rapid Adaptation to Eccentric Exercise-Induced Muscle Damage. Int. J. Sports Med. 1995, 16, 352–356. [Google Scholar] [CrossRef]
- Lieber, R.L.; Fridén, J. Morphologic and Mechanical Basis of Delayed-Onset Muscle Soreness. J. Am. Acad. Orthop. Surg. 2002, 10, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Gaitanos, G.C.; Williams, C.; Boobis, L.H.; Brooks, S. Human muscle metabolism during intermittent maximal exercise. J. Appl. Physiol. 1993, 75, 712–719. [Google Scholar] [CrossRef] [Green Version]
- Machado, A.F.; Ferreira, P.H.; Micheletti, J.K.; de Almeida, A.C.; Lemes, Í.R.; Vanderlei, F.M.; Netto Junior, J.; Pastre, C.M. Can Water Temperature and Immersion Time Influence the Effect of Cold Water Immersion on Muscle Soreness? A Systematic Review and Meta-Analysis. Sport. Med. 2016, 46, 503–514. [Google Scholar] [CrossRef] [Green Version]
- Cochrane, D.J. Effectiveness of using wearable vibration therapy to alleviate muscle soreness. Eur. J. Appl. Physiol. 2017, 117, 501–509. [Google Scholar] [CrossRef]
- Haun, C.T.; Roberts, M.D.; Romero, M.A.; Osburn, S.C.; Mobley, C.B.; Anderson, R.G.; Goodlett, M.D.; Pascoe, D.D.; Martin, J.S. Does external pneumatic compression treatment between bouts of overreaching resistance training sessions exert differential effects on molecular signaling and performance-related variables compared to passive recovery? An exploratory study. PLoS ONE 2017, 12, e0180429. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, M.D.; Badowski, N.; Chin, J.; Stuempfle, K.J. A Randomized Controlled Trial of Massage and Pneumatic Compression for Ultramarathon Recovery. J. Orthop. Sport. Phys. Ther. 2016, 46, 320–326. [Google Scholar] [CrossRef] [Green Version]
- Field, A.; Harper, L.D.; Chrismas, B.C.R.; Fowler, P.M.; McCall, A.; Paul, D.J.; Chamari, K.; Taylor, L. The use of recovery strategies in professional soccer: A worldwide survey. Int. J. Sports Physiol Perform. 2021, 1, 1804–1815. [Google Scholar] [CrossRef]
- Sands, W.A.; Murray, M.B.; Murray, S.R.; McNeal, J.R.; Mizuguchi, S.; Sato, K.; Stone, M.H. Peristaltic Pulse Dynamic Compression of the Lower Extremity Enhances Flexibility. J. Strength Cond. Res. 2014, 28, 1058–1064. [Google Scholar] [CrossRef]
- Sands, W.A.; McNeal, J.R.; Murray, S.R.; Stone, M.H. Dynamic Compression Enhances Pressure-to-Pain Threshold in Elite Athlete Recovery. J. Strength Cond. Res. 2015, 29, 1263–1272. [Google Scholar] [CrossRef] [Green Version]
- Muluk, S.C.; Hirsch, A.T.; Taffe, E.C. Pneumatic Compression Device Treatment of Lower Extremity Lymphedema Elicits Improved Limb Volume and Patient-reported Outcomes. Eur. J. Vasc. Endovasc. Surg. 2013, 46, 480–487. [Google Scholar] [CrossRef] [Green Version]
- Martin, J.S.; Friedenreich, Z.D.; Borges, A.R.; Roberts, M.D. Acute Effects of Peristaltic Pneumatic Compression on Repeated Anaerobic Exercise Performance and Blood Lactate Clearance. J. Strength Cond. Res. 2015, 29, 2900–2906. [Google Scholar] [CrossRef]
- Cochrane, D.; Booker, H.; Mundel, T.; Barnes, M. Does Intermittent Pneumatic Leg Compression Enhance Muscle Recovery after Strenuous Eccentric Exercise? Int. J. Sports Med. 2013, 34, 969–974. [Google Scholar] [CrossRef]
- Chleboun, G.S.; Howell, J.N.; Baker, H.L.; Ballard, T.N.; Graham, J.L.; Hallman, H.L.; Perkins, L.E.; Schauss, J.H.; Conatser, R.R. Intermittent pneumatic compression effect on eccentric exercise-induced swelling, stiffness, and strength loss. Arch. Phys. Med. Rehabil. 1995, 76, 744–749. [Google Scholar] [CrossRef]
- Zuj, K.A.; Prince, C.N.; Hughson, R.L.; Peterson, S.D. Enhanced muscle blood flow with intermittent pneumatic compression of the lower leg during plantar flexion exercise and recovery. J. Appl. Physiol. 2018, 124, 302–311. [Google Scholar] [CrossRef]
- Kevin Stetter, E.H. An Intermittent Pneumatic Compression Device Reduces Blood Lactate Concentrations More Effectively Than Passive Recovery after Wingate Testing. J. Athl. Enhanc. 2013, 2, 18–25. [Google Scholar] [CrossRef] [Green Version]
- Waller, T.; Caine, M.; Morris, R. Intermittent Pneumatic Compression Technology for Sports Recovery in The Engineering of Sport 6; Springer: New York, NY, USA, 2006; pp. 391–396. [Google Scholar]
- Brown, D. A Review of the PubMed PICO Tool: Using Evidence-Based Practice in Health Education. Health Promot. Pract. 2020, 21, 496–498. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- Review Manager (RevMan) [Computer Program]; Version 5.4.1; The Cochrane Collaboration: London, UK, 2020.
- Haun, C.T.; Roberts, M.D.; Romero, M.A.; Osburn, S.C.; Healy, J.C.; Moore, A.N.; Mobley, C.B.; Roberson, P.A.; Kephart, W.C.; Mumford, P.W.; et al. Concomitant external pneumatic compression treatment with consecutive days of high intensity interval training reduces markers of proteolysis. Eur. J. Appl. Physiol. 2017, 117, 2587–2600. [Google Scholar] [CrossRef]
- Collins, R.; McGrath, D.; Horner, K.; Eusebi, S.; Ditroilo, M. Effect of External Counterpulsation on Exercise Recovery in Team Sport Athletes. Int. J. Sports Med. 2019, 40, 511–518. [Google Scholar] [CrossRef]
- Draper, S.N.; Kullman, E.L.; Sparks, K.E.; Little, K.; Thoman, J. Effects of Intermittent Pneumatic Compression on Delayed Onset Muscle Soreness (DOMS) in Long Distance Runners. Int. J. Exerc. Sci. 2020, 13, 75–86. [Google Scholar]
- Northey, J.M.; Rattray, B.; Argus, C.K.; Etxebarria, N.; Driller, M.W. Vascular Occlusion and Sequential Compression for Recovery After Resistance Exercise. J. Strength Cond. Res. 2016, 30, 533–539. [Google Scholar] [CrossRef]
- Heapy, A.M.; Hoffman, M.D.; Verhagen, H.H.; Thompson, S.W.; Dhamija, P.; Sandford, F.J.; Cooper, M.C. A randomized controlled trial of manual therapy and pneumatic compression for recovery from prolonged running—An extended study. Res. Sport. Med. 2018, 26, 354–364. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Montalvo, Z.; Torrontegi, E.; Sánchez-Martínez, G.; Lucia, A.; de la Villa, P. Enhanced External Counterpulsation and Recovery From a Plyometric Exercise Bout. Clin. J. Sport Med. 2018, 30, 416–419. [Google Scholar] [CrossRef]
- Oliver, A.; Driller, M. The Use of Upper-Body Intermittent Sequential Pneumatic Compression Arm Sleeves on Recovery From Exercise in Wheelchair Athletes. Am. J. Phys. Med. Rehabil. 2021, 100, 65–71. [Google Scholar] [CrossRef]
- Cranston, A.W.; Driller, M.W. Investigating the Use of an Intermittent Sequential Pneumatic Compression Arm Sleeve for Recovery After Upper-Body Exercise. J. Strength Cond. Res. 2020. [CrossRef]
- Wiecha, S.; Jarocka, M.; Wiśniowski, P.; Cieśliński, M.; Price, S.; Makaruk, B.; Kotowska, J.; Drabarek, D.; Cieśliński, I.; Sacewicz, T. The efficacy of intermittent pneumatic compression and negative pressure therapy on muscle function, soreness and serum indices of muscle damage: A randomized controlled trial. BMC Sports Sci. Med. Rehabil. 2021, 13, 144. [Google Scholar] [CrossRef] [PubMed]
- Halson, S.L. Nutrition, sleep and recovery. Eur. J. Sport Sci. 2008, 8, 119–126. [Google Scholar] [CrossRef]
- Kerksick, C.M.; Arent, S.; Schoenfeld, B.J.; Stout, J.R.; Campbell, B.; Wilborn, C.D.; Taylor, L.; Kalman, D.; Smith-Ryan, A.E.; Kreider, R.B.; et al. International society of sports nutrition position stand: Nutrient timing. J. Int. Soc. Sports Nutr. 2017, 14, 33. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.K.; Oikawa, S.Y.; Halson, S.; Stephens, J.; O’Riordan, S.; Luhrs, K.; Sopena, B.; Baker, L.B. In-Season Nutrition Strategies and Recovery Modalities to Enhance Recovery for Basketball Players: A Narrative Review. Sport. Med. 2021, 36, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, M.S.; Baker, L.B. Recovery interventions and strategies for improved tennis performance. Br. J. Sports Med. 2014, 48, i18–i21. [Google Scholar] [CrossRef]
- Brancaccio, P.; Maffulli, N.; Limongelli, F.M. Creatine kinase monitoring in sport medicine. Br. Med. Bull. 2007, 81–82, 209–230. [Google Scholar] [CrossRef]
- Noakes, T.D. Effect of Exercise on Serum Enzyme Activities in Humans. Sport. Med. 1987, 4, 245–267. [Google Scholar] [CrossRef]
- Malm, C.; Sjödin, B.; Sjöberg, B.; Lenkei, R.; Renström, P.; Lundberg, I.E.; Ekblom, B. Leukocytes, cytokines, growth factors and hormones in human skeletal muscle and blood after uphill or downhill running. J. Physiol. 2004, 556, 983–1000. [Google Scholar] [CrossRef]
- Baird, M.F.; Graham, S.M.; Baker, J.S.; Bickerstaff, G.F. Creatine-Kinase- and Exercise-Related Muscle Damage Implications for Muscle Performance and Recovery. J. Nutr. Metab. 2012, 2012, 960363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehlers, G.G.; Ball, T.E.; Liston, L. Creatine Kinase Levels are Elevated During 2-A-Day Practices in Collegiate Football Players. J. Athl. Train. 2002, 37, 151–156. [Google Scholar] [PubMed]
- Fallon, K.E.; Sivyer, G.; Sivyer, K.; Dare, A. The biochemistry of runners in a 1600 km ultramarathon. Br. J. Sports Med. 1999, 33, 264–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hortobágyi, T.; Denahan, T. Variability in Creatine Kinase: Methodological, Exercise, and Clinically Related Factors. Int. J. Sports Med. 1989, 10, 69–80. [Google Scholar] [CrossRef]
- Kratz, A.; Lewandrowski, K.B.; Siegel, A.J.; Chun, K.Y.; Flood, J.G.; Van Cott, E.M.; Lee-Lewandrowski, E. Effect of Marathon Running on Hematologic and Biochemical Laboratory Parameters, Including Cardiac Markers. Am. J. Clin. Pathol. 2002, 118, 856–863. [Google Scholar] [CrossRef]
- Cheung, K.; Hume, P.A.; Maxwell, L. Delayed Onset Muscle Soreness. Sport. Med. 2003, 33, 145–164. [Google Scholar] [CrossRef]
- Armstrong, R.B. Mechanisms of exercise-induced delayed onset muscular soreness: A brief review. Med. Sci. Sports Exerc. 1984, 16, 529–538. [Google Scholar] [CrossRef]
- Hamill, J.; Freedson, P.S.; Clarkson, P.M.; Braun, B. Muscle Soreness during Running: Biomechanical and Physiological Considerations. Int. J. Sport Biomech. 1991, 7, 125–137. [Google Scholar] [CrossRef]
- Orchard, J.; Marsden, J.; Lord, S.; Garlick, D. Preseason Hamstring Muscle Weakness Associated with Hamstring Muscle Injury in Australian Footballers. Am. J. Sports Med. 1997, 25, 81–85. [Google Scholar] [CrossRef]
- Edgerton, V.R.; Wolf, S.L.; Levendowski, D.J.; Roy, R.R. Theoretical basis for patterning EMG amplitudes to assess muscle dysfunction. Med. Sci. Sport. Exerc. 1996, 28, 744–751. [Google Scholar] [CrossRef]
- Wessel, J.; Wan, A. Effect of Stretching on the Intensity of Delayed-Onset Muscle Soreness. Clin. J. Sport Med. 1994, 4, 83–87. [Google Scholar] [CrossRef]
- Gulick, D.T.; Kimura, I.F. Delayed Onset Muscle Soreness: What Is It and How Do We Treat It? J. Sport Rehabil. 1996, 5, 234–243. [Google Scholar] [CrossRef]
- Vairo, G.L.; Miller, S.J.; Rier, N.M.C.I.; Uckley, W.B.I. Systematic Review of Efficacy for Manual Lymphatic Drainage Techniques in Sports Medicine and Rehabilitation: An Evidence-Based Practice Approach. J. Man. Manip. Ther. 2009, 17, 80E–89E. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, A.J.; Keane, S.P.; Coglan, J. Force-Velocity Relationship and Stretch-Shortening Cycle Function in Sprint and Endurance Athletes. J. Strength Cond. Res. 2004, 18, 473. [Google Scholar] [CrossRef] [PubMed]
- Loturco, I.; Pereira, L.A.; Cal Abad, C.C.; D’Angelo, R.A.; Fernandes, V.; Kitamura, K.; Kobal, R.; Nakamura, F.Y. Vertical and Horizontal Jump Tests Are Strongly Associated With Competitive Performance in 100-m Dash Events. J. Strength Cond. Res. 2015, 29, 1966–1971. [Google Scholar] [CrossRef]
- Sattler, T.; Hadžić, V.; Dervišević, E.; Markovic, G. Vertical Jump Performance of Professional Male and Female Volleyball Players. J. Strength Cond. Res. 2015, 29, 1486–1493. [Google Scholar] [CrossRef]
- Castagna, C.; Castellini, E. Vertical Jump Performance in Italian Male and Female National Team Soccer Players. J. Strength Cond. Res. 2013, 27, 1156–1161. [Google Scholar] [CrossRef]
- Kons, R.L.; Ache-Dias, J.; Detanico, D.; Barth, J.; Dal Pupo, J. Is Vertical Jump Height an Indicator of Athletes’ Power Output in Different Sport Modalities? J. Strength Cond. Res. 2018, 32, 708–715. [Google Scholar] [CrossRef]
- Canavan, P.K.; Vescovi, J.D. Evaluation of Power Prediction Equations: Peak Vertical Jumping Power in Women. Med. Sci. Sports Exerc. 2004, 36, 1589–1593. [Google Scholar] [CrossRef]
- Potteiger, J.A.; Lockwood, R.H.; Haub, M.D.; Dolezal, B.A.; Almuzaini, K.S.; Schroeder, J.M.; Zebas, C.J.; Lockwood, R.; Dolezal, B.; Almuzaini, K.; et al. Muscle Power and Fiber Characteristics Following 8 Weeks of Plyometric Training. J. Strength Cond. Res. 1999, 13, 275–279. [Google Scholar]
- Bobbert, M.F. Drop Jumping as a Training Method for Jumping Ability. Sports Med. 1990, 9, 7–22. [Google Scholar] [CrossRef]
- Markovic, G.; Markovic, G. Does Plyometric Training Improve Vertical Jump Height? A Meta-Analytical Review. Br. J. Sports Med. 2007, 41, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Morrison, A.; Polisena, J.; Husereau, D.; Moulton, K.; Clark, M.; Fiander, M.; Mierzwinski-Urban, M.; Clifford, T.; Hutton, B.; Rabb, D. The Effect of English-Language Restriction on Systematic Review-Based Meta-Analyses: A Systematic Review of Empirical Studies. Int. J. Technol. Assess. Health Care 2012, 28, 138–144. [Google Scholar] [CrossRef] [PubMed]



| Author/Country | Design/Publication Year | Participant Cohort (Training Status, Sex, Age) | Sample Size (n) | Experimental vs. Control Condition | DOMS Induction Intervention | Outcome Variables and Time of Measurement Post-Exercise (hrs) | Main Effects [* p < 0.05: Pre-Post (× Time)] | Total Exposition Time | Therapy Parameters |
|---|---|---|---|---|---|---|---|---|---|
| Hoffman et al. [12]/USA | RCT/2016 | participants in the 2015 161-km Western States Endurance Run, men (IPC:43 ± 8 years, Massage:46 ± 10 years, con.:45 ± 9 years) | n = 72 n = 24 exp. (IPC) n = 25 exp. (Massage) n = 23 con. | 45 min post exercises IPC (20 min), 45 min post-exercise Massage (20 min) vs. Placebo therapy (20 min) | 161 km ultramarathon race | 400-m run times, Muscle Pain and Soreness, Overall Fatigue (prerace, postrace, posttreatment, 24–168 h post-race day) | 400-m run times (pre↔, post 72 h↑, 120 h↓) Lower-Body Muscle Pain and Soreness (pre↔, postrace↑*, posttreatment↑*#, post 24–96 h↑*, post-120–168 h↑ Time and interaction effect* (no group effect) Muscular Fatigue (pre↔, postrace↑, post-treatment↑*#, postrace 24–168 h↑) Time and interaction effect* (no group effect) | 20 min ISPC 20 min Massage 20 min Con. | ISPC—80 mmHg Massage—(the 30 s—calf and hamstring, 1 min—quadriceps), compression (2 min—calf and quadriceps, 3 min hamstring), tapotement (30 s leg and quadriceps) |
| Haun et al. [27]/USA | RCT/2017 | endurance-trained male, participating in ≥72 h per week of endurance exercise for at least 3 months. (EPC:21 ± 0.4 years, con:21.1 ± 0.6 years) | n = 18 n = 9 exp. (EPC) n = 9 con. | 24 h, 48 h, 72 h post-exercises EPC (1 h) vs. Placebo therapy (1 h) 96 h, 120 h treatments only EPC (1 h) vs. placebo therapy (1 h) | 6 km run on the treadmill at an incline of 1% (pre and 16 h) | CK, Muscle Pain, and Soreness (pre-exercises, 72 h to 168 h), Flexibility (pre-exercises, 72 h to 168 h), 6-km run times (pre-exercises, 168 h) | CK (pre, 72 h↑, 96 h↑*, 120 h↑, 144 h↑, 168 h↔) Time effect* (No group or group × interaction effect) Muscle Soreness (pre, 72 h↓*, 96 h↓, 120 h↓*, 144 h↓, 168 h↔) Time effect* (No group or —group effect) Flexibility (pre, 72 h↑, 96 h↔, 120 h↔, 144 h↔,168 h↓) 6 km run time (pre, 168 h↓) | 300 min EPC 300 min EPC Con | EPC—70 mmHg (inflation—30 s/deflation—30 s) |
| Cochrane et al. [18]/NZ | RCO/2013 | 10 healthy males, involved in physical activity (21.0 ± 1.7 years) | n = 10 n = 10 exp. (IPC) n = 10 con. | Immediately post-exercises, 24 h post-exercise, 48 h post-IPC (30 min) vs. Placebo therapy (30 min) | 3 sets × 100 rep. strenuous bout of eccentric exercise on BIODEX | CK, VJ, Muscle Dynamometry ISO 75° - CON 30°/s; 180°/s - ECC 30°/s; 180°/s) (Pre, 24 h, 48 h, post 72 h) | CK (pre, 24 h↑*, 48 h↑, 72 h↑) VJ height (pre, 24 h↓, 48 h↑, 72 h↑) VJ peak power (pre, 24 h↓, 48 h↓, 72 h↓) Peak ISO (pre, 24 h↓*, 48 h↑*, 72 h↑*) Peak CON 30° (pre, 24 h↓*, 48 h↓, 72 h↓) Peak CON 180° (pre, 24 h↓, 48 h↓, 72 h↓) Peak ECC 30° (pre, 24 h↓, 48 h↑, 72 h↑) Peak ECC 180° (pre, 24 h↓, 48 h↑, 72 h↑) Ave ISO 75° (pre, 24 h↓, 48 h↑, 72 h↑) Ave CON 30° (pre, 24 h↓*, 48 h↓, 72 h↓) Ave CON 180° (pre, 24 h↓, 48 h↓, 72 h↓) Ave ECC 30° (pre, 24 h↓, 48 h↑, 72 h↑) Ave ECC 180° (pre, 24 h↓, 48 h↑, 72 h↑) | 90 min IPC 90 min Con | IPC—cell 1 (distal)—70 mmHg, cells 2–4 80 mmHg, cell 5 (proximal) 60 mmHg/deflation—30 s. |
| Collins et al. [28]/IE | RCT/2019 | 21 male team sport athletes (21.6 ± 3.4 years) | n = 21 n = 11 exp. n = 10 con. | Pre-, post-, 24 h post- exercises ECP (20 min) vs. Placebo therapy (20 min) | Max CMJ, 2 × 20 sprint, and second max CMJ | CK, C, T, IgA, sAA, VAS, CMJ height (Pre, post, 24 h post) | CK (pre, post↑*, 24 h↑*) Main effect for time* Cortisol (pre, post↑, 24 h↓) Testosterone (pre, post↑*, 24 h↓*) Main effect for time Alpha-Amylase (pre, post↑*#, 24 h↑*#) Main effect for time, and group Immunoglobulin—A (pre, post↑, 24 h↓) VAS (pre, post↑, 24 h↑*) Main effect for time CMJ (pre, post↓*#, 24 h↑*#) | 60 min ECP 60 min Con | ECP—235.3 ± 26.9 mmHg |
| Draper et al. [29]/USA | RCO/2020 | 10 runners, endurance-trained males (38.7 ± 11.2 years) | n = 10 n = 10 exp. n = 10 con. | 1 h, 24 h, 48 h, 72 h, 96 h, 120 h post- IPC (1 h) vs. 1 h, 24 h, 48 h, 72 h, 96 h, 120 h post- Placebo therapy (1 h) | 2 × 20 mile runs at 70% VO2 max separated by 3 or 4 weeks | CRP, VAS (pre, post, and 24 h, 48 h, 72 h, 96 h, 120 h post) | CRP (pre-, post-run ↔, 24 h↑*, 48 h↑, 72 h↑,96 h↔120) Main effect of time VAS (pre, post-run↑*, 24 h↑*, 48 h↑*, 72 h↑,96 h↑, 120 h↔ pre-run) | 6 h IPC 6 h Con | IPC—90 mmHg for cell 1 (distal) and cell 5 (proximal) and 100 mmHg for cells 2–4 (compression 30 s) |
| Northey et al. [30]/AU | RCO/2016 | 12 strength-trained male (24.0 ± 6.3 years) | n = 12 n = 12 exp. n = 12 con. | 1 h post-exercises SIPC (45 min) vs. Placebo therapy (45 min) | 10 sets × 10 rep. of back squats at 70% 1 repetition maximum | VAS, CON (peak of quadriceps), SJ, CMJ (Pre, post, 1 h, 24 h) | CON peak (pre, post↓*, 1 h↓*, 24 h↔) SJ (pre, post↓*, 1 h↓*, 24 h↓*) CMJ (pre, post↓*, 1 h↓*, 24 h↓) VAS (pre, post↑*, 1 h↑*, 24 h↑*) | 12 min OCC -2 sets × 3 min (per leg) 45 min SIPC 45 min Con | SIPC—80 mmHg (deflation—15 s) OCC 220 mmHg (inflation 3 min) |
| Heapy et al. [31]/NZ | RCT/2018 | 56 ultramarathoners (con. = 19; 42 ± 9 years), (IPC = 18; 41 ± 8 years), (Massage = 19; 43 ± 9 years), men | n = 56 n = 18 exp. (IPC) n = 19 exp. (Massage) n = 19 con. | Post-race, 24 h, 48 h, 72 h post-race IPC (20 min) post-race, 24 h, 48 h, 72 h post-race Massage (25 min) vs. Placebo therapy (20 min) | Run race—three distance options of 62.7 km, 87.4 km, and 102.8 km | 400 m run times (pre-race 1, pre-race 2, post-race at 72 h, 120 h, 168 h, and 336 h), VAS, Fatigue Scores (pre, post, day 24–168 h post and 336 h post) | 400 m run times (pre-race 1, pre-race 2↔, 72 h↑, 120 h↑, 168 h↔, 336 h↔) Time effect* (No group, or interaction effect) VAS (pre-race, post-race↑*, 24 h↑*, 48 h↑, 72 h↑,96 h↑, 120 h↑, 144 h↔, 168 h↔, 336 h↔) Time effect* (No group or interaction effect) Muscle Fatigue (pre-race, post-race↑*, 24 h↑*, 48 h↑*, 72 h↑*#, 96 h↑#, 120 h↑#, 144 h↔, 168 h↔, 336 h↔) Time and interaction effect* (No group effect) | 80 min IPC 100 min Massage 80 min Con. | IPC—80 mmHg |
| Chleboun et al. [19]/USA | RCT/1995 | 22 college women students (21.7 ± 0.7 years) | n = 22 n = 22 exp. (IPC) n = 10 con. (passive rest) | Post-exercise, 24 h, 48 h, 72 h, 96 h, 120 h post IPC (20 min) vs. Placebo therapy (20 min) | 3 sets of ECC exercise performed with weights equal to 90%, 80%, and 70% of the ISO MVC | Pain (five-point pain-rating scale), Swelling (post, day 1 to 5), Stiffness, and Isometric Strength (pre-exercise, pre-, post-IPC days 1 to 5) | Pain (post, 24 h↑, 48 h↑, 72 h↑, 96 h↑, 120 h↑) Swelling (post, pre IPC (post IPC), 24 h↑ (24 h↑*), 48 h↑ (48 h↑*), 72 h↑ (72 h↑*), 96 h↑ (96 h↑), 120 h↑ (120 h↑*)) Stiffness (post-, pre-IPC, (post-IPC), 24 h↑ (24 h↑), 48 h↑ (48 h↓*), 72 h↓ (72↓*), 96 h↓ (96 h↓), 120 h↓ (120 h↓)) Strength (post-, pre-IPC (post-IPC) 24 h↓ (24 h↓), 48 h↓ (48 h↓), 72 h↓ (72 h↓), 96 h↑ (96 h↑), 120 h↑ (120 h↑)) | 120 min IPC | IPC—60 mmHg (inflation 40 s/deflation 20 s) |
| Velanzuela et al. [32]/ES | RCO/2018 | 10 healthy participants (27 ± 4 years), 7 men, 3 females | n = 10 n = 10 exp. n = 10 con. | Post-exercises, 24 h post-EECP (30 min) vs. Placebo therapy (30 min) | Plyometric exercise bout (10 sets of 10 jumps) | Muscle Soreness (VAS), CK, CMJ, RSI (pre and 24 and 48 h post) | Muscle Soreness (pre, 24 h post↑, 48 h post↑) CK (pre, 24 h post↑, 48 h post↑) CMJ (pre, 24 h post↓, 48 h post↔) RSI (pre, 24 h post↓, 48 h post↔) | 60 min EECP 60 min Con. | EECP—80 mmHg |
| Haun C.T. et al. [11]/USA | RCT/2017 | 20 resistance-trained male (21.6 ± 2.4 years) | n = 10 n = 10 exp. (EPC) n = 10 con. | 48 h, 72 h, 96 h, 120 h, 144 h post-EPC (1 h) vs. Placebo therapy (1 h) | 10 sets of five rep. at 80% of back squat 1 RM | CK, Flexibility (pre, 48–168 h post) CRP (pre, 8–168 h post) | CK (pre, 72 h↑*, 96 h↑*, 120 h↑*, 144 h↑, 168 h↑) Flexibility (pre, 72 h↑*#, 96 h↑, 120 h↑*, 144 h↑, 168 h↓) CRP (pre, 48 h↑, 72 h↑, 96 h↑, 120 h↑, 144 h↑, 168 h↑) | 5 h EPC 5 h Con. | EPC—70 mmHg (inflation—30 s/deflation—30 s) |
| Oliver et al. [33]/NZ | RCO/2021 | 11 well-trained wheelchair basketball and rugby athletes (33 ± 10 years), men | n = 11 n = 11 exp. n = 11 con. | post exercises ISPC (20 min) vs. Placebo therapy (30 min) | 10 wheelchair court sprints (28 m). Ten times figure of eight agility drill (the 30 s). Ten sprints (28 m) immediately followed by three medicine ball chest throws | Medicine Ball Throw (m), Wheelchair Sprint, 5, 10, 15 (m) (pre-ex, post-ex, post-rec) Muscle Soreness 0–10 scale and Muscle Fatigue 0–10 scale (pre-ex, post-ex, post-rec, 24 h post-rec) Blood Lactate (post-ex, post-rec) | Medicine Ball Throw (pre-ex, post-ex↓, post-rec↑), Wheelchair Sprint: (5 m) (pre-ex, post-ex↑, post-rec↑) (10 M) (pre-ex, post-ex↑, post-rec↑) (15 m) (pre-ex, post-ex↑, pot-rec↑) Muscle Soreness (pre-ex, post-ex↑, post-rec↑, 24 h post↑) Muscle Fatigue (pre-ex, post-ex↑, post-rec↑, 24 h post↑) Blood Lactate (post-ex, post-rec↓) | 20 min ISPC 30 min Con. | ISPC—80 mmHg (inflation 30 s/deflation 15 s) |
| Cranston et al. [34] | RCT/2020 | 50 resistance-trained athletes (27 ± 4 years), 37 men, 13 females | n = 50 n = 25 exp. n = 25 con. | post exercises ISPC (30 min) vs. Placebo therapy (30 min) | Fatiguing Exercise Circuit (consisted of five different exercises): 1. Reverse grip battle rope waves (the 60 s) 2. 20 m Farmers carry (20 kg for women and 30 kg for men) 3. Chin-ups (maximum number of repetitions) 4. Chin-up bar hangs (long as possible with their hands in a pronated grip) 5. Handgrip crushers (as many times as possible) | Grip Strength Dynamometer (kg), Single-Arm Medicine Ball Throw (m), Preacher Bench Bicep Curls- max repetitions (pre-ex, post-ex, post-rec) | Grip Strength Dynamometer (pre-ex, post-ex↓, post-rec↓) Single-Arm Medicine Ball Throw (pre-ex, post-ex↓, post-rec↑) Max. Rep. Single-Arm Preacher Bench Bicep Curls (pre-ex, post-ex↓, post-rec↓) Triceps Brachii Long Head Soreness (pre-ex, post-ex↑, post-rec↑#, 24 h post-rec↑#) Biceps Brachii Soreness (pre-ex, post-ex↑, post-rec↓#, 24 h post-rec↑#) Extensor Digitorum Soreness (pre-ex, post-ex↑, post-rec↓#, 24 h post-rec↑#) Flexor Carpi Radialis Soreness (pre-ex, post-ex↑, post-rec↓#, 24 h post-rec↑#) | 30 min ISPC 30 min Con. | ISPC—80 mmHg (inflation—26 s/deflation—15 s) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiśniowski, P.; Cieśliński, M.; Jarocka, M.; Kasiak, P.S.; Makaruk, B.; Pawliczek, W.; Wiecha, S. The Effect of Pressotherapy on Performance and Recovery in the Management of Delayed Onset Muscle Soreness: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 2077. https://doi.org/10.3390/jcm11082077
Wiśniowski P, Cieśliński M, Jarocka M, Kasiak PS, Makaruk B, Pawliczek W, Wiecha S. The Effect of Pressotherapy on Performance and Recovery in the Management of Delayed Onset Muscle Soreness: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2022; 11(8):2077. https://doi.org/10.3390/jcm11082077
Chicago/Turabian StyleWiśniowski, Paweł, Maciej Cieśliński, Martyna Jarocka, Przemysław Seweryn Kasiak, Bartłomiej Makaruk, Wojciech Pawliczek, and Szczepan Wiecha. 2022. "The Effect of Pressotherapy on Performance and Recovery in the Management of Delayed Onset Muscle Soreness: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 11, no. 8: 2077. https://doi.org/10.3390/jcm11082077
APA StyleWiśniowski, P., Cieśliński, M., Jarocka, M., Kasiak, P. S., Makaruk, B., Pawliczek, W., & Wiecha, S. (2022). The Effect of Pressotherapy on Performance and Recovery in the Management of Delayed Onset Muscle Soreness: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 11(8), 2077. https://doi.org/10.3390/jcm11082077








